Back to Journals » Infection and Drug Resistance » Volume 17
Extracorporeal Membrane Oxygenation in Septic Cardiomyopathy Caused by Aspergillus Infection: The First Case Report
Authors Wu N, Shen X, Li J, Chen M, Wu G, Niu H, Liang H, Yang T
Received 14 November 2023
Accepted for publication 23 March 2024
Published 30 March 2024 Volume 2024:17 Pages 1303—1307
DOI https://doi.org/10.2147/IDR.S449491
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Nengwen Wu,* Xiaoqing Shen,* Jianwei Li, Miaolian Chen, Guishen Wu, Haiming Niu, Hongkai Liang, Ting Yang
Department of Critical Care Medicine, Zhongshan People’s Hospital, Zhongshan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongkai Liang; Ting Yang, Department of Critical Care Medicine, Zhongshan People’s Hospital, Zhongshan, 528400, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Septic cardiomyopathy (SCM) is often associated with bacterial infections but also occurs with infections with viruses such as influenza and spirochetes, including syphilis. However, there has been no systematic investigation into whether Aspergillus infections can cause septic cardiomyopathy. We report on such a case for the first time in a patient without immunodeficiency. Therefore, clinicians should be concerned with septic cardiomyopathy caused by some atypical or rare pathogens when admitting such patients.
Keywords: septic cardiomyopathy, aspergillus, extracorporeal membrane oxygenation, metagenomic next-generation sequencing
Introduction
Septic cardiomyopathy (SCM) is a complication of sepsis-associated reversible myocardial dysfunction characterized by a reduced ejection fraction (EF <50%) and ventricular dilatation. SCM is accompanied by elevated levels of several cardiac markers, which can be reversed within seven to ten days.1 Numerous pathogenic infections have been associated with SCM.2–6 To our knowledge, there are no published reports on Aspergillus as a cause of septic cardiomyopathy, so we performed the first case report.
Case
A 19-year-old male patient was transferred to our hospital from a lower hospital with the aid of extracorporeal membrane oxygenation (ECMO). This patient presented with a high fever, shortness of breath, and mental changes for approximately 12 hours after surgery for appendicitis. Appendicular perforation with peritonitis was detected on abdominal computed tomography (CT) when the patient was admitted to a lower hospital with metastatic right lower quadrant pain, high fever, nausea, and vomiting. An appendectomy was performed immediately. After surgery, the patient’s blood pressure remained low (mean arterial pressure < 60 mmHg) under continuous norepinephrine and dopamine treatment, fluid resuscitation, and antibiotic therapy. Laboratory test results revealed the following parameters: a white blood cell count of 3.44 × 109/L, a neutrophil ratio of 80.4%, procalcitonin (PCT) > 100 ng/mL, creatine kinase (CK) at 2849 U/L, high sensitivity troponin (hs-TnT) at 136 ng/L, and N-terminal pro-brain natriuretic peptide (NT-proBNP) at 8713 pg/mL. Echocardiography showed cardiac insufficiency, left ventricular enlargement, weakened wall activity, an EF of 20%, and normal valvular activity. Cardiogenic shock was considered. Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO) was initiated immediately, and the patient was transferred to our hospital.
On admission, the patient’s blood pressure was 89/50 mmHg (norepinephrine was administered at 1 ug/kg/min), and the heart rate was 138 beats per minute, the pulse oxygen saturation (SPO2) under invasive mechanical ventilation was 99%. Confusion was observed in the patient, and moist rales were heard in both lungs. The patient expressed pain on abdominal palpation, and the abdominal muscles were slightly tense. The abdominal drainage fluid was yellowish, and his extremities were clammy. Laboratory tests showed that the white blood cell count was 1.44 × 109/L, the neutrophil ratio was 85.4%, hemoglobin was 96 g/L, the platelet count was 29 × 109/L, interleukin-6 > 5000 pg/mL, procalcitonin > 100 ng/mL, a lactate level of 5.7 mmol/L, the creatine kinase (CK) was 2175 U/L, a creatine kinase isoenzyme value (CK-MB) of 36 U/L, and the cardiac troponin T value (cTNT) was 170 ng/l. An electrocardiogram revealed the presence of sinus tachycardia, and an echocardiogram suggested cardiac insufficiency with generalized weakening of the left ventricular wall motion, left ventricular enlargement, and an EF of 17%. A chest CT showed solid compression changes in both lower lungs (Figure 1). An abdominal CT demonstrated free air in the abdominal cavity, retroperitoneum, and peritoneal effusion. A cardiology consultation was requested to rule out the possibility of other cardiac diseases, and the final diagnosis was septic cardiomyopathy. To determine the pathogen as rapidly as possible, we collected blood, bronchoalveolar lavage fluid, and peritoneal effusion samples for culture and metagenomic sequencing (mNGS) immediately after admission. Considering the patient exhibited an abdominal infection with pneumonia, we initiated intravenous infusion of imipenem (1 g q8 h) combined with tigecycline (50 mg q12 h, with an initial dose of 100 mg) as empirical anti-infective therapy. Additional treatments included organ support, appropriate analgesia and sedation, and continuous renal replacement therapy.
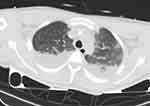 |
Figure 1 Chest CT showed compression atelectasis and consolidation in bilateral lower lung. |
On the morning of the second day, the patient’s blood pressure was 99/67 mmHg, and the SPO2 was 99%. At that time, the norepinephrine administration was 0.5 ug/kg/min, the ECMO rotation speed was 2800 rpm, and the flow rate was 2.45 L/min. Echocardiography showed moderate mitral and tricuspid insufficiency, and the left ventricular wall activity was generally weakened. The EF was 27%. On the same day, the NGS results of the blood and bronchoalveolar lavage fluid revealed the presence of Aspergillus fumigatus. To treat the fungal infection, we utilized an intravenous drip of Amphotericin B Cholesterol Sulfate Complex (3 mg/kg/d) and atomization inhalation (25 mg/q12h) antifungal therapy, starting on the same day. During this period, dynamic monitoring of laboratory parameters indicated a progressive decreasing trend in white blood cell count, procalcitonin, CK, and CK-MB values (Figure 2).
 |
Figure 2 Dynamic evolution of inflammatory markers, cardiogenic markers, and echocardiographic EF values during the first 10 days in intensive care unit. |
On the 6th day of ECMO treatment, the patient’s blood pressure was 105/57 mmHg without vasoactive drugs, the ECMO rotation speed was 1500 rpm, and the flow rate was 2.0 L/min. Echocardiography indicated that the left ventricular systolic function recovered after ECMO flow reduction, the EF was 46%, and the patient’s vital signs were stable. ECMO was terminated on the same day. Echocardiography on the second day after cessation of ECMO showed no abnormalities in ventricular wall motion and valvular structure, the EF was 66%, and the cardiac parameters decreased to the normal range. NGS examination of blood and bronchoalveolar lavage fluid showed no positive results. The patient was transferred from the ICU to a general ward for rehabilitation on day 23 and was discharged on day 42 after improvement was observed.
Discussion
Septic cardiomyopathy (SCM) is considered a potential complication of septic shock. Although there is no broad consensus on the definition of SCM, most scholars believe that septic cardiomyopathy has the following three clinical features: 1) decreased ejection fraction, with most clinical studies using a left ventricular ejection fraction (LVEF) that is < = 50% or an EF value that is reduced by more than 10% compared with the baseline level. 2) The presence of left ventricular dilatation, and 3) an EF and cardiac function recovery time of seven to ten days.1 In previous studies, septic shock with SCM is associated with a poor prognosis because it significantly increases patient mortality.7,8 Also, due to the lack of uniform diagnostic criteria and large-scale studies, the reported incidence of SCM in sepsis/septic shock varies from 13.8 to 40%.8,9
Although the specific pathogenesis of SCM is unknown, existing studies have shown that SCM is due to multiple factors, including mediation of inflammatory mediators, effects of exosomes, and mitochondrial dysfunction in cardiomyocytes.7,10 Septic cardiomyopathy has been repeatedly observed in sepsis and septic shock, but there have been no case reports of septic cardiomyopathy due to Aspergillus infection. Therefore, this is the first report of a case of septic cardiomyopathy associated with Aspergillus infection, in which a patient with suppurative appendicitis and perforation without underlying diseases rapidly progressed into cardiogenic shock. The primary clinical signs observed in this patient were a decreased ejection fraction and left ventricular dilatation accompanied by elevated cardiac markers. Normal cardiac function was restored within ten days by VA ECMO treatment. Other cardiac diseases were excluded, and the only positive etiological result in the blood analysis was A. fumigatus, which confirmed the diagnosis of SCM associated with Aspergillus infection.
Aspergillus fumigatus is a ubiquitous fungus in nature, and it is primary transmission route is airborne, first involving the lungs and, in severe cases, additional organs via hematogenous routes.11 When immunocompetent people inhale the conidia, they are rapidly eliminated by the immune system. However, immunocompromised or immunodeficient individuals are particularly susceptible to developing Aspergillus infections and invasive aspergillosis (IA), with a mortality rate of 50% – 95%.12 However, it has been reported to occurs in non-immunocompromised individuals. In fact, foreign studies have shown that 60% of patients who develop IA in the ICU have no traditional IA risk factors.13 Specifically, ICU patients with Aspergillus infections often lack obvious clinical manifestations in the early stage, which can delay early diagnosis and treatment, increasing the risk of death. We described a patient in this case with a history of smoking but had no underlying disease, immunodeficiency, or other predisposing factors. Although the specific route of A. fumigatus infection remains unclear, we speculate that the likely route in this patient was inhalation of airborne fungal spores. Then, an impaired immune function due to perforation and gangrene associated with appendicitis increases the risk of infection. Furthermore, the possible epithelial cell damage caused by smoking could have increased this patient’s susceptibility to A. fumigatus, which progressed into aspergillosis.
The patient presented with rapidly progressive heart failure at the onset, and his circulation remained difficult to stabilize even with high doses of vasoactive drugs. We initiated VA ECMO when the patient was seen initially, which provided valuable time for patient treatment. Currently, there are no recommended guidelines or treatment strategies for SCM due to multiple factors associated with the disease and its unclear pathogenesis. Similar to septic shock, septic cardiomyopathy treatment primarily includes infection control, fluid therapy, the use of pressor drugs, the application of inotropic drugs and others, as well as other ancillary treatments, including ECMO and Intra-Aortic Balloon Pump (IABP). VA ECMO is typically utilized as an alternative treatment.14 A retrospective study by Vogel et al concluded that the survival rate of SCM patients treated with venous extracorporeal membrane oxygenation was 75%.15 Additional reports have discussed the application of aortic balloon counterpulsation in SCM.16 However, due to the limited data associated with this study, a larger cohort study is needed to evaluate its efficacy and feasibility.
Conclusions
Septic cardiomyopathy is commonly encountered in clinical practice. Clinicians should consider the presence of little-known or atypical pathogens in SCM. Finally, VA ECMO might be an effective treatment modality for rapidly progressing SCM.
Ethics Approval and Consent
The design and methods of the research are in accordance with the requirements of related regulations and procedures as well as the ethical principles. The study approved by the Ethical Committee of Zhongshan People’s Hospital (Medical Ethics 2023-009). The patient information in this article is anonymous and written informed consent was obtained from the patient’s immediate family for the publication of this case report and accompanying images.
Consent for Publication
Written informed consent was obtained from the patient himself for publication of this case report and accompanying images. A copy of the written consent form is available for review by the Editor-in-Chief of this journal. All authors read and approved the final manuscript.
Acknowledgments
We would like to thank the members of our department for their insight and support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or Critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors have read and approved the manuscript.
Funding
No funding was received for this study.
Disclosure
Nengwen Wu and Xiaoqing Shen are co-first authors for this study. The authors declare that they have no competing interests in this work.
References
1. Sato R, Nasu M. A review of sepsis-induced cardiomyopathy. J Intensive Care. 2015;3:48. doi:10.1186/s40560-015-0112-5
2. Endo A, Shiraishi A, Aiboshi J, Hayashi Y, Otomo Y. A case of purpura fulminans caused by Hemophilus influenzae complicated by reversible cardiomyopathy. J Intensive Care. 2014;2(1):13. doi:10.1186/2052-0492-2-13
3. Lin AN, Shaikh A, Lin S, Misra D. A reminder of Escherichia coli sepsis-induced reversible cardiomyopathy. BMJ Case Rep. 2017;2017:bcr–2017. doi:10.1136/bcr-2017-220556
4. Fujioka M, Suzuki K, Iwashita Y, et al. Influenza-associated septic shock accompanied by septic cardiomyopathy that developed in summer and mimicked fulminant myocarditis. Acute medicine & surgery. Apr. 2019;6(2):192–196. doi:10.1002/ams2.394
5. Lee SI, Hwang HJ, Lee SY, et al. Veno-veno-arterial extracorporeal membrane oxygenation for acute respiratory distress syndrome with septic-induced cardiomyopathy due to severe pulmonary tuberculosis. J Artif Organs. 2017;20(4):359–364. doi:10.1007/s10047-017-0982-5
6. Guo S, Guo Q. Syphilis-associated septic cardiomyopathy: case report and review of the literature. BMC Infect Dis. 2021;21(1):33. doi:10.1186/s12879-020-05722-z
7. Ehrman RR, Sullivan AN, Favot MJ, et al. Pathophysiology, echocardiographic evaluation, biomarker findings, and prognostic implications of septic cardiomyopathy: a review of the literature. Critical Care. 2018;22(1):1–14. doi:10.1186/s13054-018-2043-8
8. Li Y, Li H, Zhang D. Analysis of incidence and risk factors of septic cardiomyopathy. Chin J Emerg Med. 2019;2019:836–840.
9. Liang YW, Zhu YF, Zhang R, Zhang M, Ye XL, Wei JR. Incidence, prognosis, and risk factors of sepsis-induced cardiomyopathy. World J Clin Cases. 2021;9(31):9452–9468. doi:10.12998/wjcc.v9.i31.9452
10. Ravikumar N, Sayed MA, Poonsuph CJ, Sehgal R, Shirke MM, Harky A. Septic cardiomyopathy: from basics to management choices. Curr Prob Cardiol. 2021;46(4):100767. doi:10.1016/j.cpcardiol.2020.100767
11. Gu X, Hua Y-H, Zhang Y-D, Bao D, Lv J, Hu H-F. The pathogenesis of, host defense mechanisms, and the development of AFMP4 antigen as a vaccine. Pol J Microbiol. 2021;70(1):3–11. doi:10.33073/pjm-2021-003
12. Waring P, Eichner RD, Müllbacher A, Sjaarda A. Gliotoxin induces apoptosis in macrophages unrelated to its antiphagocytic properties. J Biol Chem. 1988;263(34):18493–18499. doi:10.1016/S0021-9258(19)81385-6
13. Vandewoude KH, Blot SI, Depuydt P, et al. Clinical relevance of Aspergillus isolation from respiratory tract samples in critically ill patients. Critical Care. 2006;10(1):1–10. doi:10.1186/cc4823
14. Liu C, Zhu R, Zhou Z, et al. Sepsis-induced cardiomyopathy complicated with cardiogenic shock patients supported with extracorporeal membrane oxygenation. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017;29(12):1140–1143. doi:10.3760/cma.j.issn.2095-4352.2017.12.018
15. Vogel DJ, Murray J, Czapran AZ, et al. Veno-arterio-venous ECMO for septic cardiomyopathy: a single-centre experience. Perfusion. 2018;33(1_suppl):57–64. doi:10.1177/0267659118766833
16. Takahashi Y, Sonoo T, Naraba H, Hashimoto H, Nakamura K. Effect of intra-arterial balloon pumping for refractory septic cardiomyopathy: a case series. Indian J Crit Care Med. 2019;23(4):182. doi:10.5005/jp-journals-10071-23150
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
