Back to Journals » Infection and Drug Resistance » Volume 16
Extended-Spectrum β-Lactamase and Carbapenemase-Producing Gram-Negative Bacteria and Associated Factors Among Patients Suspected of Community and Hospital-Acquired Urinary Tract Infections at Ayder Comprehensive Specialized Hospital, Tigrai, Ethiopia
Authors Gebremedhin MG, Weldu Y, Kahsay AG , Teame G , Adane K
Received 13 March 2023
Accepted for publication 21 June 2023
Published 23 June 2023 Volume 2023:16 Pages 4025—4037
DOI https://doi.org/10.2147/IDR.S412350
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Mulu Gebretsadik Gebremedhin,1 Yemane Weldu,2 Atsebaha Gebrekidan Kahsay,2 Gebrecherkos Teame,3 Kelemework Adane4
1Ayder Comprehensive Specialized Hospital, Mekelle University, Mekelle, Tigrai, Ethiopia; 2Department of Medical Microbiology and Immunology, College of Health Sciences, Mekelle University, Mekelle, Tigrai, Ethiopia; 3Department of Biomedical Research and Technology Transfer, Tigray Health Research Institute, Mekelle, Ethiopia; 4Department of Microbiology, Immunology, and Parasitology, School of Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Atsebaha Gebrekidan Kahsay, Department of Medical Microbiology and Immunology, Mekelle University, P. O. Box: 1871, Mekelle, Tigrai, Ethiopia, Email [email protected]
Background: Little is known about bacteria that produce extended-spectrum beta-lactamases (ESBLs) and carbapenemase in patients with urinary tract infections (UTIs) in Tigrai, Ethiopia. The aim of this study was to describe the magnitude of ESBL- and carbapenemase -producing gram-negative bacteria among patients suspected of community- and hospital-acquired UTIs at a referral hospital in Tigrai, Ethiopia.
Methods: A cross-sectional study was conducted at Ayder Comprehensive Specialized hospital from January 2020 to June 2020. A 10– 20 mL sample of morning mid-stream and catheter urine was collected from consenting participants. Urine samples were cultured on cysteine lactose electrolyte deficient medium and MacConkey agar, and bacteria were identified using standard microbiological protocols. The Kirby-Bauer disk diffusion method was used for antimicrobial susceptibility testing. The combination disk and modified Hodge tests were used detect ESBL and carbapenemase production, respectively. The data was entered into EPI 3.1 software and analyzed using SPSS version 21.
Results: Overall, 67 gram-negative bacteria were recovered from 64 participants. Escherichia coli was the predominant isolate (68.6%), followed by Klebsiella pneumoniae (22.4%), while ESBL production was found in both Escherichia coli and Klebsiella pneumoniae (52.2% and 86.7%, respectively). Isolates recovered from patients with hospital-acquired UTIs were more likely to produce ESBLs (AOR= 16.2; 95% CI: 2.95– 89.5). Carbapenemase was produced by 4.3% of E. coli and 20% of Klebsiella pneumoniae isolates. High resistance rates were found against tetracycline (84.8%), ampicillin (78.3%), amoxicillin/clavulanic acid (58.7%) for Escherichia coli isolates and against ampicillin (93.3%), sulphamethexazole trimethoprim (93.3%), cefotaxime (86.6%), and ceftazidime (86.6%), and tetracycline (73.3%) for Klebsiella pneumoniae.
Conclusion: Most UTIs were caused by ESBL-producing bacteria, especially those that were related to healthcare. Microbiological-based therapy for patients with UTIs is essential at our study site due to high rates of ESBL and significant carbapenemase production with concomitant high rates of drug resistance to several antibiotics.
Keywords: community-acquired infections, carbapenemase, extended-spectrum β-lactamases, gram-negative bacteria, hospital-acquired infections, urinary tract infections
Introduction
Urinary tract infections (UTIs) are one of the major causes of morbidity and mortality among the global population and ranked next to upper respiratory infections.1 Globally, an estimated 150 million people suffer from UTIs every year.1 Members of the Enterobactericeae family such as Escherichia coli (E.coli), Klebsiella species, Proteus species and other non-fermenter gram-negative rods such as Pseudomonas species are the predominant etiologic agents of UTIs.2 The human intestinal system is the primary reservoir for E.coli, and these pathogens use specialized virulence factors such as adhesions, siderophores, and toxins to colonize and invade the urinary tract in an ascending pattern.3 The presence of capsular polysaccharides, type 1 and type 3 pili, aggregative adhesion factors, and siderophores all play key roles in Klebsiella pneumoniae (K. pneumoniae) infections.4
Despite the efforts of infection control and antibiotic stewardship, the incidence of carbapenem-resistant and extended-spectrum beta-lactamase-producing bacteria is rising globally and untreatable superbugs are emerging.5,6 World Health Organization (WHO) predicted that deaths associated with antibiotic resistance will be 10 million per year by 2050 globally unless appropriate control and prevention strategies are implemented.7 Multidrug resistance (MDR) has grown around the world, threatening the public’s health. Recent studies have revealed the emergence of multidrug-resistant bacterial infections, which warrants a prudent use of antibiotics and laboratory-based patient care.8–10 Empirical treatment of UTIs results in an increase in medication resistance among patients, making management of community and hospital-acquired bacterial UTIs challenging.8
On top of this, bacteria causing UTIs are known for producing extended-spectrum beta-lactamase (ESBLs) and are often resistant to third-generation cephalosporins.11 Extended-spectrum beta-lactamase can hydrolyze and become resistant to the latest generation of cephalosporin and monobactams.12 Additionally, because some uropathogens produce carbapenemase enzymes, resistance to last-resort antibiotics like carbapenemase is rising.13 The proportion of carbapenemase-producing bacterial isolates reported from hospital-based studies in sub-Saharan African countries ranged from 9% to 60%.14,15 A significant incidence of ESBL-producers, carbapenem-resistant, and MDR bacteria from patients with UTIs has also been documented in several earlier studies in various contexts, notably from sub-Saharan African countries.14–16
In Ethiopia, where several studies have documented the range and antibiotic resistance pattern of bacteria causing UTIs, there are significant changes in the etiology and resistance profiles of bacterial pathogens across geographical areas and time.17–22 In a 2015 study at Gondar University Hospital in Northwest Ethiopia, 160 (87.4%) of the patients were MDR where the most common isolates were K. pneumoniae and E.coli. Five isolates (2.7%) were carbapenemase producers, and all carbapenemase strains were 100% ESBL producers.22 In a 2016 study at Jimma University Specialized Hospital in southwest Ethiopia, 23% of the uropathogens were ESBL producers where E. coli and K. pneumoniae were the most prevalent. Cefotaxime (100%), ceftriaxone (100%), and ceftazidime (70.6%) resistance was observed in ESBL-producing phenotypes.21
Even though the different etiologies could have an impact on treatment options, the bulk of research in Ethiopia is mainly concentrated on UTIs that were acquired in hospitals, neglecting the bacterial range and antibiotic resistance profiles uropathogens that could be acquired in the community. In addition, although the Ethiopian Ministry of Health has published a national action plan to prevent and control antimicrobial resistance, antibiotic stewardship programs have not been systematically implemented in many Ethiopian hospitals, including our study site.23 Antibiotics are also generally available to the general public over the counter in Ethiopia and are used to empirically treat a variety of bacterial diseases, which may lead to an increase in antimicrobial resistance from time to time.24 Therefore, this study was conducted to describe ESBL- and carbapenemase- producing gram-negative bacteria and associated factors among patients suspected of community and hospital acquired UTIs at Ayder comprehensive specialized hospital, Tigrai, Ethiopia.
Materials and Methods
Study Setting
A cross-sectional prospective study was conducted from January 1, 2020 to June 30, 2020 at Ayder Comprehensive Specialized hospital. The hospital is located in Mekelle, a city located 783 kilometers north of Addis Ababa. The hospital’s primary function is to provide medical care, but it also conducts medical education and research. The hospital has a capacity of more than 500 beds and serves 350 patients on average per day at all outpatient departments and in various admission units. More than 9 million people from Tigrai and bordering regions of Amhara and Afar are served by the hospital.
Study Population and Recruitment
The study populations were all patients suspected of having community and/or hospital acquired UTIs at Ayder Comprehensive Specialized hospital during the study period. Patients of any age group who provided written consent and assent and with suspected UTIs (both community- and hospital-acquired UTI) were included. Following that, data on socio-demographic characteristics of study participants such as age, gender, and other sociodemographic parameters were obtained prospectively using a standardized questionnaire prior to urine sample collection. The clinical profile data for the participants was extracted using a standardized recording format from the patients’ medical recoding document retrospectively.
Sample Size Determination
The sample size was determined using a single proportion formula, n1 = z2 p (1-p)/d2, where n1 was the initial sample size, with a confidence level of 95%, an estimated proportion of 50%, and a margin of error of 5%. After applying a finite population correction, n2 = n1/(1 + (n1/N)), where N was the total number of patients suspected with UTI at study site for a three months period (N = 1300), we obtained a final sample size of 297. The sample was then proportionally distributed, with 126 participants coming from hospital and 171 from the community acquired groups, respectively. A convenient sampling technique was employed to recruit the study participants.
Study Variables and Definitions
Dependent variables in this study were microbiologically confirmed UTIs (community- and hospital-acquired), ESBL production, and drug resistance pattern (susceptible, resistant, and intermediate). Community-acquired infections are defined as infections that have an onset within 48 hours of hospital admission or that present in the outpatient setting.25 Hospital acquired infections, on the other hand, are newly acquired bacteria contracted within a hospital environment after 48 hours of admission or within three days after discharge from other healthcare, and within a month following surgery.26 Age, sex, and other sociodemographic factors were considered as independent variables. In addition, several clinical characteristics and related factors were taken into consideration, such as having a urinary catheterization, having benign prostate hyperplasia, having an underlying disease, undergoing dialysis, having stones in the kidneys, being pregnant, and if the participant has chronic renal fever.
Urine Sample Collection
A sterile, dry, wide-necked, 100 mL container with a leak-proof seal was provided to study participants for the collection of 10–20 mL of morning mid-stream for the ambulatory patients. A trained nurse also aseptically collected catheter urine from inpatients.
Bacterial Isolation and Identification
Collected urine samples were inoculated onto cysteine lactose electrolyte deficient (CLED) (Oxoid, UK) and MacConkey agar (Oxoid, UK) media using a calibrated loop (1μL) and incubated at 35 °C±2°C for 24 hours. A participant was declared positive for UTIs if the colony count was > 103 CFU/mL in symptomatic ambulatory patients, whereas the presence of any possible bacterial pathogens in catheterized patients was considered positive for UTIs. The isolates were identified using sequential microbiological procedures such as gram staining and a series of biochemical tests including triple sugar iron test, lysine decarboxylase test, sulfide and indole positivity test, motility and citrate utilization tests, urease production, and oxidase positivity tests.27
Antimicrobial Susceptibility Testing
For antimicrobial susceptibility testing, we used the disk diffusion method in accordance with the 2014 Clinical Laboratory Standard Institute’s (CLSI) guidelines.28,29 Following species identification, three to five colonies of the same morphological type were chosen from CLED agar (Oxoid, UK). The growth was transferred into a tube containing 4 to 5 mL of normal saline and turbidity was adjusted to 0.5 McFarland standards as assessed by turbidometer. A sterile cotton swab was submerged and swirled several times before being pushed against the upper test tube wall. It was then swabbed 60 degree across the Mueller Hinton Agar (Oxoid, UK) surface. The antibiotic classes we considered were penicillins (ampicillin, amoxicillin/clavulanic acid), cephalosporins (cefotaxime and ceftazidime), aminoglycosides (amikacin and gentamicin), carbapenems (meropenem), tetracyclines (tetracycline), floroquinolones (ciprofloxacin), and nitrofuran (nitrofurantoin) and sulfonamides (trimethoprim sulfamethoxazole). All of the antibiotic discs used were from Oxoid, UK and specifically included cefotaxime (30 μg), ampicillin (30 μg), amikacin (30 μg), ceftazidime (30 μg), amoxicillin/clavulanic acid (20/10 μg), ciprofloxacin (30 µg), gentamicin (10 μg), meropenem (30μg), nitrofurantoin (300 ug), tetracycline (30 μg), and trimethoprim sulfamethoxazole (20 μg). These antibiotics were chosen based on CLSI guidelines and availability.28 Antimicrobial impregnated paper disks were placed on the plate and incubated aerobically at 35°C±2 °C for 18 hours. The isolates were then categorized as sensitive, intermediate, or resistant using the CLSI’s defined charts.28 Multidrug resistance (MDR) is defined as resistance to three or more classes of antibiotics.30
Screening for Potential ESBL-Producing Isolates
The ESBL screening test was performed by the standard disk diffusion method by using ceftazidime (30 µg) and cefotaxime (30 µg) (Oxoid, UK) discs as recommended by CLSI guidelines.28 Freshly grown colonies were suspended in normal saline, and the suspension’s turbidity was set at 0.5 McFarland’s standard. The suspension was then inoculated onto Mueller-Hinton agar (Oxoid, UK) using a sterile cotton swab, and the two antibiotic discs were placed at a gap of 20 mm and incubated at 35 2°C for 16–18 hours. The isolates that showed inhibition zone size of ≤ 22 mm for ceftazidime (30 μg) and ≤ 27 mm for cefotaxime (30 μg) were considered as potential ESBL-producers. Further confirmation for ESBLs production was made by using combination disk test (CDT) as recommended by CLSI guidelines as described below.28
Phenotypic Confirmation of ESBL Producers
A suspension of pure bacterial culture matched with 0.5 McFarland turbidity standards was distributed on a Mueller-Hinton agar plate (Oxoid, UK), and amoxicillin-clavulanic acid (Oxoid, UK), was added, along with cefotaxime (30 g) and ceftazidime (30 g) (Oxoid, UK). The plate was incubated at 35oC for 24 hours before being inspected for an enhancement of the oxyimino-β-lactam caused by the inhibitory zone induced by the clavulanate synergy in the amoxicillin-clavulanate disk. An increase in inhibition zone diameter > 5 mm for a combination of the discs relative to ceftazidime or cefotaxime disc alone was recognized as confirmation of ESBL producer according to CLSI criteria.28
Phenotypic Detection of Carbapenemase Production
The Modified Hodge test (MHT) was used to detect carbapenemase production in all meropenem-resistant and meropenem-intermediate Enterobactericeae and P. aeruginosa isolates in this study. Briefly, E. coli ATCC 25922 was prepared as a 0.5 McFarland dilution in 5 mL of broth and a Mueller Hinton agar plate (Oxoid, UK) was streaked with a 1:10 dilution as lawn. In the middle of the test area, a 10 μg disc of meropenem was positioned. From the edge of the disk to the edge of the plate, the test organism was streaked in a straight line. The plate was then incubated for 24 hours at 35°C. When E.coli ATCC 25922 grew around the streak organism and showed indentation, the isolate was recorded as a carbapenemase producer, whereas no growth of E. coli ATCC 25922 along the streak organism indicated a negative test and the isolate was not a carbapenemase producer.28,31
Quality Control
The quality of microbiological analysis was ensured by following standard quality assurance protocols. Commercially available E. coli (ATCC 25922), S. aureus (ATCC 25923), and P. aeruginosa (ATTC 27853) from the Ethiopian Public Health Institute (EPHI) were utilized as controls in each batch of media prepared and during microbiological identification. Mueller-Hinton agar was also tested for quality, with a thickness of 4mm and a pH of 7.2–7.4.
Data Analysis
Data were entered into EPI 3.1 software (http://www.epidata.dk) and analyzed by SPSS version 21 ((IBM Corporation, Armonk, NY, USA). Bivariate and multivariate logistic regression models were used to examine the potential predictor variables with the outcomes of interest. Covariates with p-values of ≤ 0.25 in a bivariate analysis and co-linearity matrix index of ≤ 0.7 were considered for inclusion in the multivariate model. P-value < 0.05 with 95% confidence interval was considered as statistically significant.
Ethical Considerations
Ethical clearance was obtained from the Institutional Review Board (IRB) of Mekelle University, College of Health Sciences with reference number (ERC) 1484/2020. The study was carried out in accordance with relevant national, international and scientific guidelines’ along with our study was conducted in accordance with the Declaration of Helsinki. After briefing the objectives of the study and before collecting the data, informed consent and assent were collected from adult participants and minors’ guardians, respectively. The data and samples were kept confidential and used for the specified objectives only and finally, the specimens were discarded following the infection prevention guideline.
Results
Socio-Demographic and Clinical Characteristics
Table 1 shows the sociodemographic and clinical characteristics of the study participants. In total, 297 urine samples were collected from 126 and 171 patients suspected of having UTIs in the community and hospital, respectively. The participants’ median age was 30 years, with an interquartile range of 18–47 years. Eighty-eight (33%) of the participants were between the ages of 16 and 30, and 162 (54.5%) were females. The majority of participants, 213 (71.7%), were from urban areas. A large number of the urine samples, 131 (44.1%), were taken from medical outpatient departments (OPDs), followed by 77 (26%) from medical wards. Furthermore, 112 (37.7%) of the participants had underlying disease status, with chronic renal disease and diabetes mellitus accounting for 28 (9.4%) and 31 (10.4%) of the cases, respectively. More than one-third of the participants (34.4%) had a history of urinary catheterization, and 146 (49.2%) had previously taken antibiotics, with 17.2% and 13.5% using at least ceftriaxone and ceftazidime, respectively. Seventy-eight (26.3%) of the samples was collected from patients who had recurrent UTIs twice.
Prevalence of Gram- Negative Bacteria
A total of 67 gram-negative bacteria were found among 64 (21.5%) participants, 3 of which were mixed infections. The most common isolate was E. coli 46 (68.6%), followed by K. pneumoniae 15 (22.4%) and P. aeruginosa 3 (4.5%), Figure 1. The majority of E. coli isolates (63%) were from patients who had UTIs in the community. In contrast, 12 (80%) of K. pneumoniae isolates were recovered from patients with hospital acquired UTIs. All carbapenemase producing bacteria 7 (22.6%) were recovered from patients with hospital acquired UTIs, Table 2.
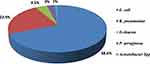 |
Figure 1 Prevalence of gram negative bacterial isolates among patients suspected for community and hospital- acquired UTI in ACSH, Tigrai, Ethiopia. |
Phenotypic Characteristics of the Recovered Isolates
ESBL production was observed in 24 (52.2%) of E. coli, 13 (86.7%) of K. pneumoniae, and 2 (100%) of E. cloacae isolates. None of the isolated P. aeruginosa or Acinetobacter species produced ESBL. On the other hand, 2 (4.3%) of E.coli, 3 (20%) of K. pneumoniae, and 1 (33.3%) of P. aeruginosa isolates produced carbapenemase, Table 2.
Antimicrobial Resistance Pattern of the Recovered Isolates
High resistance rates were found against tetracycline 39/46 (84.8%), ampicillin (78.3%), amoxicillin/clavulanic acid (58.7%) for Escherichia coli isolates and against ampicillin (93.3%), sulphamethexazole trimethoprim (93.3%), cefotaxime (86.6%), and ceftazidime (86.6%), and tetracycline (73.3%) for Klebsiella pneumoniae. E. coli showed the lowest rates of resistance to amikacin (4.3%) and meropenem (4.3%), whereas K. pneumoniae showed the lowest rates of resistance to amikacin (13.3%), meropenem (26.7%) and nitrofurantoin (33.3%). Out of the 3 P. aeruginosa isolates, 1 (33.3%) was resistant to ciprofloxacin, 1 (33.3%) for gentamicin, and another 1 (33.3%) for meropenem. None of the isolates of P. aeruginosa and Acinetobacter Spp were resistant to amikacin and ciprofloxacin, respectively, Table 3.
The antibiotic resistance pattern was further divided to the community and hospital isolates, as shown in Table 4. Tetracycline (79.4% vs 86.7%), ampicillin (76.5% vs 90%), and tetracycline (79.4% vs 86.7%) had higher rates of resistance in isolates recovered from both community- and hospital-acquired UTIs. Twenty-eight (90.3%) of the resistant isolates in the hospital- acquired and 11 (30.5%) in the community- acquired groups were ESBL producers.
Multidrug Resistant of Gram Negative Bacterial Isolates
The overall multidrug resistant gram negative bacteria in our study were 56 (83.6%). Thirteen (19.4%) and nine (9%) isolates showed MDRs to seven and eight antimicrobial classes, respectively. The multidrug resistance rates of E. coli and K. pneumonia were 37 (80.4) and 14 (93.3) respectively. Seven (15.2%) and six (40%) isolates of E. coli and K. pneumonia showed resistant to seven classes of antibiotics respectively. Three isolates (6.5%) of E. coli showed resistant to eight classes of antibiotics. The only two isolates of Enterobacter cloacae and only one isolates of Enterobacter spp showed MDRs to eight classes of antibiotics but 1 (33.3%) isolate of Pseudomonas showed MDR to seven classes of antibiotics, Table 5.
Factors Associated with Urine Culture-Proven Community- and Hospital-Acquired UTIs
In a multivariate logistic regression analysis, being female [AOR= 2.072; 95% CI: 1.065–4.031], living in a rural area [AOR= 2.061; 95% CI: 1.352–5.003], having underlying disease [AOR= 2.145; 95% CI: 1.116–4.123], and having a frequency of UTI recurrence greater than two times [AOR= 4.287; 95% CI: 1.872–9.821] were significantly associated with having a microbiologically confirmed community and hospital acquired UTIs, Table 6.
 |
Table 6 Factors Associated with Having a Microbiologically-Confirmed Community- and Hospital -Acquired UTIs at a Referral Hospital, Tigrai, Ethiopia from January 2020 to June 2020 (n = 297) |
Factors Associated with ESBL Production Among the Recovered Isolates
In a multivariate analysis, isolates recovered from patients with hospital acquired UTIs were significantly more likely to produce ESBL [AOR= 16.237; 95% CI: 2.947, 89.473] as compared to those recovered from patients with community acquired UTIs, Table 7.
Discussion
In this study, a total of 67 gram-negative bacteria were recovered from 64 participants with community-and hospital-acquired UTIs. E. coli made up the majority of the isolates (68.6%), followed by K. pneumoniae (22.4%), while ESBL production was found in isolates from both K. pneumoniae and E. coli (86.7% and 52.2%, respectively). Carbapenemase was produced by 4.3% of E. coli and 20% of K. pneumoniae, indicating that carbapenemase-mediated resistance is becoming a concern in our study setting. High resistance rates were observed against sulphamethexazole trimethoprim, amoxicillin/clavulanic acid, ampicillin, and tetracycline.
Our study’s overall observed ESBL production rate (58.2%) is higher than earlier studies in different parts of Ethiopia, most notably a study conducted in southwest Ethiopia (23%)21 and Adama hospital in Central Ethiopia (25%).32 The discrepancies could be attributed to differences in extent of antibiotic use and participant characteristics. In the southwest Ethiopian study, for example, only 33.8% of the isolates were recovered from patients with UTIs at a healthcare facility, but in our study, nearly half (46.3%) of the isolates were obtained from hospital associated UTIs. Studies show that ESBL production and antimicrobial resistance are much higher in health-care associated infections than in the community.33–35 This was also demonstrated in our study, where isolates from UTIs acquired in hospitals were substantially more likely to produce ESBL than isolates from UTIs acquired in communities. It is also worth noting that our study period coincides with the COVID-19 pandemic, and because antibiotics may have been used to empirically treat febrile patients to prevent secondary bacterial infections, this may have increased ESBL and carbapenemase production and associated drug resistance including in patients with UTIs.36 Our results might indicate that ESBL-producing gram-negative bacteria are expanding quickly in Ethiopia over time.
When compared to findings from other countries, such as those from Nepal (55.2%),37 Italy (42.9%),38 and Sri Lanka (40.2%),39 ESBL production is also higher in our study. Although these studies included patients with healthcare-associated UTIs like ours, the discrepancies may be due to variations in antibiotic use policies, infection prevention practices, and sample size. Contrary to those countries, Ethiopia allows people to purchase antibiotics over-The-counter and use them against infections, which could promote the production of ESBLs and eventually result in antimicrobial resistance.24 Our finding, however, is lower than one from Ethiopian children, where 79% of the isolates were found to be ESBL producers.20 This high level of ESBL production in the previous study could be attributed to the fact that the majority of study participants (74%) were inpatients.
K. pneumoniae was the most common ESBL producing gram- negative bacteria (88.2%). This is comparable with previous studies done in Ethiopia17 Nigeria, North America40 & Europe41 and Bangladesh.42 On the other hand, our findings contradicted other reports from Ethiopia,21 Sri Lanka,39 and India.43 This disparity in the findings could be attributed to differences in the use of antibiotics, corticosteroid use, and hospitalization. The prevalence of carbapenemase producing gram negative bacteria was 10.5%, which is line with reports from northwest Ethiopia and sub-Saharan Africa17,19 but lower than the studies from China44 and Nigeria.40 The increasing incidence of carbapenemase-producing strains is a big worry, particularly in countries like Ethiopia where antibiotics are available over the counter and can be used without prescription.
Public health concerns exist in the study site and likely in Ethiopia due to high rates of resistance against tetracycline (84.8%), ampicillin (78.3%), amoxicillin/clavulanic acid (58.7%) for ESBL-producing E. coli isolates and against ampicillin (93.3%), sulphamethexazole trimethoprim (93.3%), cefotaxime (86.6%), and ceftazidime (86.6%), and tetracycline (73.3%) for K. pneumoniae. This may imply that using this class of antibiotics to treat UTIs at our study site may result in treatment failure. Antibiotics that are effective against ESBL-producing pathogens are typically few and may provide serious treatment challenges in the future.45,46 In our investigation, E. coli showed the lowest rates of resistance to amikacin (0.4%) and meropenem (0.4%), whereas K. pneumoniae showed the lowest rates of resistance to amikacin (1.3%), meropenem (2.6%), and nitrofurantoin (3.3%). This further supports the need to use antibiotic susceptibility testing results to help manage patients with UTIs at our study site.
Females, those living in rural areas, those with underlying diseases, and those who had recurrent UTIs more than twice were more likely to have bacteriologically proven UTIs in a multivariate analysis, which is consistent with previous reports.47,48 Instead of relying just on clinical grounds and empirical treatment, such clients presenting with UTI symptoms at our study site should be managed based on microbiological analysis, including antibiotic susceptibility testing results.
Although this study provided important information on the etiologic agents of UTIs and antimicrobial resistance profile of uropathogens at our study site, it was not without limitations. First, we were unable to undertake a molecular analysis due to financial constraints. Given the scarcity of data in developing countries like Ethiopia, we believe that our findings, albeit just phenotypic, will be beneficial to health programmers, particularly those aiming to prevent antimicrobial resistance. Second, we used odds ratios to estimate associations, and as previous research has shown, odds ratios may overestimate the association in cross-sectional studies, and Cox or Poisson regressions may be preferable for this sort of study.49
Conclusions
The majority of UTIs at our study sites were caused by ESBL producing bacteria and especially those acquired in the healthcare. The documented high rate of ESBL and significant carbapenemase production with concomitant high rates of drug resistance, including multidrug resistance to many antibiotic classes, highlights the importance of using microbiological-based therapy for patients with UTIs at the study sites. The associations between UTIs and being female, living in rural areas, and having underlying diseases suggest that such clients presenting with UTI symptoms at our study site should be managed based on microbiological analysis, including antibiotic susceptibility testing results, rather than just on clinical grounds and empirical treatment.
Abbreviations
AMR, Antimicrobial resistance; ATCC, American Type culture Collection; CFU, Colony Forming Unite; CDT, Combination disk test; CLSI, Clinical laboratory standards institute; CLED, Cysteine lysine electrolyte deficiencies; CPE, Carbapenemase producing Enterobacteriaceae; CRE, Carbapenemase resistance Enterobacteriaceae; ESBL, Extended-spectrum β-lactamase; H2S, Hydrogen peroxide; IQR, Inter quartile range; MHA, Muller Hinton Agar; OPD, Outpatient Diagnosis; SIM, Sulfide Indole Motility; WHO, World Health Organization; UTI, Urinary tract infection.
Acknowledgment
We would like to forward our gratitude to administrative office of Ayder Comprehensive Specialized Hospital, College of Health Sciences of Mekelle University for their collaborating in giving ethical clearance and letters of support to conduct the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–284. doi:10.1038/nrmicro3432
2. Mortazavi-Tabatabaei SAR, Ghaderkhani J, Nazari A, Sayehmiri K, Sayehmiri F. Pattern of antibacterial resistance in urinary tract infections: a systematic review and meta-analysis. Int J Preventive Med. 2019;10.
3. Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7(12):653–660. doi:10.1038/nrurol.2010.190
4. Candan ED, Klebsiella pneumoniae: characteristics of carbapenem resistance and virulence factors. Acta Biochimica Polonica. 2015;62(4):56.
5. Viale P, Giannella M, Bartoletti M, Tedeschi S, Lewis R. Considerations about antimicrobial stewardship in settings with epidemic extended-spectrum β-lactamase-producing or carbapenem-resistant Enterobacteriaceae. Infectious Dis Therapy. 2015;4:65–83. doi:10.1007/s40121-015-0081-y
6. Li C, Claeys KC, Justo JA. No Crystal Ball? Using Risk Factors and Scoring Systems to Predict Extended-Spectrum Beta-Lactamase Producing Enterobacterales (ESBL-E) and Carbapenem-Resistant Enterobacterales (CRE) Infections. Entropy. 2022;24(11):147–158. doi:10.3390/e24020147
7. World Health Organization. Addressing the rising prevalence of hearing loss. World Health Organization; 2018.
8. Shafiq M, Zeng M, Permana B, et al. Coexistence of blaNDM–5 and tet (X4) in international high-risk Escherichia coli clone ST648 of human origin in China. Front Microbiol. 2022;13:1135614.
9. Kareem SM, Al-Kadmy IM, Kazaal SS, et al. Detection of gyrA and parC mutations and prevalence of plasmid-mediated quinolone resistance genes in Klebsiella pneumoniae. Infection Drug Resist. 2021;14:555–563. doi:10.2147/IDR.S275852
10. Algammal A, Hetta HF, Mabrok M, Behzadi PJFi M. Emerging multidrug-resistant bacterial pathogens “superbugs”: a rising public health threat. Front Microbiol. 2023;14:1135614.
11. Osthoff M, McGuinness SL, Wagen AZ, Eisen DP. Urinary tract infections due to extended-spectrum beta-lactamase-producing Gram-negative bacteria: identification of risk factors and outcome predictors in an Australian tertiary referral hospital. Int J Infect Dis. 2015;34:79–83. doi:10.1016/j.ijid.2015.03.006
12. Rawat D, Nair DJ. Extended-spectrum β-lactamases in Gram Negative Bacteria. J Global Infect Dis. 2010;2(3):263. doi:10.4103/0974-777X.68531
13. Kanj SS, Kanafani ZA Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum β-lactamase–producing enterobacteriaceae, carbapenem-resistant enterobacteriaceae, and multidrug-resistant Pseudomonas aeruginosa.
14. Gales AC, Sader HS, Jones RN. Urinary tract infection trends in Latin American hospitals: report from the SENTRY antimicrobial surveillance program (1997–2000). Diagnostic Microbiol Infect Dis. 2002;44(3):289–299. doi:10.1016/s0732-8893(02)00470-4
15. Wangai FK, Masika MM, Lule GN, et al. Bridging antimicrobial resistance knowledge gaps: the East African perspective on a global problem. PLoS One. 2019;14(2):e0212131. doi:10.1371/journal.pone.0212131
16. de Andrade SS, Gales AC, Sader HS. Antimicrobial resistance in Gram-negative bacteria from developing countries. Antimicrob Resist Dev Countries. 2010;249–266.
17. Moges F, Eshetie S, Abebe W, et al. High prevalence of extended-spectrum beta-lactamase-producing Gram-negative pathogens from patients attending Felege Hiwot Comprehensive Specialized Hospital, Bahir Dar, Amhara region. PLoS One. 2019;14(4):e0215177. doi:10.1371/journal.pone.0215177
18. Teklu DS, Negeri AA, Legese MH, et al. Extended-spectrum beta-lactamase production and multi-drug resistance among Enterobacteriaceae isolated in Addis Ababa, Ethiopia. Antimicrob Resist Dev Countries. 2019;8:1–12.
19. Beyene D, Bitew A, Fantew S, Mihret A, Evans MJ. Multidrug-resistant profile and prevalence of extended spectrum β-lactamase and carbapenemase production in fermentative Gram-negative bacilli recovered from patients and specimens referred to National Reference Laboratory, Addis Ababa, Ethiopia. PloS one. 2019;14(9):e0222911.
20. Legese MH, Weldearegay GM, Asrat DJI, resistance D. Extended-spectrum beta-lactamase-and carbapenemase-producing Enterobacteriaceae among Ethiopian children. Infect Drug Resist. 2017;10:27–34. doi:10.2147/IDR.S127177
21. Abayneh M, Tesfaw G, Abdissa A. Isolation of extended-spectrum β-lactamase-(ESBL-) producing Escherichia coli and Klebsiella pneumoniae from patients with community-onset urinary tract infections in Jimma University Specialized Hospital, Southwest Ethiopia. Canadian J Infect Dis amp. 2018;2018. doi:10.1155/2018/4846159
22. Eshetie S, Unakal C, Gelaw A, et al. Multidrug resistant and carbapenemase producing Enterobacteriaceae among patients with urinary tract infection at referral Hospital, Northwest Ethiopia. Antimicrob Resist Dev Countries. 2015;4(1):1–8.
23. Health-Ethiopia. Mo. Antimicorbial Resistance Prevention and Containment Strategic Plan: the One-Health Approach, 2021–2025. Addis Ababa, Ethiopia; 2021. Available from: https://e-library.moh.gov.et/library/wp-content/uploads/2021/11/Stakeholder-Engagement-Plan-1-2_AF_draft-Nov-15.pdf.
24. Muhie OA. Antibiotic use and resistance pattern in Ethiopia: systematic review and meta-analysis. Int J Microbiol. 2019;2019.
25. Friedman ND, Kaye KS, Stout JE, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Int Med. 2002;137(10):791–797. doi:10.7326/0003-4819-137-10-200211190-00007
26. Lobdell KW, Stamou S, Sanchez JA. Hospital-acquired infections. Surg Clin North Am. 2012;92(1):65–77. doi:10.1016/j.suc.2011.11.003
27. Cheesbrough M. District Laboratory Practice in Tropical Countries, Part 2. Cambridge university press; 2005.
28. Wayne P. Clinical and Laboratory Standards Institute, Performance Standards for Antimicrobial Susceptibility Testing. 2014;TwentyFourth Informational Supplement. CLSI document M100-S24. Clinical and Laboratory Standards Institute; 2010.
29. Wayne PA. Clinical and Laboratory Standards Institute: Performance standards for antimicrobial susceptibility testing: 20th informational supplement. Clinical and Laboratory Standards Institute; 2010.
30. Magiorakos A, Srinivasan A, Carey R, et al. Bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical Microbiology and Infection: the Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2011;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
31. Pierce VM, Simner PJ, Lonsway DR, et al. Modified carbapenem inactivation method for phenotypic detection of carbapenemase production among Enterobacteriaceae. J Clin Microbiol. 2017;55(8):2321–2333. doi:10.1128/JCM.00193-17
32. Mulisa G, Selassie L, Jarso G, et al. Prevalence of extended Spectrum Beta-lactamase producing Enterobacteriaceae: a cross sectional study at Adama hospital, Adama, Ethiopia. J Emerg Infect Dis. 2016;1(1):1–6.
33. Gashaw M, Berhane M, Bekele S, et al. Emergence of high drug resistant bacterial isolates from patients with health care associated infections at Jimma University medical center: a cross sectional study. Antimicrob Resist Dev Countries. 2018;7:1–8.
34. Huang L-F, Lo Y-C, Su L-H, Chang C-L. Antimicrobial susceptibility patterns among Escherichia coli urinary isolates from community-onset health care-associated urinary tract infection. J Formosan Med Assoc. 2014;113(12):970–973. doi:10.1016/j.jfma.2014.01.009
35. Cho YH, Jung SI, Chung HS, et al. Antimicrobial susceptibilities of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in health care-associated urinary tract infection: focus on susceptibility to fosfomycin. Int Urol Nephrol. 2015;47(7):1059–1066. doi:10.1007/s11255-015-1018-9
36. Adebisi YA, Alaran AJ, Okereke M, et al. COVID-19 and antimicrobial resistance: a review. Infect Dis. 2021;14:11786337211033870. doi:10.1177/11786337211033870
37. Baral P, Neupane S, Marasini BP, Ghimire KR, Lekhak B, Shrestha BJ. High prevalence of multidrug resistance in bacterial uropathogens from Kathmandu, Nepal. BMC Res. 2012;5(1):1–9.
38. Arnoldo L, Migliavacca R, Regattin L, et al. Prevalence of urinary colonization by extended spectrum-beta-lactamase Enterobacteriaceae among catheterised inpatients in Italian long term care facilities. BMC Infect Dis. 2013;13(1):1–8.
39. Tillekeratne LG, Vidanagama D, Tippalagama R, et al. Extended-spectrum ß-lactamase-producing Enterobacteriaceae as a common cause of urinary tract infections in Sri Lanka. Infect amp. 2016;48(3):160–165. doi:10.3947/ic.2016.48.3.160
40. Ibrahim Y, Sani Y, Saleh Q, Saleh A, Hakeem GJ. Phenotypic detection of extended spectrum beta lactamase and carbapenemase co-producing clinical isolates from two tertiary hospitals in Kano, North West Nigeria. Ethiopian J Health Sci. 2017;27(1):3–10. doi:10.4314/ejhs.v27i1.2
41. Hoban DJ, Lascols C, Nicolle LE, et al. Antimicrobial susceptibility of Enterobacteriaceae, including molecular characterization of extended-spectrum beta-lactamase–producing species, in urinary tract isolates from hospitalized patients in North America and Europe: results from the SMART study 2009–2010. Diagnostic Microbiol Infect Dis. 2012;74(1):62–67. doi:10.1016/j.diagmicrobio.2012.05.024
42. Sultan A, Rizvi M, Khan F, Sami H, Khan HM. Increasing antimicrobial resistance among uropathogens: is fosfomycin the answer? Urol Ann. 2015;7(1):26. doi:10.4103/0974-7796.148585
43. Singh V, Tuladhar R. Beta lactamase producing Escherichia coli, Klebsiella pneumoniae and methicillin resistant Staphylococcus aureus among uropathogens. Nepal J Sci Technol. 2015;16(1):105–112.
44. Li B, Xu XH, Zhao ZC, Wang MH, Cao Y. High prevalence of metallo-β-lactamase among carbapenem-resistant Klebsiella pneumoniae in a teaching hospital in China. Canadian J Microbiol. 2014;60(10):691–695. doi:10.1139/cjm-2014-0291
45. Medina E, Pieper DH. Tackling threats and future problems of multidrug-resistant bacteria. Curr Topics Microbiol Immunol. 2016;398:3–33. doi:10.1007/82_2016_492
46. Bader MS, Loeb M, Leto D. Treatment of urinary tract infections in the era of antimicrobial resistance and new antimicrobial agents. Postgraduate Med. 2020;132(3):234–250.
47. van Nieuwkoop C, van der Starre WE, Stalenhoef JE, et al. Treatment duration of febrile urinary tract infection: a pragmatic randomized, double-blind, placebo-controlled non-inferiority trial in men and women. BMC Med. 2017;15:1–9.
48. Alevizakos M, Nasioudis D, Mylonakis EJ. Urinary tract infections caused by ESBL‐producing Enterobacteriaceae in renal transplant recipients: a systematic review and meta‐analysis. Transplant Infect Dis. 2017;19(6):e12759.
49. Antay-Bedregal D, Camargo-Revello E, Alvarado GF. Associated factors vs risk factors in cross-sectional studies. Patient Preference Adherence. 2015;9:1635–1636. doi:10.2147/PPA.S98023
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






