Back to Journals » Journal of Inflammation Research » Volume 17
Expression Profiles of Matrix Metalloproteinases and Their Inhibitors in Nasal Polyps
Authors Duan C , Yu X, Feng X , Shi L , Wang D
Received 3 October 2023
Accepted for publication 28 December 2023
Published 3 January 2024 Volume 2024:17 Pages 29—39
DOI https://doi.org/10.2147/JIR.S438581
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Chen Duan,1 Xuemin Yu,2 Xin Feng,1 Li Shi,3 Deyun Wang4
1Department of Otorhinolaryngology, Qilu Hospital of Shandong University, National Health Commission (NHC) Key Laboratory of Otorhinolaryngology, Shandong University, Jinan, Shandong, 250000, People’s Republic of China; 2Department of Otorhinolaryngology, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, NHC Key Laboratory of Otorhinolaryngology (Shandong University), Qingdao, Shandong, 250000, People’s Republic of China; 3Department of Otolaryngology, The Second Hospital of Shandong University, Jinan, Shandong, 250000, People’s Republic of China; 4Department of Otolaryngology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
Correspondence: Li Shi, Department of Otolaryngology, The Second Hospital of Shandong University, Jinan, Shandong, 250000, People’s Republic of China, Email [email protected] Xin Feng, Department of Otorhinolaryngology, Qilu Hospital of Shandong University, National Health Commission (NHC) Key Laboratory of Otorhinolaryngology, Shandong University, Jinan, Shandong, 250000, People’s Republic of China, Email [email protected]
Purpose: Nasal polyp (NP) is characterized by inflammation of the sinonasal mucosa with predominant inflammatory cell infiltration. Matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) are recognized to play an important role in leukocyte migration in airway inflammation. Herein, efforts were made to confirm the expression levels of MMPs/TIMPs and study the relationship between the infiltration of inflammatory cells and local expression levels of MMPs/TIMPs in NPs.
Patients and Methods: NP tissues were obtained from 42 Chinese patients with bilateral nasal polyps during the endoscopic sinus surgery. Inferior turbinate (IT) tissues from 19 patients with septal deviation were taken during the rhinoplasty surgery as controls. mRNA and protein levels of MMP1, MMP9, MMP10, MMP12, TIMP1 and TIMP3 were assessed by quantitative PCR and immunohistochemistry.
Results: Eosinophilia (72%, 23/32 samples), neutrophilia (41%, 13/32 samples), and increase in macrophages (38%, 12/32 samples) were found in NP tissues. mRNA expression of MMP1 (10.9-fold), MMP9 (4.1-fold), MMP10 (6.7-fold) and MMP12 (3.5-fold) were significantly up-regulated, while TIMP1 (1.5-fold) and TIMP3 (6.0-fold) were significantly down-regulated in NPs (n=42) as compared to the controls (n=19). The immunostaining levels of all 4 MMPs and two TIMPs were higher in NPs than those in controls. The co-localization of MMP1/MMP10/MMP12 and macrophages were identified in NPs. MMP9 was mainly expressed in neutrophils, while TIMP1 or TIMP3 were mostly found in eosinophils in NPs.
Conclusion: The results of our study indicate that tissue remodeling is significant in NPs, where MMPs/TIMPs play important roles in both tissue remodeling and inflammatory cells infiltration.
Keywords: remodeling, inflammatory cells, extracellular matrix, matrix metalloproteinases
Introduction
Chronic rhinosinusitis (CRS) is divided into CRS with nasal polyp (CRSwNP) and CRS without NP (CRSsNP) by the presence or absence of NP.1 NPs are common chronic inflammatory disease that severely affect the quality of life of the patients.2 The etiology and pathophysiology of NPs are still not fully understood, and its medical treatment remains unsatisfactory. NPs are characterized by mucosal inflammation, high concentrations of inflammatory cells, and tissue remodeling including epithelial remodeling, edema, and extracellular matrix (ECM).3,4 However, the tissue remodeling and inflammatory cells infiltration patterns in NPs are still not fully understood.
Matrix metalloproteinases (MMPs) belong to the zinc-dependent endopeptidase family, which can break down the ECM.5 Moreover, MMPs are considered to play an important role in leukocyte migration in airway inflammation, which, according to their degradation substrates, can be divided into six categories: collagenase (MMP1, MMP8, MMP13, MMP18), gelatinase (MMP2, MMP9), matrix hydrolases (MMP3, MMP10, MMP11), membrane-type MMPs (MMP14, MMP15, MMP16, MMP17), and other MMPs (MMP7, MMP12, MMP19, and MMP20).6 MMPs can be specifically inhibited by tissue inhibitor of metalloproteinases (TIMPs)7 including TIMP1, TIMP2, TIMP3, and TIMP4.6 The dynamic balance of MMPs and TIMPs maintains the integrity of ECM.8 Previous studies have demonstrated higher levels of MMPs in NP tissues,9,10 also the possible role that these proteins play in sinonasal polyposis.11 MMP1 was found to be significantly higher in CRSwNP patients than that in controls,12 however, other studies found that the presence of MMP1 in the NP epithelium was debated.13,14 In NPs, significant high expressions of MMP9 and MMP10 were observed compared to those in controls.15 An animal experiment revealed that MMP12 was significantly up-regulated at 3 months in a murine model of chronic rhinosinusitis.16 However, another study found that the protein levels of MMP12 showed no statistical significance.17 Patients with CRSwNP showed significantly lower concentration of TIMP1 compared with patients with CRSsNP, whereas TIMP3 was not detectable.18 In contrast, another study demonstrated the increased expression of TIMP1 in asthmatics.8 Fazilat Mohammed et al19 claimed that TIMP3 was a physiological regulator of inflammation,20 but studies on TIMP3 in NPs have been rarely reported. These studies have shown that the expression of MMPs and TIMPs in airway inflammation is still unclear. Moreover, their relationship with inflammatory cells has not been fully understood too.
The present study aimed to investigate the expression levels of MMP1, MMP9, MMP10 and MMP12, which belong to four major categories of the MMPs family and TIMP1, TIMP3 in NPs. In particular, the relationship between the inflammatory cells and MMPs/TIMPs in NPs was highlighted.
Materials and Methods
Patients and Tissue Samples
A total of 61 patients from the Department of Otolaryngology Department of Qilu Hospital of Shandong University were enrolled. The diagnosis of NP was based upon the presence of relevant clinical symptoms, computed tomographic scans of the sinuses, as well as endoscopic findings. The diagnostic criteria for CRSwNP have been established by following the document of European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS2012).21 NP tissues from 42 patients with bilateral polyps were obtained during the endoscopic sinus surgery. Tissue biopsies of inferior turbinate (IT) from 19 patients with nasal septal deviation were taken during the rhinoplasty surgery as controls. Because some samples were too small, we have prioritized in retaining RNA samples, therefore only 32 NPs and 15 ITs underwent staining experiments. None of the NP patients and control subjects had any history of malignancy, cystic fibrosis, ciliary dyskinesia, allergic fungal sinusitis, maxillary antrochoanal polyps, upper or lower respiratory tract infections within 2 weeks preoperatively, or any other autoimmune diseases. Written consent forms were obtained from all participants, and the procedure to conduct the study was approved by The Medical Ethics Committee of Qilu Hospital of Shandong University (No.2015086).
Evaluation of Nasal Epithelium and Inflammatory Cell Infiltration
In the microscopic evaluation of the epithelium structure, epithelium with more than 4 layers of cells was determined as epithelial hyperplasia.22 Goblet cell hyperplasia was defined as more than 2 layers of goblet cells in the epithelium. Squamous metaplasia was identified in specimens where the epithelium had lost its pseudostratified columnar epithelial structure with absence of goblet cells and ciliated cells, which was replaced by squamous epithelium. Eosinophils and neutrophils were randomly counted in three high power fields (HPF, 400× magnification) from the lamina propria. Eosinophilia or neutrophilia or macrophage cells infiltration was respectively defined as eosinophils or neutrophils or macrophage cells exceeding 10.3
mRNA Expression in Nasal Tissues
A portion of NP or control nasal mucosa was stored in RNAlater solution (Applied Biosystems, Foster City, CA). Total cellular RNA was isolated from nasal tissues with RiboPure Kit (Applied Biosystems), followed by cDNA reverse transcription. mRNA levels of the selected genes were determined by TaqMan gene expression assays (Applied Biosystems) including MMP1 (Hs00899658_m1), MMP9 (Hs00957562_m1), MMP10 (Hs00233987_m1), MMP12 (Hs00159178_m1), TIMP1 (Hs00159178_m1) and TIMP3 (Hs00165949_m1). Relative gene expression was calculated using the comparative 2−ΔΔCt method,23 with GAPDH (Hs02786624_g1) as a house keeping gene.
Histological, Immunohistochemical, and Immunofluorescent Staining
NP tissues were fixed with formalin and embedded in paraffin. The samples were sectioned with microtome (Leica, Wetzlar, Germany), and slides were stained with hematoxylin and eosin (H&E) for general histology evaluation eosinophil infiltration. Goblet cells were highlighted by staining with Alcian Blue periodic acid–Schiff (PAS). The sections were immersed in Alcian Blue pH 2.5 stain solution for 30 minutes followed by periodic acid solution for 5 minutes and Schiff reagent for 15 minutes, which were also counterstained with hematoxylin. The control group was given the same procession.
Immunohistochemical staining was performed using a modified horseradish peroxidase (HRP) technique with the Dako Cytomation EnVision+System-HRP (Dako A/S) in NP and control groups. The sections were processed with Target Retrieval Buffer (Dako A/S), and endogenous peroxidase activity was blocked with 0.3% H2O2. Sections were then stained with primary antibodies at 4°C overnight. The slides were then incubated with Dako EnVision+SystemHRP (Dako A/S) at room temperature for 30 minutes, followed by applying HRP substrate (diaminobenzidine) for color development. All slides were counterstained with hematoxylin. The observers who read the slides were blinded to clinical information of the subjects. The primary antibodies included MMP1 and MMP9, rabbit anti-human pAB (Abcam, Cambridge, MA); MMP10, rabbit anti-human pAB (ABclone, Wuhan, CHINA); MMP12, rabbit anti-human pAB (Proteintech, Chicago, US); and TIMP1/TIMP3: mouse anti-human mAB (Santa Cruz Biotechnology, Dallas, US). Neutrophils were stained with mouse anti-human neutrophil elastase monoclonal antibody (mAb) (Dako A/S, Glostrup, Denmark), and macrophages with mouse anti-human CD68 mAb (Abcam, Cambridge, UK).
Specimens in NP were also assessed by immunofluorescence staining. Tissue sections were processed with Target Retrieval Buffer (Dako A/S). The slides were incubated with primary antibodies at 4°C overnight and then incubated with Alexa Fluor 488 or 594 conjugated secondary antibodies (goat-anti mouse or rabbit immunoglobulin G [IgG], H+L; Molecular Probes, Carlsbad, CA) at 1:400 in the dark at room temperature for 1 hour, which were later mounted with Antifade reagent with DAPI (molecular probes). In addition to the primary antibodies mentioned above, there is also a primary antibody labeled with eosinophils, which is rabbit anti-human (Eosinophil peroxidase) EPX polyclonal antibody (pAb) (EPO) (Biorbyt, Wuhan, CHINA).
Statistical Analysis
Data generated in this study were analyzed using GraphPad Prism 8 and SPSS version 26.0 (SPSS Inc., Chicago, IL). Continuous data were represented as mean with standard deviation (SD) and were assessed for normality and equal variation. In order to compare the demographic distribution and clinical variables among the different groups, Chi-square test was applied for categorical variables and clinical variables between 42 NP patients and 19 controls and Mann–Whitney U-test was carried out to assess for the staining differences between 32 NP patients and 15 controls. Correlation analysis was performed using Spearman correlation by comparing smoking and squamous metaplasia. Statistical significance was determined by a p value of <0.05, and confidence intervals (CIs) were established at 95%.
Results
Demographic and Clinical Characteristics
Demographic and clinical characteristics of the NP and control groups are summarized in Table 1. Atopy was confirmed by either skin prick test or serum specific immunoglobulin E (Ig E) test to common inhalant allergens. Smokers were defined as current cigarette smokers who consume 1 or more packs of cigarettes a day, averaged over 1 year. A regular drinker was defined as a person who drank alcoholic beverages at least once a month. No significant differences were observed between the two groups in age, sex, allergy, asthma, and drinking, but smoking was considered statistically different between the two groups (p<0.05) (Table 1). Then, we performed the correlation analysis in NP group and found a correlation between smoking and squamous metaplasia (correlation coefficient is 0.494, p<0.01).
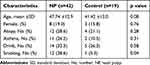 |
Table 1 Characteristics of Subjects Enrolled in the Study |
Tissue Remodeling Patterns in NPs
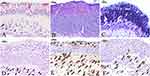
Epithelial remodeling was more prominent in NP patients than those in controls (Figure 1A–D), with 88% (28/32) of NP samples showing hyperplastic epithelium, 25% (8/32) exhibiting squamous cell metaplasia, and 19% (6/32) presenting goblet cell hyperplasia (Figure 1B–D and Table 2). The percentages of epithelium hyperplasia and squamous metaplasia were significantly larger in NPs than those in control group (p<0.05) (Table 2), while no significant difference was observed in the epithelium goblet cell hyperplasia between the two groups (p>0.05). A significant increase in eosinophilia (72%, 23/32 samples), neutrophilia (41%, 13/32 samples), and macrophages (38%, 12/32 samples) was found in NP tissues as compared to those in controls (p<0.05, Figure 1D–F and Table 2).
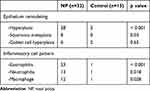 |
Table 2 Histopathological Patterns |
Expression of MMP1, MMP9, MMP10, MMP12 in NPs and Controls
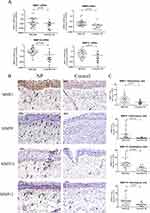
The mRNA expression of MMP1 (10.9-fold), MMP9 (4.1-fold), MMP10 (6.7-fold) and MMP12 (3.5-fold) were significantly up-regulated in NPs (n=42) as compared to those in controls (n=19) (p<0.05) (Figure 2A). The immunostaining showed that these MMPs were mainly expressed in epithelium, ECM, and subepithelial cells in which NP group displayed a strong positive staining of all these MMPs. Furthermore, we also quantified the positive cells numbers in ECM, which also presented higher levels in NPs as compared to controls (p<0.05) (Figure 2B and C).
Expression of TIMP1 and TIMP3 in NPs
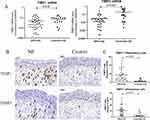
The mRNA level and immunostaining expression of TIMP1 and TIMP3 in NPs and controls are shown in Figure 3. TIMP1 (1.5-fold) and TIMP3 (6.0-fold) were significantly downregulated in NPs (n=42) as compared to controls (p<0.05) (n=19) (Figure 3A). However, the immunostaining levels of the two TIMPs were higher in NPs than those in controls (p<0.05) (Figure 3B and C). Considering changes in the translational process from mRNA to protein, this inconsistence in the expression level of mRNA and immunohistochemical might reflect the complexity of the abnormal regulation mechanism involved in NPs.
Relationships Between MMPs/ TIMPs and Inflammatory Cells in NPs
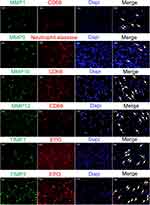
In order to understand the relationships between MMPs/TIMPs and positive cells in ECM, immunofluorescence staining of MMPs/TIMPs and inflammatory cells were performed. The co-localization of MMP1/MMP10/MMP12 and macrophages were identified in nasal polyps. Furthermore, MMP9 was mainly expressed in neutrophils which were identified by neutrophil elastase, while TIMP1 or TIMP3 were mostly found in eosinophils in NPs (Figure 4).
Discussion
Herein, the clinical and histological characteristics of the study subjects were analyzed, and it was found that there were more smokers in NPs than in controls. Moreover, a statistical difference in squamous metaplasia was observed between these two groups. Then, we perform the correlation analysis in NPs and found a correlation between smoking and squamous metaplasia. This result was consistent with our previous study that smoking has a strong association with squamous metaplasia in NPs.24 Besides, in this study, NP samples showed more hyperplastic epithelium, squamous cell metaplasia, and goblet cell hyperplasia than controls, indicating that the epithelial remodeling was common in NPs.
Furthermore, we found that eosinophil infiltration was predominant in NPs (72%), and the increase of neutrophilia (41%) and macrophage (38%) was also found in NP tissues. Besides, mixed infiltration of multiple inflammatory cells was also common in NPs. Although previous studies have demonstrated that a significant proportion of NPs in Asians present a neutrophil-predominant cellular infiltration instead of the eosinophil-predominant cellular infiltration in Caucasians,25–27 the percentage of type 2 signature disease in patients with CRS has been increasing (“eosinophilic shift”) in several Asian countries over the last 20 years.28 Determining the causes and pathophysiology for this eosinophilic shift still requires additional research. Besides, there are other findings indicating that neutrophils are also activated in eosinophilic CRSwNP, and the eosinophil–neutrophil dualism has been revaluated.29,30 CRS endotypes have stressed the complexity of CRS with a frequent presentation of mixed inflammatory patterns and cellular diversity, making it obvious that type 1 or type 2 inflammation alone is not sufficient to explain the pathophysiology,29 Chinese studies found that 35.8% of the patients with CRSwNP displayed a mixed phenotype, associated with type 2 inflammation.31 The results of our study are consistent with this opinion.
Differential expression patterns of MMPs and TIMPs in NPs and control subjects were demonstrated that MMPs and their inhibitors are critical in tissue repair and remodeling,32–40 but the involvement and role of MMPs/TIMPs in NP pathology is controversial.17 Our study further confirmed that the mRNA expression and protein levels of MMP1, MMP9, MMP10, MMP12 were higher in NPs than controls. Moreover, we also demonstrated that these MMPs were mainly expressed in ECM and subepithelial cells, in which NP group displayed a strong positive staining of all these MMPs, suggesting that the formation of NP is closely related to the high expression of MMPs.
As the inhibitor of MMPs, TIMPs also mattered considerably in NP remodeling, but the expression level of TIMP1 was still controversial,17 and few studies were conducted on the expression of TIMP3 in nasal inflammatory diseases. In our study, the mRNA expression levels of TIMP1 and TIMP3 were significantly down-regulated in NPs as compared to controls. However, TIMPs displayed a strong positive staining in subepithelial cells and the numbers of positive cells presented higher levels in NPs as compared to controls. mRNA expression represents the transcriptional level of TIMPs in the entire polyp tissue, while the immunostaining is able to show the expression levels of TIMPs in the individual inflammation cells. Perhaps, some biological changes may occur during the transcriptional process of mRNA to protein in NPs, which need to be studied in future. Besides, it may suggest that MMPs/TIMPs are active in both matrix degradation and synthesis in NPs. MMPs/TIMPs interact and balance each other. When the ratio of the two is imbalanced, it can cause obstacles to the decomposition and synthesis of ECM, leading to the tissue remodeling in NPs. In addition, the mechanisms by which MMPs/TIMPs are synthesized need to be further investigated. We can further investigate the subepithelial cells which MMPs/TIMPs expressed.
MMPs and TIMPs have been reported to be secreted by a wide variety of cells, including epithelial cells, fibroblasts and inflammatory cells such as macrophages and neutrophils in response to a noxious stimulus.41,42 Neutrophils are a major source of MMP9 that have been implicated in the process of dentinogenesis. MMP9 has been reported to be associated with neutrophilic inflammation.43–45 In addition, macrophages form the main source of MMP9 in normal lungs, but neutrophils secrete MMP9 in chronic obstructive lung disease.46 Macrophages are an important source of MMPs, such as MMP10 and MMP12 in urolithiasis.47 Various studies have been conducted to elucidate the relationship between MMPs and macrophages.48 M1 macrophages could release MMP10 to induce vascular remodeling and pulmonary arterial hypertension.49 MMP12, also called macrophage elastase, could mediate a variety of pathological processes. Besides, MMPs secreted by other cells can induce macrophage infiltration. Several MMPs, including MMP1, MMP9, and MMP12, can process on macrophages to its active form, which might evoke the constitutive release of TNF from macrophages to induce tissue damage.50 Another study showed that TIMP1 was associated with eosinophilic cells.51 However, these studies did not identify the main MMPs/TIMPs sources in NPs. In our study, immunofluorescence was performed on MMPs and inflammatory cells, which showed that MMP1/MMP10/MMP12 positive cells were identified in macrophages with positive expression of CD68, suggesting that macrophages might produce these MMPs in NPs. Then, we performed co-staining of MMP9 and neutrophils elastase, which showed that neutrophils are the main source of MMP9 in NPs. Furthermore, up-regulation of TIMP1 or TIMP3 positive cells was mostly found in eosinophilic NPs.
Overall, this study provides evidence for the pathogenic roles of MMPs and TIMPs in the formation of NPs. Our findings are endowed with the potential to identify key factors enhancing tissue remodeling, making it necessarily important to further reveal the pathogenesis of NP. Meanwhile, these findings indicate that tissue remodeling is significant in NPs, where MMPs/TIMPs play an important role in tissue remodeling. Furthermore, we found that inflammatory cells can secrete MMPs/TIMPs, which may consequently promote the infiltration of inflammatory cells. Therefore, there may be a possibility to reduce tissue remodeling via the pharmacological interventions on certain MMPs or TIMPs in the inflammatory cells. We may also reduce inflammatory cell infiltration by controlling the expressional levels of MMPs/TIMPs. However, these hypothesizes need to be further investigated and confirmed in NPs.
There are several limitations to this study. The pathophysiology and pathogenesis of nasal polyps are complex. There are many types of inflammatory cells infiltrated in NPs, and we only investigate three of them. In the future, we need to include other inflammatory cells, such as lymphocytes and mast cells. Second, the PCR results of TIMPs were inconsistent with immunohistochemical staining. It indicates that some changes may occur during the translational process from mRNA to protein, which was not investigated in this study. Finally, this study was performed only in the Chinese patients with a relatively small sample size, which may limit the external validity of this research. Further studies with large sample size and an ethnically diverse population are needed to validate the outcomes of this study.
Conclusion
The results of our study indicate that tissue remodeling is significant in NPs and MMPs/TIMPs play important roles in both tissue remodeling and inflammatory cells infiltration in NPs. In addition, MMPs/TIMPs-related pathways could be regarded as new therapeutic targets in the treatment of epithelial hyperplasia in patients with inflammatory.
Abbreviations
CRS, Chronic rhinosinusitis; CRSwNP, CRS with NP; CRSsNP, CRS without NP; NP, Nasal polyp; MMPs, Matrix metalloproteinases; ECM, extracellular matrix; TIMPs, tissue inhibitor of metalloproteinases; IT, inferior turbinate; H&E, hematoxylin and eosin; PAS, Alcian Blue periodic acid–Schiff; mAb, monoclonal antibody; pAb, polyclonal antibody; HRP, horseradish peroxidase; EPX, Eosinophil peroxidase; EPO, EPX polyclonal antibody; SD, standard deviation; CIs, confidence intervals; No, number; RT-PCR, reverse transcription polymerase chain reaction.
Data Sharing Statement
The datasets generated and analyzed during the current study are not publicly available but are available from Li Shi on reasonable request.
Ethics Approval and Informed Consent
The studies involving human participants were reviewed and approved by The Medical Ethics Committee of Qilu Hospital of Shandong University (No.2015086). Our study complies with the Declaration of Helsinki.
Acknowledgments
We wish to thank Chunwei Li at the department of Otolaryngology of The First Affiliated Hospital of Sun Yat-sen University, for the assistance with the experiments.
The abstract of this paper was presented at the European Academy of Allergy and Clinical Immunology (EAACI) Conference name Expression profiles of matrix metalloproteinases and their inhibitors in nasal polyps as a conference talk with interim findings.
Funding
This study is supported by Shandong Natural Science Foundation (ZR2020QH151), the National Natural Science Foundation of China (82171106 and 81700890), and Taishan Scholar Program of Shandong Province (tsqn202103166).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1–464. doi:10.4193/Rhin20.401
2. Li X, Tao Y, Li X. Expression of MMP-9/TIMP-2 in nasal polyps and its functional implications. Int J Clin Exp Pathol. 2015;8(11):14556–14561.
3. Duan C, Li CW, Zhao L, et al. Differential expression patterns of EGF, EGFR, and ERBB4 in nasal polyp epithelium. PLoS One. 2016;11(6):e0156949. doi:10.1371/journal.pone.0156949
4. Guerra G, Testa D, Salzano FA, et al. Expression of matrix metalloproteinases and their tissue inhibitors in chronic rhinosinusitis with nasal polyps: etiopathogenesis and recurrence. Ear Nose Throat J. 2021;100(5_suppl):597S–605S. doi:10.1177/0145561319896635
5. Feng X, Payne SC, Borish L, Steinke JW. Differential expression of extracellular matrix components in nasal polyp endotypes. Am J Rhinol Allergy. 2019;33(6):665–670. doi:10.1177/1945892419860634
6. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. doi:10.1161/01.RES.0000070112.80711.3D
7. Mavrogonatou E, Angelopoulou MT, Kletsas D. The catabolic effect of TNFα on bovine nucleus pulposus intervertebral disc cells and the restraining role of glucosamine sulfate in the TNFα-mediated up-regulation of MMP-3. J Orthop Res. 2014;32(12):1701–1707. doi:10.1002/jor.22725
8. Profita M, Gagliardo R, Di Giorgi R, et al. In vitro effects of flunisolide on MMP-9, TIMP-1, fibronectin, TGF-beta1 release and apoptosis in sputum cells freshly isolated from mild to moderate asthmatics. Allergy. 2004;59(9):927–932. doi:10.1111/j.1398-9995.2004.00516.x
9. Yao Y, Xie S, Yang C, Zhang J, Wu X, Sun H. Biomarkers in the evaluation and management of chronic rhinosinusitis with nasal polyposis. Eur Arch Otorhinolaryngol. 2017;274(10):3559–3566. doi:10.1007/s00405-017-4547-2
10. Kim DK, Jin HR, Eun KM, et al. The role of interleukin-33 in chronic rhinosinusitis. Thorax. 2017;72(7):635–645. doi:10.1136/thoraxjnl-2016-208772
11. Bachert C, Gevaert P, Holtappels G, Cuvelier C, van Cauwenberge P. Nasal polyposis: from cytokines to growth. Am J Rhinol. 2000;14(5):279–290. doi:10.2500/105065800781329573
12. Lygeros S, Danielides G, Grafanaki K, Riga M. Matrix metalloproteinases and chronic rhinosinusitis with nasal polyposis. Unravelling a puzzle through a systematic review. Rhinology. 2021;59(3):245–257. doi:10.4193/Rhin20.578
13. Eyibilen A, Cayli S, Aladag I, Koç S, Gurbuzler L, Atay GA. Distribution of matrix metalloproteinases MMP-1, MMP-2, MMP-8 and tissue inhibitor of matrix metalloproteinases-2 in nasal polyposis and chronic rhinosinusitis. Histol Histopathol. 2011;26(5):615–621. doi:10.14670/HH-26.615
14. de Borja Callejas F, Picado C, Martínez-Antón A, et al. Differential expression of remodeling markers by tissue structure in nasal polyposis. Am J Rhinol Allergy. 2013;27(3):e69–74. doi:10.2500/ajra.2013.27.3908
15. Rostkowska-Nadolska B, Kapral M, Fraczek M, Kowalczyk M, Gawron W, Mazurek U. A microarray study of gene expression profiles in nasal polyps. Auris Nasus Larynx. 2011;38(1):58–64. doi:10.1016/j.anl.2010.05.002
16. Sautter NB, Delaney KL, Hausman FA, Trune DR. Tissue remodeling gene expression in a murine model of chronic rhinosinusitis. Laryngoscope. 2012;122(4):711–717. doi:10.1002/lary.22148
17. Lygeros S, Danielides G, Kyriakopoulos GC, et al. Evaluation of MMP-12 expression in chronic rhinosinusitis with nasal polyposis. Rhinology. 2022;60(1):39–46. doi:10.4193/Rhin21.320
18. Li X, Meng J, Qiao X, et al. Expression of TGF, matrix metalloproteinases, and tissue inhibitors in Chinese chronic rhinosinusitis. J Allergy Clin Immunol. 2010;125(5):1061–1068. doi:10.1016/j.jaci.2010.02.023
19. Mohammed FF, Smookler DS, Taylor SE, et al. Abnormal TNF activity in Timp3-/- mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet. 2004;36(9):969–977. doi:10.1038/ng1413
20. Black RA. TIMP3 checks inflammation. Nat Genet. 2004;36(9):934–935. doi:10.1038/ng0904-934
21. Fokkens WJ, Lund VJ, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;23(3):1–298.
22. Li CW, Shi L, Zhang KK, et al. Role of p63/p73 in epithelial remodeling and their response to steroid treatment in nasal polyposis. J Allergy Clin Immunol. 2011;127(3):
23. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
24. Gao T, Ng CL, Li C, et al. Smoking is an independent association of squamous metaplasia in Chinese nasal polyps. Int Forum Allergy Rhinol. 2016;6(1):66–74. doi:10.1002/alr.21631
25. Tecimer SH, Kasapoglu F, Demir UL, Ozmen OA, Coskun H, Basut O. Correlation between clinical findings and eosinophil/neutrophil ratio in patients with nasal polyps. Eur Arch Otorhinolaryngol. 2015;272(4):915–921. doi:10.1007/s00405-014-3174-4
26. Cao PP, Li HB, Wang BF, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009;124(3):
27. Tikaram A, Prepageran N. Asian nasal polyps: a separate entity. Med J Malaysia. 2013;68(6):445–447.
28. Zhang Y, Gevaert E, Lou H, et al. Chronic rhinosinusitis in Asia. J Allergy Clin Immunol. 2017;140(5):1230–1239. doi:10.1016/j.jaci.2017.09.009
29. Delemarre T, Bochner BS, Simon HU, Bachert C. Rethinking neutrophils and eosinophils in chronic rhinosinusitis. J Allergy Clin Immunol. 2021;148(2):327–335. doi:10.1016/j.jaci.2021.03.024
30. Gelardi M, Giancaspro R, Duda L, et al. Eosinophil-mast cell pattern of intraepithelial infiltration as a marker of severity in CRSwNP. Sci Rep. 2023;13(1):12101. doi:10.1038/s41598-023-39149-8
31. Wei Y, Zhang J, Wu X, et al. Activated pyrin domain containing 3 (NLRP3) inflammasome in neutrophilic chronic rhinosinusitis with nasal polyps (CRSwNP). J Allergy Clin Immunol. 2020;145(3):1002–1005.e16. doi:10.1016/j.jaci.2020.01.009
32. Yoshizaki T, Sato H, Furukawa M. Recent advances in the regulation of matrix metalloproteinase 2 activation: from basic research to clinical implication (Review). Oncol Rep. 2002;9(3):607–611.
33. Miller TL, Touch SM, Shaffer TH. Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase expression profiles in tracheal aspirates do not adequately reflect tracheal or lung tissue profiles in neonatal respiratory distress: observations from an animal model. Pediatr Crit Care Med. 2006;7(1):63–69. doi:10.1097/01.PCC.0000192320.87416.1A
34. Chen P, Farivar AS, Mulligan MS, Madtes DK. Tissue inhibitor of metalloproteinase-1 deficiency abrogates obliterative airway disease after heterotopic tracheal transplantation. Am J Respir Cell Mol Biol. 2006;34(4):464–472. doi:10.1165/rcmb.2005-0344OC
35. Strup-Perrot C, Vozenin-Brotons MC, Vandamme M, Benderitter M, Mathe D. Expression and activation of MMP −2, −3, −9, −14 are induced in rat colon after abdominal X-irradiation. Scand J Gastroenterol. 2006;41(1):60–70. doi:10.1080/00365520510023963
36. Mercer PF, Shute JK, Bhowmik A, Donaldson GC, Wedzicha JA, Warner JA. MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation. Respir Res. 2005;6(1):151. doi:10.1186/1465-9921-6-151
37. Misugi F, Sumi T, Okamoto E, et al. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinase in uterine endometrial carcinoma and a correlation between expression of matrix metalloproteinase-7 and prognosis. Int J Mol Med. 2005;16(4):541–546.
38. Ko FW, Diba C, Roth M, et al. A comparison of airway and serum matrix metalloproteinase-9 activity among normal subjects, asthmatic patients, and patients with asthmatic mucus hypersecretion. Chest. 2005;127(6):1919–1927. doi:10.1378/chest.127.6.1919
39. John M, Jaworski C, Chen Z, et al. Matrix metalloproteinases are down-regulated in rat lenses exposed to oxidative stress. Exp Eye Res. 2004;79(6):839–846. doi:10.1016/j.exer.2004.08.025
40. Krane SM. Clinical importance of metalloproteinases and their inhibitors a. Ann N Y Acad Sci. 1994;732(1):1–10. doi:10.1111/j.1749-6632.1994.tb24719.x
41. Malinsky RR, Valera FC, Cavallari FE, et al. Matrix metalloproteinases and their impact on sinusal extension in chronic rhinosinusitis with nasal polyps. Eur Arch Otorhinolaryngol. 2013;270(4):1345–1348. doi:10.1007/s00405-012-2219-9
42. Lechapt-Zalcman E, Coste A, d’Ortho MP, et al. Increased expression of matrix metalloproteinase-9 in nasal polyps. J Pathol. 2001;193(2):233–241. doi:10.1002/1096-9896(2000)9999:9999<::AID-PATH771>3.0.CO;2-W
43. Liu X, Dong H, Wang M, et al. IL-1α-induced microvascular endothelial cells promote neutrophil killing by increasing MMP-9 concentration and lysozyme activity. Immunol Res. 2016;64(1):133–142. doi:10.1007/s12026-015-8731-4
44. Ardi VC, Van den Steen PE, Opdenakker G, Schweighofer B, Deryugina EI, Quigley JP. Neutrophil MMP-9 proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway. J Biol Chem. 2009;284(38):25854–25866. doi:10.1074/jbc.M109.033472
45. Vlahos R, Wark PA, Anderson GP, Bozinovski S. Glucocorticosteroids differentially regulate MMP-9 and neutrophil elastase in COPD. PLoS One. 2012;7(3):e33277. doi:10.1371/journal.pone.0033277
46. Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol. 2003;28(1):12–24. doi:10.1165/rcmb.2002-0166TR
47. Lee YY, Li YC, Hung SL, Chen YC, Lee YH, Yang SF. Mineral trioxide aggregate induces the release of matrix metalloproteinase-9 by human neutrophils. J Dental Sci. 2013;8(4):378–384. doi:10.1016/j.jds.2012.12.010
48. Hong SY, Jiang HC, Xu WC, Zeng HS, Wang SG, Qin BL. Bioinformatics analysis reveals the potential role of matrix metalloproteinases in immunity and urolithiasis. Front Immunol. 2023;14:1158379. doi:10.3389/fimmu.2023.1158379
49. Chi PL, Cheng CC, Hung CC, et al. MMP-10 from M1 macrophages promotes pulmonary vascular remodeling and pulmonary arterial hypertension. Int J Biol Sci. 2022;18(1):331–348. doi:10.7150/ijbs.66472
50. Mohan MJ, Seaton T, Mitchell J, et al. The tumor necrosis factor-alpha converting enzyme (TACE): a unique metalloproteinase with highly defined substrate selectivity. Biochemistry. 2002;41(30):9462–9469. doi:10.1021/bi0260132
51. Janulaityte I, Januskevicius A, Rimkunas A, Palacionyte J, Vitkauskiene A, Malakauskas K. Asthmatic eosinophils alter the gene expression of extracellular matrix proteins in airway smooth muscle cells and pulmonary fibroblasts. Int J Mol Sci. 2022;23(8):4086. doi:10.3390/ijms23084086
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.