Back to Journals » Infection and Drug Resistance » Volume 12
Extensively drug-resistant Gram-negative bacterial bloodstream infection in hematological disease
Authors Zhou L , Feng S, Sun G, Tang B, Zhu X, Song K, Zhang X, Lu H, Liu H, Sun Z, Zheng C
Received 19 October 2018
Accepted for publication 18 January 2019
Published 26 February 2019 Volume 2019:12 Pages 481—491
DOI https://doi.org/10.2147/IDR.S191462
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Li Zhou,1,* Shanglong Feng,1,2,* Guangyu Sun,1 Baolin Tang,1 Xiaoyu Zhu,1 Kaidi Song,1 Xuhan Zhang,1 Huaiwei Lu,3 Huilan Liu,1 Zimin Sun,1 Changcheng Zheng1,2
1Department of Hematology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China; 2Department of Hematology, Anhui Provincial Hospital, Anhui Medical University, Hefei, China; 3Department of Clinical Laboratory, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China
*These authors contributed equally to this work
Background: Extensively drug-resistant Gram-negative bacterial (XDR-GNB) bloodstream infection (BSI) is difficult to treat and is associated with a high mortality rate in patients with hematological diseases. The aim of this study is to investigate the predisposing risk factors and the efficacy of the antibiotic treatment in these patients, including exploration of efficacy and adverse effects of high-dose tigecycline.
Methods: Between January 2013 and December 2017, 27 XDR-GNB BSI patients with hematological diseases were diagnosed and retrospectively reviewed in the current study.
Results: Clinical response in patients with severe complications (such as severe neutropenia >10 days, grade III–IV acute graft-versus-host disease (aGVHD), and concurrent pneumonia) was significantly lower than in patients without or with only mild complications (P=0.033). The efficacy rate was 62.5% (10/16) in patients with tigecycline-based combination therapy regimen, 77.8% (7/9) with a high-dose tigecycline regimen, and 42.9% (3/7) with a standard-dose tigecycline regimen (P=0.36). The 30-day survival rates of patients undergoing high-dose or standard-dose tigecycline treatment were 66.7% (95% CI: 28.2–87.8) and 57.1% (95% CI: 17.2–83.7), respectively, (P=0.603). Patients with mild complications were associated with superior 30-day survival rates than patients with severe complications (93.8% vs 36.4%, P=0.001), >10 days of neutropenia (90.9% vs 33.3%, P=0.012), severe aGVHD (100% vs 40%, P=0.049), and concurrent pneumonia (84.6% vs 57.1%, P=0.048).
Conclusion: Our study indicated that XDR-GNB BSI in patients of hematological diseases with severe complications, such as long duration of neutropenia (>10 days) and severe aGVHD were associated with poor clinical response and short survival. We first indicated that these patients undergoing high-dose tigecycline treatment had an improved clinical response and an increased 30-day survival rate compared with the standard-dose group, although the differences were not statistically significant. This might be due to more severe complicated patients enrolled in high-dose group and the limited number size in our study.
Keywords: carbapenem resistant bacterial infection, bloodstream infection, high-dose tigecycline, hematological malignancies
Introduction
Extensively drug-resistant Gram-negative bacterial (XDR-GNB) infection refers to infection by Gram-negative bacteria that are only susceptible to tigecycline or polymyxin. The extended use of high-dose glucocorticoids, immunosuppressants, and broad-spectrum antibiotics, especially the wide application of carbapenems, has led to the emergence of XDR bacteria. This type of infection is difficult to treat and is associated with a high mortality rate, and it is generally more severe to have XDR-GNB bloodstream infection (BSI) in patients with hematological diseases concurrent with severe neutropenia. Currently, all major guidelines recommend a combination treatment plan based mainly on tigecycline or polymyxin for the treatment of infections caused by XDR-GNB.1 In China, polymyxin antibiotics are not available for clinical use, and combination therapy based on tigecycline is used most often. However, a previous study reported that treating XDR-GNB BSI with tigecycline at the standard dose resulted in relatively high failure and mortality rates.2 Therefore, it remains controversial whether tigecycline is effective for treating bacteremia. In the current study, we retrospectively analyzed the incidence, risk predisposing factors, and efficacy of the antibiotic treatment of XDR-GNB BSI in patients with hematological diseases, including exploration of efficacy and adverse effects of high-dose tigecycline, in order to determine an optimal strategy for the treatment of XDR-GNB BSI.
Methods and materials
Patients’ selection and laboratory methods
Twenty-seven patients diagnosed with XDR-GNB BSI were retrospective reviewed in this study between January 1, 2013, and December 31, 2017 in Department of Hematology, the First Affiliated Hospital of USTC (University of Science and Technology of China). XDR-GNB was defined as resistant to almost all classes of antibiotics except one or two classes of antibiotics (mainly polymyxin and tigecycline).3–5 The BacT/ALERT 3D automated blood culture system (BioMérieux, Lyon, France) was used to process all blood samples. All strains were identified by Gram stain and the VITEK 2-compact automated system (BioMerieux). Antimicrobial susceptibility testing was also carried out using the VITEK 2-compact system. The susceptibility of the organism tested was determined according to the Clinical and Laboratory Standards Institute recommendations 2013 edition. Quality control strains were included in each batch of antimicrobial testing to ensure the accuracy of the results.
Treatment regimens
Patients with XDR-GNB BSI were treated with double or triple antibiotic regimens. A total of 16 patients received a tigecycline-based combination therapy regimen, which including nine patients received a high-dose tigecycline regimen. Patients who were treated with tigecycline at 50 mg every 12 hours after a 100 mg initial dose were defined as a standard-dose group (SD). Patients who were treated with tigecycline at 100 mg doses every 12 hours after a 200 mg initial dose were defined as the high-dose group (HD). For Enterobacter (Escherichia coli and Klebsiella pneumoniae), administration of carbapenems or not was determined based on the minimum inhibitory concentration (MIC) value. Fosfomycin or aminoglycoside antibiotics were also administered if MIC values indicated sensitively. Non-fermentative Gram-negative bacilli were treated with sulbactam combined with fosfomycin or aminoglycoside antibiotics based on tigecycline treatment. Pseudomonas aeruginosa was treated with β-lactams and fosfomycin or aminoglycoside antibiotics without tigecycline.
Efficacy evaluation
Clinical symptoms, vital signs, and drug-related adverse events were recorded daily during treatment. Routine blood tests were performed daily. Liver and kidney functional values, electrolytes, and fasting plasma glucose levels were determined at least twice every week. Infection markers, including C-reactive protein and procalcitonin, were checked every other day. Blood samples were cultured at least once each week to determine the status of bacterial clearance. A comprehensive evaluation of the clinical efficacy was determined based on the aforementioned indicators.
Definitions and statistical analysis
Definitions of XDR-GNB, BSI, neutropenia, and GVHD were defined according to previously published criteria.3–8 Patient-, disease-, and treatment-related variables were measured using chi-squared test (categorical variables) or Mann–Whitney U test (continuous variables). Survival curves were established by the Kaplan–Meier method, and rank sum tests were performed with 95% CIs for the respective ORs calculated. R statistical software was used for statistical analysis (R Foundation for Statistical Computing). Differences were considered statistically significant at P<0.05.
Ethics
The Institutional Ethics committee of the First Affiliated Hospital of USTC approved this clinical study, and written informed consent of high-dose tigecycline had been provided by the patients (or a parent/legal guardian if the patient was <18 years of age or unconscious), in accordance with the Declaration of Helsinki.
Results
Clinical characteristics
Between January 2013 and December 2017, 27 patients of XDR bacterial BSI were diagnosed and retrospectively reviewed in the current study. The average length of the hospital stay was 29.5 days (range: 3–62). The median age was 33.7 years, with a range of 14.0–70.0 years. Eleven patients were diagnosed with acute myeloid leukemia, eight with acute lymphoblastic leukemia, three with severe aplastic anemia, two with mixed acute leukemia, two with myelodysplastic syndrome, and one with anaplastic large cell lymphoma. A total of 21 patients had deep venous catheters (77.8%), and 10 patients underwent hematopoietic stem cell transplantation (37.0%). A total of 13 patients were infected with E. coli (48.1%), 10 with K. pneumonia (37.1%), 2 with Acinetobacter baumannii (7.4%), and 2 with P. aeruginosa (7.4%). All drug susceptibility tests showed resistance to imipenem, and the MIC values of imipenem were ≥16 in 18 patients (66.7%) and <16 in 9 patients (33.3%). In addition to BSI, 14 patients had concurrent lung infection (51.9%), 7 had concurrent perianal infection (25.9%), and 1 had simultaneous skin and soft-tissue infections (3.7%). All patients received prior antibiotics treatment during 2 weeks before the diagnosis of XDR-GNB, of which 24 patients received imipenem/cilastatin treatment, 12 received vancomycin, 15 cefoperazone/sulbactam, 25 received amikacin, 12 received levofloxacin, and 10 received piperacillin/tazobactam. Among the 27 patients, 12 experienced septic shock (44.4%), 11 had severe complications (40.7%), and 7 had dysfunction of one or more organs (25.9%), although 16 patients had no or mild complications (59.3%) (Table 1). We outlined the drug susceptibility assays, including the name of drugs treated, MIC in Table S1. One major concern of the study is the large time frame that could provide more insight to the variability of XDR-GNB of the perspective cohort. We subdivided the cohorts and represented them as shown in Figure S1.
  | Table 1 Clinical characteristics |
Efficacy
Following a diagnosis of XDR-GNB BSI, 8 of the 27 patients died, giving a mortality rate of 29.6% (95% CI: 10.1–44.9). There was no significant difference of clinical efficacy among the bacterial species (P=0.19) (Figure 1A), and the clinical response was similar between cohorts of MIC values of imipenem ≥16 and <16 groups (P=0.86) (Figure 1B), groups with or without transplantation (P=0.69) (Figure 1C). The cure rate in patients with severe complications (such as severe neutropenia >10 days, and grade III–IV acute GVHD [aGVHD]) was significantly lower than in patients without or with only mild complications (P=0.033) (Figure 1D). Patients combined with pulmonary infection had significantly lower responses than patients without pulmonary infection (P=0.047) (Figure 1E). A total of 16 patients received a tigecycline-based combination therapy regimen, which was effective in 10 patients, giving an efficacy rate of 62.5%. Among these 16 patients, 9 received a high-dose tigecycline regimen, which was effective in 7 patients, giving an effective rate of 77.8%. Seven patients received a standard-dose tigecycline regimen. Of these, it was effective in three patients, giving an efficacy rate of 42.9%. However, there was no statistically significant difference between these two groups (P=0.36) (Figure 1F). Carbapenems were included in the antibiotic combination therapy regimen of 12 patients (44.4%). The median duration of antibiotic use in all patients was 21 days, with a range of 3–61 days. The median duration of tigecycline use was 8 days, with a range of 3–14 days. The median treatment duration of the HD was 7 days, with a range of 3–14 days, and the median treatment duration of the SD was 8.5 days, with a range of 5–13 days.
Survival
The 30-day survival rates of patients infected by XDR E. coli, K. pneumonia, and non-fermentative Gram-negative bacilli were 85.7% (95% CI: 53.9–96.2), 55.6% (95% CI: 20.4–80.5), and 50% (95% CI: 5.78–84.5), respectively, starting from the time when the patients were diagnosed with XDR bacterial BSI. There were no statistically significant differences among these three groups (P=0.154) (Figure 2A). The 30-day survival rates of patients treated with imipenem at an MIC <16 or ≥16 were 77.8% (95% CI: 36.5–93.9) and 66.7% (95% CI: 40.4–83.4), respectively, (P=0.592) (Figure 2B). The 30-day survival rates of transplant and non-transplant patients were 70.0% (95% CI: 32.9–89.2) and 70.6% (95% CI: 43.1–86.6), respectively, (P=0.894) (Figure 2C). The 30-day survival rates of patients undergoing high-dose or standard-dose tigecycline treatment were 66.7% (95% CI: 28.2–87.8) and 57.1% (95% CI: 17.2–83.7), respectively, (P=0.603) (Figure 2D).
The 30-day survival rate of patients with mild complications was significantly higher than that of patients with severe complications, 93.8% (95% CI: 63.2–99.1) and 36.4% (95% CI: 11.2–62.7), respectively, (P=0.001) (Figure 3A). The 30-day survival rate of patients with <10 days of neutropenia was significantly higher than that of patients with >10 days of neutropenia, 90.9% (95% CI: 50.8–98.7) and 33.3% (95% CI: 4.6–67.6), respectively, (P=0.012) (Figure 3B). The 30-day survival rate of patients with mild-to-moderate aGVHD was significantly higher than that of patients with severe aGVHD, 100% and 40.0% (95% CI: 5.2–75.3), respectively, (P=0.049) (Figure 3C). The 30-day survival rate of XDR bacterial bloodstream infected patients who also had concurrent pneumonia was significantly lower than that of patients without pneumonia, 57.1% (95% CI: 28.4–78.0) and 84.6% (95% CI: 51.2–95.9), respectively, (P=0.048) (Figure 3D).
Adverse events of tigecycline treatment
In the 16 patients who received tigecycline treatment, gastrointestinal symptoms and impairment of liver function were the main adverse events. Six patients (37.5%) exhibited gastrointestinal symptoms, such as nausea, vomiting, and diarrhea, during infusion. Among these six patients, four were in the HD (44.4%) and two in the SD (28.6%). These symptoms were all alleviated after reducing the infusion speed. The liver function, including aspartate transaminase, alanine transaminase, and total bilirubin levels were elevated in five patients in the HD and in four patients in the SD. These returned to normal in five patients (55.6%) after providing supportive treatment.
Discussion
The main XDR bacterial strains involved are P. aeruginosa, A. baumannii, and K. pneumonia. Data from the CHINET surveillance indicate that the resistance rate of K. pneumonia to carbapenems is >15%, while the resistance rates of P. aeruginosa and A. baumannii are close to 30% and 70%, respectively, with the rates increasing each year.9 However, in our study, almost half of patients of XDR-GNB BSI were infected with E. coli (48.1%), 37.1% with K. pneumonia, and only 14.8% with A. baumannii or P. aeruginosa.
In current study, we found that patients with ≥10 days of neutropenia and severe aGVHD were associated with higher failure rates in clinical response and lower survival rates. Long-term neutropenia and severe aGVHD both contribute to the extensive damage of the intestinal mucosal barrier, and these are important causes of BSI in patients with hematological disease. High risks for XDR-GNB infection include prolonged duration of neutropenia, immuno-incompetent status, previous widespread broad-spectrum antibiotics treatment, hospitalized in ICU, and multiple invasive procedures. Our data indicated that if neutropenia and immune function did not return to normal within a short period, the prognosis of patients with XDR-GNB BSI in hematological disease would be poor.
Currently, major guidelines recommend a combination treatment strategy based on tigecycline or polymyxin for the treatment of XDR-GNB infections. The standard regimen for tigecycline treatment includes an initial dose at 100 mg, followed by 50 mg infusions every 12 hours. Due to its widespread tissue distribution, the plasma concentration of tigecycline is far below the MICs necessary for the treatment of Enterobacteriaceae, Acinetobacter spp. and anaerobes making it ineffective for the treatment of XDR bacterial BSI.2,10,11 However, a previous study confirmed that tigecycline exhibits characteristics of linear pharmacokinetics in healthy subjects, meaning that the area under the serum concentration–time curve (AUC) increases linearly with an increase in dose.12 Therefore, increasing the dose can improve the AUC/MIC ratio, which results in a superior antibacterial effect against BSIs. This provides a theoretical basis for using high-dose tigecycline for the treatment of XDR-GNB BSI. Multiple previous studies have shown that treatment based on high-dose tigecycline can improve efficacy in critically ill patients with difficult-to-treat infections, including hospital-associated and ventilator-associated pneumonia.2,13,14 Additionally, high-dose tigecycline did not significantly increase the incidence of adverse events in these critically ill patients.14
There are few clinical studies concerning XDR-GNB BSI in hematological diseases, and the treatment of XDR-GNB BSI with high-dose tigecycline in such patients has not been previously reported to our knowledge. In present study, we found that high-dose tigecycline was preliminarily effective for the treatment of patients with hematological diseases who have XDR-GNB BSIs. Compared with the SD, patients undergoing high-dose tigecycline treatment had an improved clinical response and an increased 30-day survival rate, although the differences were not statistically significant. This might be due to more severe patients enrolled in HD and the limited number size in our study. Therefore, a prospective study with a larger sample size is needed to confirm our findings. The tigecycline-related adverse events observed in this study were primarily gastrointestinal related. However, there was no significant difference between the HD and SD. The gastrointestinal symptoms were tolerated and the increased levels of liver enzymes reduced to acceptable levels after supportive treatment, which did not affect the patients’ continued treatment. We believe that the benefits of high-dose tigecycline treatment far outweigh the deficits caused by drug-induced adverse events, especially for critically ill patients.
Conclusion
Our study indicated that E. coli and K. pneumonia might be the main pathogenic microbes of XDR-GNB BSI in hematological diseases. Patients with long duration of neutropenia (>10 days) and severe aGVHD were associated with poor clinical response and short survival. This study suggested that a combination treatment regimen based on high-dose tigecycline might improve the efficacy of treatment of XDR-GNB BSI. This study is a small-scale, retrospective and non-controlled clinical study, so a large prospective controlled clinical trial is needed to further verify this conclusion.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81570159) and the Fundamental Research Funds for the Central Universities of China (no. WK9110000003).
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Paul M, Carmeli Y, Durante-Mangoni E, et al. Combination therapy for carbapenem-resistant gram-negative bacteria. J Antimicrob Chemother. 2014;69(9):2305–2309. | ||
Falagas ME, Vardakas KZ, Tsiveriotis KP, Triarides NA, Tansarli GS. Effectiveness and safety of high-dose tigecycline-containing regimens for the treatment of severe bacterial infections. Int J Antimicrob Agents. 2014;44(1):1–7. | ||
Karaiskos I, Antoniadou A, Giamarellou H. Combination therapy for extensively-drug resistant gram-negative bacteria. Expert Rev Anti Infect Ther. 2017;15(12):1123–1140. | ||
Cai Y, Chua NG, Lim TP, et al. From bench-top to bedside: a prospective in vitro antibiotic combination testing (iACT) service to guide the selection of rationally optimized antimicrobial combinations against extensively drug resistant (XDR) gram negative bacteria (GNB). PLoS One. 2016;11(7):e0158740. | ||
Chinese XDR Consensus Working Group, Guan X, He L, et al. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug-resistant gram-negative bacilli: a Chinese consensus statement. Clin Microbiol Infect. 2016;22(Suppl 1): S15–S25. | ||
Gustinetti G, Mikulska M. Bloodstream infections in neutropenic cancer patients: a practical update. Virulence. 2016;7(3):280–297. | ||
Zheng C, Tang B, Zhu X, et al. Pre-engraftment bloodstream infections in acute leukemia patients undergoing unrelated cord blood transplantation following intensified myeloablative conditioning without ATG. Ann Hematol. 2017;96(1):115–124. | ||
Dignan FL, Clark A, Amrolia P, et al. Diagnosis and management of acute graft-versus-host disease. Br J Haematol. 2012;158(1):30–45. | ||
Xu A, Zheng B, Xu YC, et al. National epidemiology of carbapenem-resistant and extensively drug-resistant gram-negative bacteria isolated from blood samples in China in 2013. Clin Microbiol Infect. 2016;22 Suppl 1:S1–S8. | ||
Fishbain J, Peleg AY. Treatment of Acinetobacter infections. Clin Infect Dis. 2010;51(1):79–84. | ||
Paul M, Carmeli Y, Durante-Mangoni E, et al. Combination therapy for carbapenem-resistant gram-negative bacteria. J Antimicrob Chemother. 2014;69(9):2305–2309. | ||
Muralidharan G, Micalizzi M, Speth J, Raible D, Troy S. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob Agents Chemother. 2005;49(1):220–229. | ||
Ramirez J, Dartois N, Gandjini H, et al. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob Agents Chemother. 2013;57(4):1756–1762. | ||
de Pascale G, Montini L, Pennisi M, et al. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care. 2014;18(3):R90. |
Supplementary materials
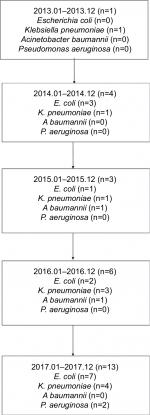  | Figure S1 Subgroups reviewed in the study according to the time frame. |
  | Table S1 Antimicrobial resistance phenotype |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



