Back to Journals » Journal of Pain Research » Volume 14
Expression of Substance P and Nerve Growth Factor in Degenerative Long Head of Biceps Tendon in Patients with Painful Rotator Cuff Tear
Authors Izumi M , Harada Y, Kajita Y, Muramatsu Y, Morimoto T, Morisawa Y, Iwahori Y, Ikeuchi M
Received 20 May 2021
Accepted for publication 27 July 2021
Published 16 August 2021 Volume 2021:14 Pages 2481—2490
DOI https://doi.org/10.2147/JPR.S320811
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Masashi Izumi,1 Yohei Harada,2,3 Yukihiro Kajita,2 Yoshitaka Muramatsu,2,4 Toru Morimoto,1 Yutaka Morisawa,5 Yusuke Iwahori,2,6 Masahiko Ikeuchi1
1Department of Orthopaedic Surgery, Kochi Medical School, Kochi University, Nankoku-City, Japan; 2Department of Orthopaedic Surgery, Aichi Medical University, Nagakute, Japan; 3Department of Orthopaedic Surgery, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan; 4Department of Orthopaedic Surgery, Saiseikai Futsukaichi Hospital, Futsukaichi, Japan; 5Department of Orthopaedic Surgery, Aki General Hospital, Aki, Japan; 6Department of Orthopaedic Surgery, Sports Medicine and Joint Center, Asahi Hospital, Kasugai, Japan
Correspondence: Masashi Izumi
Department of Orthopaedic Surgery, Kochi Medical School, Kochi University, 185-1 Kohasu, Oko-cho, Nankoku-City, Kochi Pref, 783-8505, Japan
Tel +81 88-880-2386
Fax +81 88-880-2388
Email [email protected]
Purpose: Degenerative long head of biceps tendon (LHBT) has been recognized as a notable pain source in patients with rotator cuff tear (RCT). Tenotomy or tenodesis of LHBT is frequently indicated together with arthroscopic rotator cuff repair (ARCR) aiming for complete pain relief; however, it has not been fully investigated whether resected LHBT is really a source of pain. The purpose of this study was to investigate expression levels of pain-associated mediators in LHBT and its association with preoperative pain profiles.
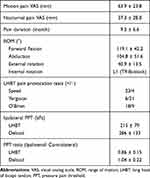
Methods: Twenty-seven RCT patients who underwent ARCR with LHBT resection were included. Each LHBT was resected due to its abnormal arthroscopic findings including tenosynovitis, hypertrophy, and partial tear. Worst macroscopic lesion of the LHBT was obtained, and expression levels of substance P (SP) and nerve growth factor (NGF) were evaluated using enzyme-linked immunosorbent assay (ELISA). Ten healthy knee flexor tendons were analyzed as non-degenerative samples. Preoperatively, subjective shoulder pain VAS and pain duration were investigated. Conventional LHBT pain provocation tests (Speed, Yergason, O’Brien) were performed. Pressure pain threshold (PPT) of bilateral LHBT on the groove was recorded.
Results: Levels of SP and NGF expression were significantly higher compared with non-degenerative tendons (P< 0.01). Shoulder pain VAS and pain duration were not directly associated with SP and NGF expression level. Patients with positive O’Brien test expressed greater SP than negative patients (P=0.001). Significant negative correlation between the PPT ratio (ipsilateral/contralateral) and SP expression level was observed (r=− 0.453, P=0.034).
Conclusion: Greater expression of SP and NGF in degenerative LHBT supported our hypothesis that it would be a pain source in RCT patients. SP was likely to be expressed highly in patients with localized pressure pain hypersensitivity and positive O’Brien test (ie, altered mechanistic pain profile of LHBT), which may help when considering simultaneous LHBT resection during ARCR.
Clinical Registration: UMIN000023943.
Keywords: long head of biceps tendon, pain, tendinopathy, substance P, nerve growth factor, pressure pain threshold
Introduction
Rotator cuff tear (RCT) is a common clinical problem that occurs in middle-aged people and the elderly causing significant shoulder pain and disability. In fact, a proportion of RCT patients suffer from severe shoulder motion pain during daily activities and nocturnal pain as well, and require medical treatment.1 Meanwhile, it has long been known that asymptomatic RCTs are highly prevalent in the general population especially in the elderly, and in some people it may become painful over time.2 Thus, pain originating from RCT still remains controversial, ie, underlying mechanisms of the difference between “painful RCT” and “painless RCT” are not fully understood yet.
Degenerative long head of biceps tendon (LHBT) has been recognized as a notable pain source in patients with RCT because it easily becomes irritated due to its unique anatomical composition and course.3–5 For complete pain relief, tenotomy or tenodesis of LHBT is frequently indicated together with arthroscopic rotator cuff repair (ARCR),6,7 and clinical outcome of the combined surgery has been reported as superior compared to isolated ARCR.8 Also, isolated tenotomy or tenodesis of LHBT could effectively treat severe pain caused by irreparable massive RCT.9 These clinical studies supported a possibility of LHBT as a pain generator in patients with RCT, however, it was not fully investigated whether resected LHBT was really a source of pain.
Nerve ingrowth and altered expression of sensory and sympathetic neuromediators play a major role in painful tendinopathy.10 Substance P (SP) is a neuropeptide mainly secreted by sensory neurons, and previous studies demonstrated increased expression of SP-positive nerve fibers in painful tendinopathies such as Achilles tendon,11 patellar tendon,12 and extensor carpi radialis brevis tendon.13 Nerve growth factor (NGF) is a member of the neurotrophin family of proteins, and commonly known as a key regulator of nociceptive pain.14 Though little has been known about its contribution to painful tendinopathy, biological effects of NGF on nociceptive processing15 such as peripheral sensitization, altered nociceptor transcription, and sprouting may be associated with the etiology of tendinopathy. Therefore, we hypothesized that degenerative LHBT, recognized as a possible pain source during ARCR, expressed higher SP and NGF than healthy tendons.
The main purpose of this research was to elucidate expression levels of SP and NGF in degenerative LHBT and its association with preoperative pain profiles. In addition, whether specific arthroscopic abnormalities and histological severities of the LHBT affected the expression levels of SP and NGF was also investigated.
Materials and Methods
Study Participants
Twenty-seven unilateral painful RCT patients (12 men and 15 women; mean age at surgery, 66.2 years; range, 47–78 years) who underwent tenotomy or tenodesis of LHBT during ARCR in two university hospitals were included. The mean duration of pain before the surgery was 9.3 (range: 2–24) months. Eleven patients (40.7%) underwent surgery ≤ 6 months after the onset of pain. The decision for LHBT resection was made by surgeons intraoperatively, based on arthroscopic findings including tenosynovitis (like ‘lip stick lesion’),16 hypertrophy, and partial rupture of the tendon (Figure 1). The criteria were standardized in two hospitals and patients who had at least one of the previously mentioned findings underwent arthroscopic tenotomy or mini-open supra-pectoral tenodesis of the LHBT. Patients who had inflammatory arthritis, connective tissue disease, psychiatric disorders, and history of surgery in the shoulder were excluded.
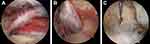 |
Figure 1 Representative arthroscopic view of the resected LHBT. (A) Tenosynovitis, (B) moderate tear (<50%), (C) severe tear (≧50%). Hypertrophy was also observed in these tendons. |
The control group comprised 10 patients (6 men and 4 women; mean age at surgery, 25.1 years; range, 14–49 years) who underwent anterior cruciate ligament reconstruction using autologous knee flexor tendons.
The study protocol was approved by the Institutional Review Board of Kochi Medical School (No. 28–55, 31–183). All participants received a verbal explanation of the study and provided written informed consent prior to the investigation. Parents of participants under 18 years of age provided informed consent. This study was conducted in compliance with the Declaration of Helsinki.
Preoperative Evaluations
Intensity of preoperative shoulder motion pain during active elevation and nocturnal pain was recorded on 100 mm visual analog scale (VAS). Active range of motion (ROM) of the shoulder, and LHBT pain provocation maneuvers, namely, Speed test, Yergason test, and O’Brien test were evaluated by surgeons.
Speed test17 was performed with patient’s forearm supinated, elbow extended, and shoulder in 60° to 90° forward flexion. The examiner applied a downward force to the patients’ resistance. Yergason test18 was performed with patient’s arm at their side, elbow flexed to 90°, and forearm in full pronation. Patients were asked to forcibly supinate against the examiners’ resistance. Positive tests were indicated by the production of pain.
O’Brien test (active compression test)19 was performed with the patient’s arm forward flexed to 90°, adducted 10 to 15°, and internally rotated such that the thumb pointed downward. The examiner applied downward force to the patient’s resistance. With the arm in the same position, the palm was then fully supinated and the maneuver was repeated. The test was considered positive if pain was elicited during the first maneuver, and was reduced or eliminated with the second. For assessment of pressure pain sensitivity, a handheld algometer (Somedic, Hörby, Sweden) with a 1-cm2 probe was used to record the pressure pain threshold (PPT) at bilateral LHBT on bicipital groove and center of deltoid muscle (control) in patients in sitting position (Figure 2). Meticulous palpation to confirm location of the bicipital groove was performed with the patient’s arm internally and externally rotated. The recording was repeated 3 times with an interval of minimum 20 s. The PPT was defined to the subject as “the time point at which the pressure sensation changed into pain”. Pressure was increased gradually at a rate of 30 kPa/s until the pain threshold was reached and the subject pressed a button. Trained physiotherapists assessed the PPTs which were blinded to the surgeons before surgery. For analyses, mean value of 3 times recording was used and ipsilateral PPT was divided by contralateral one, which was defined as “PPT ratio”.
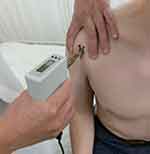 |
Figure 2 Recording of pressure pain threshold (PPT) at LHBT on the groove. Bilateral assessment was performed and PPT ratio (ipsilateral/contralateral) was calculated. |
Surgical Procedure and Tissue Sampling
All cases underwent surgery in the beach-chair position under general anesthesia. Diagnostic arthroscopy was performed with a standard posterior viewing portal. Type of RCT was classified as; isolated supraspinatus (SSP) tear, subscapularis (SSC)+SSP tear, SSP+ infraspinatus (ISP) tear, and SSC+SSP+ISP tear, respectively. Degree of RCT retraction was defined as; small/medium (<3cm) or large/massive (≧3cm) according to the length between stump of the most retracted tendon to footprint. Pathology of LHBT was carefully assessed using a probe via a standard anterior portal and abnormal findings were classified according to previous reports9,20 including tenosynovitis, hypertrophy, moderate tear (<50%), and severe tear (≥50%). Location of LHBT was also determined as; in bicipital groove, subluxation, and dislocation.
Tenotomy or tenodesis of the LHBT was performed prior to rotator cuff repair. Five early cases underwent all-arthroscopic bipolar tenotomy with removal of intra-articular portion of LHBT. For comparison of intra-articular and intra-groove lesions, the remaining 22 cases received mini-open supra-pectoral tenodesis and intra-groove LHBT was additionally resected at the exit of the groove. After the removal of LHBT, worst macroscopic lesion of the tendon with surrounding tenosynovium was harvested with a length of 1 cm as intra-articular (n=27) and intra-groove (n=22) samples (Figure 3). The samples were longitudinally split into two parts and used for ELISA and histological evaluation respectively. To compare and validate the harvested LHBT samples, 1-cm fragment of healthy (non-degenerative) knee flexor tendon discarded during preparation of the graft was obtained from patients undergoing anterior cruciate ligament reconstruction.21,22
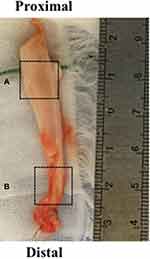 |
Figure 3 Representative macroscopic view of the resected LHBT. Tissue sampling was done from worst macroscopic lesion at intra-articular (A) and intra-groove (B) area. |
ELISA
LHBT and healthy knee flexor tendon samples were weighed and immediately stored at −80°C. In preparation for analysis, the samples were thawed and subsequently diced and lysed with RIPA buffer. The amount of added RIPA buffer was determined by the weight of each tendon to adjust the difference of sample volume. Quantification of expressed SP and NGF was analyzed using a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) kit for SP and NGF (Substance P Assay Kit; KGE007, Human beta-NGF DuoSet; DY256, R&D systems, Minneapolis, MN) according to the manufacturer's protocols. Tissue protein was assayed using BCA Protein Colorimetric Detection Kit (K041-H1, Arbor Assays, Ann Arbor, MI) according to the manufacturer's protocols, and levels of SP and NGF expression were normalized to the protein level of each tissue.
Histological Evaluation
LHBT samples were immediately placed in 10% buffered formalin. After fixation, specimens were embedded in paraffin and 4 μm sections were obtained. The sections were stained with hematoxylin and eosin (HE) and examined under light microscopy. A modified Bonar score23 was used for evaluating the extent of tendon degeneration. A grade was given from 0 (normal) to 3 (markedly abnormal) for tenocyte morphology, ground substance, collagen, and vascularity. The grades from each variable were then summed, and a total score was given that ranged between 0 (normal) and 12 (most severe abnormality).
Statistical Analysis
Statistical analyses were performed with SPSS version 26.0 software and SPSS Bootstrapping (IBM Corp. Armonk, NY, USA). Levels of SP and NGF expression among intra-articular, intra-groove samples of LHBT and healthy knee flexor tendon were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s HSD test. The modified Bonar score of intra-articular and intra-groove samples of LHBT was compared using paired t-test. Correlations between levels of SP/NGF expression of LHBT (data were given as mean value of the intra-articular and intra-groove samples) and preoperative pain intensity, ROM, PPT ratio, and modified Bonar score (data were given as mean value of the intra-articular and intra-groove samples) were assessed using Spearman’s rank correlation coefficient (r). In addition, levels of SP/ NGF expression were compared between patients who showed positive or negative LHBT pain provocation tests and specific arthroscopic findings using Student’s t-test. Estimates of mean differences of SP/NGF expression levels between groups, modified Bonar score between sampling sites and Spearman correlations with 95% confidence interval were derived from 1000 bootstrap resampling. P < 0.05 indicated statistical significance.
Results
Preoperative evaluations including pain VAS, pain duration, ROM, response to LHBT pain provocation tests, and PPT were shown in Table 1. All data were available except PPTs in 3 cases due to disorder of the algometer. Specific details of the arthroscopic findings regarding RCT and LHBT were summarized in Table 2. Twelve patients had isolated SSP tear, while fifteen patients had more than two tendons of RCT involved. Most of the LHBT showed hypertrophy that was concomitant with other abnormal findings, therefore, no LHBT was resected due to single pathology of hypertrophy. All except three cases had at least two overlapping pathologies among tenosynovitis, tear (moderate/ severe), hypertrophy, and displacement (subluxation/ dislocation) of the LHBT. The mean number of the pathologies was 2.9 (range: 1–4).
 |
Table 2 Specific Details of the Arthroscopic Findings |
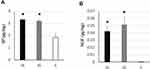
Expression level of SP and NGF in intra-articular and intra-groove LHBT was significantly greater compared with non-degenerative tendon (SP: ANOVA; F(2)=19.29, P=0.0000004, Tukey; P<0.000049, NGF: F(2)=6.74, P=0.002, Tukey; P<0.01). There were no differences between intra-articular and intra-groove samples. Expression level of NGF was quite low compared with SP, but none was detected from all control samples (Figure 4).
Preoperative shoulder motion pain and nocturnal pain VAS, pain duration before the surgery, and ROM (forward flexion, abduction, external rotation, internal rotation) were not significantly associated with SP and NGF expression level in LHBT (SP: motion pain VAS; r=0.113, P=0.616, nocturnal pain VAS; r=−0.161, P=0.475, pain duration; r=−0.232, P=0.245, ROM; r=0.048–0.403, P=0.063–0.832, NGF: motion pain VAS; r=0.130, P=0.563, nocturnal pain VAS; r=−0.361, P=0.099, pain duration; r=−0.174, P=0.384, ROM; r=0.067–0.369, P=0.058–0.766).
Among LHBT pain provocation tests, SP expression level in patients with positive O’Brien test was significantly greater than in negative patients (P=0.001), but this difference was not observed in NGF. There were no differences in SP and NGF expression level between positive and negative patients for Speed test and Yergason test (Table 3).
 |
Table 3 SP and NGF Expression Level in Patients with Positive and Negative LHBT Pain Provocation Tests |
SP expression level in LHBT was negatively correlated with PPT ratio at LHBT (r=−0.453, P=0.034) but not at deltoid. Regarding NGF, no significant correlations were observed like SP (Figure 5).
 |
Figure 5 Correlations between expression levels of SP, NGF, and PPT ratio at LHBT, deltoid (A–D). SP expression level showed significant negative correlation with PPT ratio at LHBT (A). |
Regarding arthroscopic findings, patients who showed RCT involving more than two tendons expressed greater NGF compared with isolated SSP tear (0.058±0.010 vs 0.030±0.013 pg/mg, P=0.021), but this difference was not observed in SP. The degree of RCT retraction (small-medium vs large-massive) did not make differences in SP and NGF expression level.
In terms of LHBT pathologies, no differences in SP and NGF expression level were seen between presence and absence of tenosynovitis, tear (moderate/ severe), and displacement (subluxation/ dislocation) (Table 4). The mean (range) modified Bonar score was 8.5 (6–11) for intra-articular samples and 8.3 (6–11) for intra-groove samples with no significant difference (P=0.064). The score was not associated with SP and NGF expression level (SP; r=0.051, P=0.806, NGF; r=0.231, P=0.256).
 |
Table 4 SP and NGF Expression Level in Patients with or without Tenosynovitis, Tear, and Displacement of LHBT in Arthroscopic Findings |
Discussion
This study proved our hypothesis that degenerative LHBT recognized as a possible pain source during ARCR expressed higher SP and NGF than healthy tendons. Preoperative localized pressure pain sensitivity at LHBT and positive O’Brien test were associated with the SP expression level. Not specific individual but combined arthroscopic abnormalities might contribute to the expression level of SP and NGF.
Nerve ingrowth and altered expression of nociceptive sensory neuromediators in tendinopathy of LHBT have not been sufficiently documented. Alpantaki et al24 firstly analyzed the innervation of LHBT with immunohistochemical staining and reported that a large network of nociceptive nerve fibers was identified in non-degenerative LHBTs of four fresh cadavers. They mentioned the nerve fibers were positive for SP and calcitonin gene-related peptide (CGRP) but provided only visual information and no specific data of this innervation. Singaraju et al25 confirmed SP and CGRP positive nerve fibers in six LHBTs obtained from chronic painful RCT patients, but their expression levels were not different from control cadaver tendons. More recently, Blumer et al26 analyzed, in detail, structural and molecular characteristics of nervous elements in most proximal part of LHBT obtained from six osteoarthritis and five fracture cases undergoing shoulder joint replacement. They showed that SP was exclusively expressed in unmyelinated axons in nerve fascicles close to blood vessels, and was partially co-expressed with CGRP, though it was not quantitatively assessed and not compared with control samples. In this study, we preferred to use ELISA which enables simple quantification of protein expression, and have added further information about overexpression of SP and NGF in degenerative LHBT that could be a pain source in RCT patients.
Expression level of NGF in resected LHBT was quite low compared with SP, but significantly greater than in healthy tendons, suggesting that it would be reflected in mechanisms of pain originating from LHBT. Although little has been reported on a contribution of NGF in painful human tendinopathy, clinical trials have demonstrated that NGF plays a key role in pain generation in osteoarthritis27 and chronic low back pain.28 Hence, it seems theoretically possible that NGF also works in painful tendinopathy via its biological effects on nociceptive processing such as peripheral sensitization, altered nociceptor transcription, and sprouting.15 Further, a recent study of rat RCT model showed that NGF expression levels in harvested rotator cuff were continuously elevated and there were NGF-positive synovial-like cells lining the surface of the laminated cuff tears,29 which supports the possibility that NGF plays a role in painful tendinopathy of the shoulder.
Intensity of preoperative shoulder motion pain and nocturnal pain was not directly associated with levels of SP and NGF expression in LHBT. It seems reasonable because all patients included in this study had RCT and there were many possible nociceptive inputs other than LHBT in the shoulder. Moreover, pain VAS is a highly subjective scale that can be modulated by multiple factors such as psychosocial problems.30 In contrast, PPT ratio on LHBT at bicipital groove, but not on the deltoid muscle, was associated with the level of SP expression. PPT is a threshold of pressure pain sensitivity that can directly and quantitatively evaluate altered mechanistic pain profile of specific structures.31 In addition, patients with positive O’Brien test expressed greater SP than negative patients. Taken together, our results can be interpreted that more SP was detected from mechanically hyperalgesic LHBT. From this perspective, PPT ratio (clinically, comparison of tenderness between ipsilateral and contralateral side) and O’Brien test seem to be useful markers that may help when considering simultaneous LHBT resection during ARCR.
Recently, Taylor et al32 reported the diagnostic value of several pain provocation tests for biceps-labrum complex (BLC) disease. They mentioned that O’Brien test and bicipital tunnel palpation showed higher sensitivity and negative predictive value for BLC disease in all locations (intra-articular, junctional, and bicipital tunnel) than Speed test and Yergason test. Therefore, they recommended including O’Brien test and bicipital tunnel palpation as screening tests. Interestingly, both O’Brien test and bicipital tunnel palpation (PPT), but not Speed test and Yergason test, were associated with expression level of SP in this study, which might explain a part of underlying mechanisms of their clinical results.
Patients who showed RCT involving more than two tendons expressed greater NGF compared with isolated SSP tear, which was possibly associated with altered innervation of LHBT. Although this can be explained by facilitated biomechanical inputs to the LHBT resulting from multiple rotator cuff tendons tear, it seems uncertain because this finding was not observed in SP and the degree of RCT retraction did not cause differences in SP and NGF expression level. Regarding arthroscopic abnormalities of LHBT, there was no superiority among specific individual findings such as tenosynovitis, tear, hypertrophy, and displacement for increasing the SP and NGF expressions, however, this should be taken with caution because most of the patients had overlapping pathologies that would work as confounding factors. Paradoxically, this result can be interpreted that not individual, but combined abnormal findings are clinically common in pathological LHBT that would collaboratively contribute to overexpression of SP and NGF.
The modified Bonar score represented moderate to severe histological deterioration of LHBT, which was similar to previous reports.23,33 However, the histological severity was not associated with SP and NGF expression level, which seems to be inconsistent with a recent report that documented that expression of SP evaluated by immunostaining was associated with histological severity of tendon degeneration in lateral epicondylitis.22 This discrepancy is probably due to lack of samples with mild histopathologic changes which were included in the lateral epicondylitis study. As reported, microscopic pathology was typically much worse than macroscopic findings.20 In this study, patients who had apparent arthroscopic abnormalities of LHBT were selectively included so that the lowest Bonar score was 6, which might be enough to show overexpression of SP and NGF.
This is the first study which demonstrated that intra-groove LHBT expressed comparable levels of SP and NGF with intra-articular LHBT, suggesting that both portions have similar potential as a pain generator. In contrast, several recent pieces of literature have concluded that there was no significant difference in pain relief between tenotomy and tenodesis in RCT patients.6,34–37 A plausible explanation for this discrepancy is that biomechanical environment of intra-groove LHBT changes after the tenotomy of intra-articular LHBT, which may subsequently alter nociceptive phenotype of the intra-groove LHBT, though it is beyond the scope of this study. However, our novel finding will support an idea that tenodesis after removing both portions of LHBT is preferable from the perspective of reducing nociceptive inputs as much as possible.
This study has some limitations that should be noted. First, samples harvested from healthy knee flexor tendon were not pure negative control for LHBT because of the difference in age, anatomical location, and physical loading conditions. However, from an ethical point of view, it would be difficult to obtain age-matched normal LHBT samples and hence, the same idea was used when evaluating degenerative tendons in shoulder and elbow in previous studies.21,22 Second, the number of patients and controls was small, though more patients were included than in previous similar studies with immunohistochemistry.24,25 Third, not all consecutive cases underwent tenotomy or tenodesis of LHBT during ARCR, so possible selection bias existed. In particular, this series selected patients who had apparent arthroscopic abnormalities of LHBT. The outcome might be affected when including LHBT with very mild hyperemia or fraying.
Conclusion
Degenerative LHBT, including combined arthroscopic abnormalities in most cases, expressed greater SP and NGF compared with healthy tendons, which supports our hypothesis that it would be a pain source in RCT patients. Expression levels of SP and NGF were comparable between intra-articular and intra-groove samples, indicating that both portions have similar potential as a pain generator. SP was likely to be expressed highly in patients with localized pressure pain hypersensitivity and positive O’Brien test (ie, altered mechanistic pain profile of LHBT), which may help when considering simultaneous LHBT resection during ARCR.
Abbreviations
LHBT, long head of biceps tendon; RCT, rotator cuff tear; ARCR, arthroscopic rotator cuff repair; SP, substance P; NGF, nerve growth factor; ELISA, enzyme-linked immunosorbent assay; VAS, visual analog scale; PPT, pressure pain threshold; ROM, range of motion; SSP, supraspinatus; ISP, infraspinatus; SSC, subscapularis.
Acknowledgment
We would like to thank Reika Shiraishi for invaluable technical assistance.
Author Contributions
Study design: M Izumi, YH, YK, YI. Data collection: M Izumi, YH, YK, YM, TM, YI. Data analysis: M Izumi, YH, YK, YI. Drafting or revising manuscript: M Izumi, YH, YK, YM, TM, YM, YI, M Ikeuchi. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported by JSPS KAKENHI Grant Number 16K19213 to Masashi Izumi. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflict of interest in this work.
References
1. Piper CC, Hughes AJ, Ma Y, Wang H, Neviaser AS. Operative versus nonoperative treatment for the management of full-thickness rotator cuff tears: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2018;27(3):572–576. doi:10.1016/j.jse.2017.09.032
2. Lawrence RL, Moutzouros V, Bey MJ. Asymptomatic rotator cuff tears. JBJS Rev. 2019;7(6):e9. doi:10.2106/JBJS.RVW.18.00149
3. Murthi AM, Vosburgh CL, Neviaser TJ. The incidence of pathologic changes of the long head of the biceps tendon. J Shoulder Elbow Surg. 2000;9(5):382–385. doi:10.1067/mse.2000.108386
4. Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58(6):1189–1193. doi:10.1097/01.TA.0000170052.84544.34
5. Boileau P, Ahrens PM, Hatzidakis AM. Entrapment of the long head of the biceps tendon: the hourglass biceps--a cause of pain and locking of the shoulder. J Shoulder Elbow Surg. 2004;13(3):249–257. doi:10.1016/j.jse.2004.01.001
6. Lee HJ, Jeong JY, Kim CK, Kim YS. Surgical treatment of lesions of the long head of the biceps brachii tendon with rotator cuff tear: a prospective randomized clinical trial comparing the clinical results of tenotomy and tenodesis. J Shoulder Elbow Surg. 2016;25(7):1107–1114. doi:10.1016/j.jse.2016.02.006
7. Leroux T, Chahal J, Wasserstein D, Verma NN, Romeo AA. A systematic review and meta-analysis comparing clinical outcomes after concurrent rotator cuff repair and long head biceps tenodesis or tenotomy. Sports Health. 2015;7(4):303–307. doi:10.1177/1941738114539627
8. Watson ST, Robbins CB, Bedi A, Carpenter JE, Gagnier JJ, Miller BS. Comparison of outcomes 1 year after rotator cuff repair with and without concomitant biceps surgery. Arthroscopy. 2017;33(11):1928–1936.
9. Boileau P, Baqué F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89(4):747–757. doi:10.2106/00004623-200704000-00008
10. Ackermann PW, Salo P, Hart DA. Tendon innervation. Adv Exp Med Biol. 2016;920:35–51.
11. Schubert TE, Weidler C, Lerch K, Hofstädter F, Straub RH. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis. 2005;64(7):1083–1086. doi:10.1136/ard.2004.029876
12. Lian Ø, Dahl J, Ackermann PW, Frihagen F, Engebretsen L, Bahr R. Pronociceptive and antinociceptive neuromediators in patellar tendinopathy. Am J Sports Med. 2006;34(11):1801–1808. doi:10.1177/0363546506289169
13. Ljung BO, Forsgren S, Fridén J. Substance P and calcitonin gene-related peptide expression at the extensor carpi radialis brevis muscle origin: implications for the etiology of tennis elbow. J Orthop Res. 1999;17(4):554–559. doi:10.1002/jor.1100170414
14. Seidel MF, Wise BL, Lane NE. Nerve growth factor: an update on the science and therapy. Osteoarthritis Cartilage. 2013;21(9):1223–1228. doi:10.1016/j.joca.2013.06.004
15. Denk F, Bennett DL, McMahon SB. Nerve growth factor and pain mechanisms. Annu Rev Neurosci. 2017;40(307–325):307–325. doi:10.1146/annurev-neuro-072116-031121
16. Grassbaugh JA, Bean BR, Greenhouse AR, et al. Refuting the lipstick sign. J Shoulder Elbow Surg. 2017;26(8):1416–1422. doi:10.1016/j.jse.2017.01.009
17. Crenshaw AH, Kilgore WE. Surgical treatment of bicipital tenosynovitis. J Bone Joint Surg Am. 1966;48(8):1496–1502. doi:10.2106/00004623-196648080-00003
18. Holtby R, Razmjou H. Accuracy of the Speed’s and Yergason’s tests in detecting biceps pathology and SLAP lesions: comparison with arthroscopic findings. Arthroscopy. 2004;20(3):231–236. doi:10.1016/j.arthro.2004.01.008
19. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610–613. doi:10.1177/03635465980260050201
20. Wu PT, Jou IM, Yang CC, et al. The severity of the long head biceps tendinopathy in patients with chronic rotator cuff tears: macroscopic versus microscopic results. J Shoulder Elbow Surg. 2014;23(8):1099–1106. doi:10.1016/j.jse.2013.11.013
21. Zabrzyński J, Paczesny Ł, Łapaj Ł, Grzanka D, Szukalski J. Is the inflammation process absolutely absent in tendinopathy of the long head of the biceps tendon? Histopathologic study of the long head of the biceps tendon after arthroscopic treatment. Pol J Pathol. 2017;68(4):318–325. doi:10.5114/pjp.2017.73928
22. Han SH, Kim HK, Jang Y, et al. The expression of substance P and calcitonin gene-related peptide is associated with the severity of tendon degeneration in lateral epicondylitis. BMC Musculoskelet Disord. 2021;22(1):210. doi:10.1186/s12891-021-04067-1
23. Streit JJ, Shishani Y, Rodgers M, Gobezie R. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extra-articular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;6:63–70.
24. Alpantaki K, McLaughlin D, Karagogeos D, Hadjipavlou A, Kontakis G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am. 2005;87(7):1580–1583.
25. Singaraju VM, Kang RW, Yanke AB, et al. Biceps tendinitis in chronic rotator cuff tears: a histologic perspective. J Shoulder Elbow Surg. 2008;17(6):898–904. doi:10.1016/j.jse.2008.05.044
26. Blumer R, Boesmueller S, Gesslbauer B, et al. Structural and molecular characteristics of axons in the long head of the biceps tendon. Cell Tissue Res. 2020;380(1):43–57. doi:10.1007/s00441-019-03141-4
27. Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363(16):1521–1531. doi:10.1056/NEJMoa0901510
28. Katz N, Borenstein DG, Birbara C, et al. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain. 2011;152(10):2248–2258. doi:10.1016/j.pain.2011.05.003
29. Nagura N, Kenmoku T, Uchida K, Nakawaki M, Inoue G, Takaso M. Nerve growth factor continuously elevates in a rat rotator cuff tear model. J Shoulder Elbow Surg. 2019;28(1):143–148. doi:10.1016/j.jse.2018.06.030
30. Kennedy P, Joshi R, Dhawan A. The effect of psychosocial factors on outcomes in patients with rotator cuff tears: a systematic review. Arthroscopy. 2019;35(9):2698–2706. doi:10.1016/j.arthro.2019.03.043
31. Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol. 2010;6(10):599–606. doi:10.1038/nrrheum.2010.107
32. Taylor SA, Newman AM, Dawson C, et al. The “3-pack” examination is critical for comprehensive evaluation of the biceps-labrum complex and the bicipital tunnel: a prospective study. Arthroscopy. 2017;33(1):28–38. doi:10.1016/j.arthro.2016.05.015
33. Nuelle CW, Stokes DC, Kuroki K, Crim JR, Sherman SL. Radiologic and histologic evaluation of proximal bicep pathology in patients with chronic biceps tendinopathy undergoing open subpectoral biceps tenodesis. Arthroscopy. 2018;34(6):1790–1796. doi:10.1016/j.arthro.2018.01.021
34. MacDonald P, Verhulst F, McRae S, et al. Biceps tenodesis versus tenotomy in the treatment of lesions of the long head of the biceps tendon in patients undergoing arthroscopic shoulder surgery: a prospective double-blinded randomized controlled trial. Am J Sports Med. 2020;48(6):1439–1449. doi:10.1177/0363546520912212
35. Kim J, Nam JH, Kim Y, Kim JS, Kim SH. Long head of the biceps tendon tenotomy versus subpectoral tenodesis in rotator cuff repair. Clin Orthop Surg. 2020;12(3):371–378. doi:10.4055/cios19168
36. Mardani-Kivi M, Keyhani S, Ebrahim-Zadeh MH, Hashemi-Motlagh K, Saheb-Ekhtiari K. Rotator cuff tear with concomitant long head of biceps tendon (LHBT) degeneration: what is the preferred choice? Open subpectoral versus arthroscopic intraarticular tenodesis. J Orthop Traumatol. 2019;20(1):26. doi:10.1186/s10195-019-0531-5
37. Meraner D, Sternberg C, Vega J, Hahne J, Kleine M, Leuzinger J. Arthroscopic tenodesis versus tenotomy of the long head of biceps tendon in simultaneous rotator cuff repair. Arch Orthop Trauma Surg. 2016;136(1):101–106. doi:10.1007/s00402-015-2343-2
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.