Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Expression of Endothelin-1, Endothelin Receptor-A, and Endothelin Receptor-B in facial melasma compared to adjacent skin
Authors da Silva CN, Miot HA , Grassi TF , Dias-Melício LA, Santos L, Espósito ACC
Received 30 May 2023
Accepted for publication 28 September 2023
Published 12 October 2023 Volume 2023:16 Pages 2847—2853
DOI https://doi.org/10.2147/CCID.S402168
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Carolina Nunhez da Silva,1 Hélio Amante Miot,1 Tony Fernando Grassi,2 Luciane Alarcão Dias-Melício,2– 4 Leandro Santos,2,3 Ana Cláudia Cavalcante Espósito1
1Department of Dermatology, São Paulo State University (UNESP) - Medical School of Botucatu, Botucatu, São Paulo State, Brazil; 2UNIPEX - Experimental Research Unit, São Paulo State University (UNESP) - Medical School of Botucatu, Botucatu, São Paulo State, Brazil; 3Laboratory of Immunopathology and Infectious Agents – LIAI, São Paulo State University (UNESP) - Medical School of Botucatu, Botucatu, São Paulo State, Brazil; 4Department of Pathology, São Paulo State University (UNESP) - Medical School of Botucatu, Botucatu, São Paulo State, Brazil
Correspondence: Ana Cláudia Cavalcante Espósito, Division of Dermatology and Radiotherapy, São Paulo State University (UNESP) - Medical School of Botucatu, Botucatu, São Paulo State, Brazil, Email [email protected]
Background/Objectives: Although melasma is highly prevalent, its pathogenesis is not yet fully understood. In the skin, endothelin-1 (ET-1) is primarily produced by keratinocytes in response to UVB exposure, which is mediated by an increase in IL-1α or reactive oxygen species. ET-1 plays a role in melanogenesis by binding to specific receptor B (ERB) or receptor A (ERA). However, the expression of ET-1, ERA, and ERB in melasma has not been systematically investigated. The objective of this study was to evaluate the expression of ET-1, ERA, and ERB in facial melasma compared to the adjacent unaffected skin.
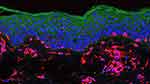
Methods: Cross-sectional study, with 40 skin samples (20: facial melasma; 20: adjacent unaffected skin) from women with facial melasma without treatment for 30 days except for sunscreen. A triple staining immunofluorescence technique was performed for anti-vimentin, DAPI, plus one of the following antibodies: (a) anti-ET1, (b) anti-ERA; (c) anti-ERB. Interfollicular areas on the slides of each topography (melasma; unaffected skin) were photographed in triplicate under confocal laser microscopy. The mean staining intensities of the image histograms (0– 255 pixels intensity) were estimated for different types of cells (suprabasal keratinocytes, basal layer, and upper dermis) and were blindly compared between topographies.
Results: The mean (SD) age of the participants was 44.9 (9.2). The expression of ET-1 was increased in the whole epidermis with melasma when compared to the adjacent skin, being 32.8% (CI95% 14.7%– 52.6%) higher in the spinous layer (p=0.013), 30.4% (CI95% 13.7%– 47.9%) higher in the basal layer (p=0.014), and 29.7% (CI95% 11.4%– 49.7%) higher in the melanocytes (p=0.006). There was no noticeable expression of ET-1 within the cells on the upper dermis. Neither ERA nor ERB resulted in differential epidermal expression between melasma and unaffected skin (p≥ 0.1).
Conclusion: ET-1 is expressed more intensely on the epidermis from the skin with facial melasma compared to the unaffected adjacent skin.
Keywords: melasma, immunofluorescence technique, endothelin-1, endothelin receptor-A, endothelin receptor-B
Introduction
Melasma is a chronic, acquired hypermelanosis with a multifactorial aetiology that mainly affects the faces of women during their childbearing years. Although melasma is highly prevalent, its pathogenesis is not yet fully understood.1 Sunlight exposure is the most relevant environmental trigger in its pathogenesis.2 However, the exact role of different wavelengths of solar radiation and the combined effects of these wavelengths are not acknowledged.3
Ultraviolet radiation (UVR) plays both a direct and indirect role in the melanogenesis process. It specifically stimulates melanocytes to produce melanin and also activates keratinocytes, mast cells, and fibroblasts, which paracrinally regulate melanogenesis.4 Exposure to UVR upregulates the production of basic fibroblast growth factor, endothelin-1 (ET-1), and GM-CSF (Granulocyte-Macrophage Colony Stimulating Factor) by keratinocytes, which in turn also stimulate the growth of melanocytes.5–8
ET-1 is a 21-amino acid peptide encoded by the EDN1 gene and expressed by various cell types, including immune cells, endothelial cells, neurons, and keratinocytes.9 Among the three endothelins described, ET-1 is the most abundant. When synthesized by endothelial cells, it acts as a potent vasoconstrictor and has been associated with the pathophysiology of several vascular diseases.10 In addition to being enhanced in the skin of patients with atopic dermatitis and psoriasis, ET-1 has been shown to induce pruritus in mice and humans via a histamine-independent mechanism.9,11,12 ET-1 is also a keratinocyte-derived factor that stimulates nearby melanocytes by binding to its receptor, either endothelin receptor A (ERA) or endothelin receptor B (ERB). This binding activates intracellular signaling cascades, which regulate melanocyte proliferation and melanogenesis, throughout ERB activation.13
In vitro studies have shown that treatment with ET-1 in a culture system of sheep skin melanocytes increases the number of melanocytes and melanin content. Furthermore, the expression of microphthalmia-associated transcription factor (MITF), melanocortin 1 receptor (MC1R), tyrosinase (TYR), and endothelin receptor B (ERB) in melanocytes was upregulated by ET-1.14
In humans, the expression of ET-1 and its receptor ERB are increased in senile lentigos, and ET-1 has been shown to induce melanogenesis and increase melanosome transport from melanocytes to keratinocytes. Inhibition of ERB function substantially reduces melanogenic ability in tissue-cultured senile lentigos. ET-1 also upregulates the expression of components necessary for early melanosome formation in these lesions, indicating its counteraction against autophagy-targeting melanosome degradation in melanocytes.15 Enhanced expression of keratinocyte-derived ET-1 has also been associated with hyperpigmentation observed in other diseases, such as pigmented basal cell carcinoma and seborrheic keratosis.16–18
The expression of ET-1, ERA, and ERB in melasma has not been systematically explored to date. Therefore, the objective of this study was to evaluate the expression of ET-1, ERA, and ERB in the epidermis and upper dermis from facial melasma and compare it with the unaffected adjacent skin.
Methods
The protocol was approved by the Institution’s Ethics Committee from Unesp Medical School, and all the participants signed the consent form. The study complies with the Declaration of Helsinki.
Twenty women with facial melasma (moderate to severe mMASI - modified Melasma Area and Severity Index), clinically diagnosed by a qualified dermatologist, without treatment for at least 30 days, except for the sunscreen use, were enrolled in the study. They were selected among patients treated at the Hospital das Clínicas da Faculdade de Medicina de Botucatu - FMB/Unesp.
Patients with concomitant facial dermatoses, photosensitive dermatoses, collagenosis, blood dyscrasias, or those using anticoagulant medication, immunosuppressive drugs, pregnant, or lactating women were excluded from the study. Phenotypic extremes, such as individuals with red hair or intensely dark skin, were also excluded since melasma is rare in these groups.
Clinical and demographic information were required from these participants. Two skin samples were obtained from each participant using a 3mm punch biopsy under local anesthesia (lidocaine 2%). One sample was taken from the facial melasma lesion, and the other was taken from adjacent unaffected skin that was exposed to UV radiation (with a maximum distance of 2cm from the lesion, having been evaluated clinically and with the aid of dermoscopy to define the limits of pigmentation). The samples were fixed in 10% buffered formalin and embedded in paraffin for histochemical staining (H&E) and immunofluorescence.
A triple immunofluorescence staining technique was performed on each skin sample using anti-vimentin (ab8978 / 1:100) and DAPI (ab104139/ 1:1), in addition to one of the following antibodies: (a) anti-ET1 (ab113697/ 1:100), (b) anti-ERA (ab76259: 1/500), or (c) anti-ERB (ab117529: 1/100). Vimentin is an intermediate filament of 52–58 kD expressed in both benign and malignant melanocytes, as well as being the primary intermediate filament of fibroblasts. Standardization of immunofluorescence protocols involved titrating the lowest concentration of antibodies that could be detected on slides of positive controls.
The areas with the highest fluorescence intensity on the slides from each location (melasma and unaffected skin) were captured using confocal microscopy equipment, specifically the LEICA TCS SP8, which was equipped with a laser with emission at 405, 488, 552, and 638 nm, as well as two photomultiplier-type detectors and a hybrid-type detector that allowed for the simultaneous acquisition of numerous fluorescence channels. TIFF-format images with a resolution of 813×813 pixels were saved for each marker channel.
In regular interfollicular areas, a quantitative analysis was performed in a blinded manner, without knowledge of the location of the sample (melasma or adjacent healthy skin), in suprabasal keratinocytes, basal layer melanocytes, and upper dermal fibroblasts. Melanocytes and dendritic cells of the upper dermis were stained with vimentin (red fluorescence) while cell nuclei were stained with DAPI (blue fluorescence), enabling topographic orientation of markings by ET-1, ERA, and ERB (green fluorescence).
To evaluate the pixel intensity associated with the fluorescence of the primary antibody, standardized photos (regarding laser intensity) were taken. The average color histogram intensity (ranging from 0 to 255 shades of gray) within the cytoplasm of the target cells was computed across various sites. These intensities were adjusted by dividing the cytoplasmic intensity by the background intensity (represented by the black area) for each photograph, yielding the relative pixel intensity, which served as the primary outcome of this study. Therefore, a relative intensity of 30 represents a semi-quantitative measurement indicating that the cytoplasmic intensity of the antibody is 30 times greater than that of the dark background.
The thickness of the epidermis was calculated by digital histomorphometry. ImageJ 1.51 was the software used for the semiquantitative digital analysis.
Statistical Analysis
The normality of the data obtained was assessed using the Shapiro–Wilk test.19 Data were compared between topographies using a linear model generalized mixed effects, with robust covariance structure, distribution of probability adjusted to each sample (normal or gamma) and post hoc analysis by the sequential Šidák algorithm.20
The correlation between the fluorescence of ET-1 and histomorphometric parameters was assessed by the Spearman rank correlation coefficient.21
The analysis was performed using the IBM SPSS 22v software, and significance was defined for p<0.05.22 The sample size was based on the expectation of difference between the topographies of more than 20% of the expressions among the sampled skins, with alpha 0.05 and beta 0.2.
Results
The main clinical and demographic information from the 20 participants are presented in the Table 1.
 |
Table 1 Main Clinical and Demographic Data from the Participants (n = 20 Women) |
The relative fluorescence of ET-1 in whole epidermis was higher in melasma, than in the adjacent skin (Figures 1 and 2, Supplementary Table 1), being 32.8% (CI95% 14.7%─52.6%) higher in the spinous layer (p=0.013), 30.4% (CI95% 13.7%─47.9%) higher in the basal layer (p=0.014), and 29.7% (CI95% 11.4%─49.7%) higher in the melanocytes (p=0.006). Neither ERA nor ERB resulted in differential epidermal expression between melasma and unaffected skin (p>0.1).
There was no relevant fluorescence in upper dermis for ET-1, ERA, and ERB.
There was a positive correlation between the expression of ET-1 in the epidermis of melasma with the thickness of the epidermis (rho=0.51; p=0.02), and with age (rho=0.58; p=0.01), but not with mMASI scores (rho=0.18; p=0.45), nor with the receptors ERA and ERB (p>0.3).
Discussion
Our study demonstrated that ET-1, derived from keratinocytes and melanocytes in the epidermis, is overexpressed in skin with melasma compared to adjacent non-lesional skin exposed to sunlight. This suggests that epidermal ET-1, but not from upper dermis, may play a role in the augmented melanogenesis observed in melasma.13,23
Upon exposure to UV radiation, endothelin is produced in the epidermis, which in turn stimulate the ET1-ERB pathway in melanocytes, which is prolonged up to 10 days after UVB exposition.24 This pathway modulates MITF phosphorylation, leading to pigmentation. However, the inhibition of ERA does not result in melanogenesis.25,26 Our results suggest that there is an increase in ET1 expression in the epidermis of skin with melasma, but without a compensatory increase in the expression of ERB or ERA receptors.
Endothelin receptors are G-protein coupled receptors expressed on the surface of melanocytes and play a critical role in their survival and homeostasis. During prenatal life, ERB is the receptor for ET-3 and is expressed on melanoblasts. In postnatal life, ERB is the main receptor for ET-1, which has the same binding affinity and effects as ET-3. Activation of ERB by ET-1 leads to increased intracellular Ca2+ mobilization and PKC activity.27
Extensive research on ET-1 has primarily focused on endothelial secretion, given that it is a potent vasoconstrictor agent. In the epidermis, melanogenesis, keratinocyte proliferation, pruritus and pain can be mediated by ET-1.28,29 It is a relevant cytokine in the pathogenesis of pigmented seborrheic keratosis, lentigo senilis and pigmented basal cell carcinoma.18,30,31 Our study confirmed the higher expression of ET-1 in the epidermis in sites with increased melanogenesis (melasma) and it was correlated with greater epidermal thickness.
UV irradiation induces a dose-dependent increase in melanocyte dendritic percentage and dendrite length, which is promoted by keratinocyte-derived ET-1.32 However, in vitro irradiation of endothelial cells with UVB or UVA leads to a more intense synthesis of SCF (stem cell factor), which is also a pro-melanogenic factor. In another study, the use of 590 nm LED light, which targets vascular components, led to an improvement in skin pigmentation and erythema in melasma patients by inhibiting the expression of vascular growth factors and SCF in dermal endothelial cells.33
Despite the presumable role of dermal ET-1 produced by endothelial cells from the prominent vascular component in the upper dermis in melasma, epidermal ET-1 is more relevant for melanogenesis in healthy and melasma skin. The present study reinforces this finding, as it did not show noticeable ET-1 expression in the upper dermis from photoexposed skin samples, neither in melasma nor in the unaffected adjacent skin. However, fibroblasts from melasma display a senescent phenotype and overexpress ET-3, which also activates ERB, contributing to the sustained melanogenesis in melasma.34
ET-1, as well as keratinocyte plasmin, has been identified as a target of tranexamic acid (TA) in topical formulations that have shown promising results in clearing melasma.35 Given the limited skin penetration of topical formulations, targeting the epidermis rather than the dermis may be the key to the positive results observed in this therapy approach, which is consistent with our findings. Other agents which acts as direct ET-1 inhibitors are being prospected as potential treatments for pigmentary disorders.36,37 To date, no specific topical ERB blockers are available, while potent ERA antagonist (eg, bosentana) have no role in melanogenesis.
Recently, sebocytes have been demonstrated to be responsible for some epidermal ET-1 production, as well as the secretion of other inflammatory mediators, melanogenic factors, and senescence markers observed in melasma. This discovery makes sebocytes a potential target for treatment.38
As melasma is more prevalent among women at childbearing age, some researchers suggest that estrogen can regulate the production of endothelin-1 in skin cells by modulating the activity of enzymes involved in the synthesis and degradation of ET-1. In addition, estrogen can also modulate the sensitivity of skin cells to the effects of ET-1. Nevertheless, the interaction between estrogen receptors (through the noncanonical pathway) and endothelin-1 in the skin is complex and still not fully understood.39,40
Melasma skin present a reduced autophagy in basal layer melanocytes compared to the adjacent unaffected skin.41 Furthermore, ET-1 also induces a decrease in autophagy, which is well documented in cardiac hypertrophy and contractile dysfunction and can be another action in melasma skin.42
Other changes found in melasma skin are damage to the basement membrane zone, including disruptions, gaps, lower density and thinning in the lamina densa and damage in anchoring fibrils from lamina lucida, in addition to an increase in oxidative stress in upper dermis.43–45 There is a proinflammatory and cellular repair environment in melasma skin, which may contribute to maintaining the increase in ET-1 in the epidermis, as it is also released in repair situations.46
The leading hypothesis behind the overexpression of ET-1 in the epidermis of melasma is associated with the local production of nitric oxide (NO). The oxidative and proinflammatory conditions in the upper dermis stimulate NO release, resulting in vasodilation.47 ET-1 is produced as a countermeasure to the vasodilatory effects induced by NO and other inflammatory mediators. Given the increased expression of iNOS in melasma-afflicted skin, which leads to heightened local NO production, there is a potential for sustained production of ET-1 to counteract the effects of NO, leading to a sustained hipermelanogenesis.48,49 A study that concurrently examines the skin expression of ET-1 and NO is warranted.
This study is limited in that only women were included, although this does ensure homogeneity of the sample. Furthermore, the overall severity of melasma, as assessed by the mMASI score, is not particularly high. However, this factor does not undermine the likelihood of detecting a significant difference among the examined topographies.
Additional research is required to explore the underlying mechanisms responsible for the overexpression of ET-1 in melasma. Moreover, it is crucial to develop therapeutic approaches that specifically target epidermal ET-1 to achieve efficient depigmentation and long-term resolution of melasma.
Conclusion
The expression of ET-1 is more pronounced in the epidermis of facial skin affected by melasma compared to the adjacent unaffected skin.
Funding
FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) – grant 2021/08361-9.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Espósito ACC, Cassiano DP, da Silva CN, et al. Update on melasma-part I: pathogenesis. Dermatol Ther. 2022;12(9):1967–1988. doi:10.1007/s13555-022-00779-x
2. Ortonne JP, Arellano I, Berneburg M, et al. A global survey of the role of ultraviolet radiation and hormonal influences in the development of melasma. J Eur Acad Dermatol Venereol. 2009;23(11):1254–1262. doi:10.1111/j.1468-3083.2009.03295.x
3. Alcantara GP, Esposito ACC, Olivatti TOF, Yoshida MM, Miot HA. Evaluation of ex vivo melanogenic response to UVB, UVA, and visible light in facial melasma and unaffected adjacent skin. An Bras Dermatol. 2020;95(6):684–690. doi:10.1016/j.abd.2020.02.015
4. Sklar LR, Almutawa F, Lim HW, Hamzavi I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: a review. Photochem Photobiol Sci off J Eur Photochem Assoc Eur Soc Photobiol. 2013;12(1):54–64.
5. Halaban R, Langdon R, Birchall N, et al. Basic fibroblast growth factor from human keratinocytes is a natural mitogen for melanocytes. J Cell Biol. 1988;107(4):1611–1619. doi:10.1083/jcb.107.4.1611
6. Imokawa G. Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Res. 2004;17(2):96–110. doi:10.1111/j.1600-0749.2003.00126.x
7. Yohn JJ, Morelli JG, Walchak SJ, Rundell KB, Norris DA, Zamora MR. Cultured human keratinocytes synthesize and secrete endothelin-1. J Invest Dermatol. 1993;100(1):23–26. doi:10.1111/1523-1747.ep12349932
8. Roméro-Graillet C, Aberdam E, Clément M, Ortonne JP, Ballotti R. Nitric oxide produced by ultraviolet-irradiated keratinocytes stimulates melanogenesis. J Clin Invest. 1997;99(4):635–642. doi:10.1172/JCI119206
9. Nakahara T, Kido-Nakahara M, Ohno F, et al. The pruritogenic mediator endothelin-1 shifts the dendritic cell-T-cell response toward Th17/Th1 polarization. Allergy. 2018;73(2):511–515. doi:10.1111/all.13322
10. Halili L, Singh MS, Fujii N, Alexander LM, Kenny GP. Endothelin‐1 modulates methacholine‐induced cutaneous vasodilatation but not sweating in young human skin. J Physiol. 2016;594(12):3439–3452. doi:10.1113/JP271735
11. Gomes LO, Hara DB, Rae GA. Endothelin-1 induces itch and pain in the mouse cheek model. Life Sci. 2012;91(13–14):628–633. doi:10.1016/j.lfs.2012.03.020
12. Katugampola R, Church MK, Clough GF. The neurogenic vasodilator response to endothelin-1: a study in human skin in vivo. Exp Physiol. 2000;85(6):839–846. doi:10.1017/S0958067000020893
13. Imokawa G, Yada Y, Miyagishi M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem. 1992;267(34):24675–24680. doi:10.1016/S0021-9258(18)35817-4
14. Pang Y, Geng J, Qin Y, et al. Endothelin-1 increases melanin synthesis in an established sheep skin melanocyte culture. Vitro Cell Dev Biol Anim. 2016;52(7):749–756. doi:10.1007/s11626-016-0042-0
15. Murase D, Hachiya A, Kikuchi-Onoe M, et al. Cooperation of endothelin-1 signaling with melanosomes plays a role in developing and/or maintaining human skin hyperpigmentation. Biol Open. 2015;4(10):1213–1221. doi:10.1242/bio.011973
16. Teraki E, Tajima S, Manaka I, Kawashima M, Miyagishi M, Imokawa G. Role of endothelin-1 in hyperpigmentation in seborrhoeic keratosis. Br J Dermatol. 1996;135(6):918–923. doi:10.1046/j.1365-2133.1996.d01-1095.x
17. Wang K, Xu HN, Wang YW, et al. Ufl1 deficiency causes skin pigmentation by up-regulation of Endothelin-1. Front Cell Dev Biol. 2022;10:961675. doi:10.3389/fcell.2022.961675
18. Lan CCE, Wu CS, Cheng CM, Yu CL, Chen GS, Yu HS. Pigmentation in basal cell carcinoma involves enhanced endothelin-1 expression. Exp Dermatol. 2005;14(7):528–534. doi:10.1111/j.0906-6705.2005.00320.x
19. Miot HA. Assessing normality of data in clinical and experimental trials. J Vasc Bras. 2017;16(2):88–91. doi:10.1590/1677-5449.041117
20. Weichle T, Hynes DM, Durazo-Arvizu R, Tarlov E, Zhang Q. Impact of alternative approaches to assess outlying and influential observations on health care costs. SpringerPlus. 2013;2:614. doi:10.1186/2193-1801-2-614
21. Miot HA. Correlation analysis in clinical and experimental studies. J Vasc Bras. 2018;17(4):275–279. doi:10.1590/1677-5449.174118
22. Miola AC, Miot HA. P-value and effect-size in clinical and experimental studies. J Vasc Bras. 2021;20:e20210038. doi:10.1590/1677-5449.210038
23. Imokawa G, Miyagishi M, Yada Y. Endothelin-1 as a new melanogen: coordinated expression of its gene and the tyrosinase gene in UVB-exposed human epidermis. J Invest Dermatol Julho De. 1995;105(1):32–37. doi:10.1111/1523-1747.ep12312500
24. Hachiya A, Kobayashi A, Yoshida Y, Kitahara T, Takema Y, Imokawa G. Biphasic expression of two paracrine melanogenic cytokines, stem cell factor and endothelin-1, in ultraviolet b-induced human melanogenesis. Am J Pathol Dezembro De. 2004;165(6):2099–2109. doi:10.1016/S0002-9440(10)63260-9
25. Yuan XH, Jin ZH. Paracrine regulation of melanogenesis. Br J Dermatol. 2018;178(3):632–639. doi:10.1111/bjd.15651
26. Regazzetti C, De Donatis GM, Ghorbel HH, et al. Endothelial cells promote pigmentation through endothelin receptor B activation. J Invest Dermatol. 2015;135(12):3096–3104. doi:10.1038/jid.2015.332
27. Swope VB, Abdel-Malek ZA. Significance of the melanocortin 1 and endothelin B receptors in melanocyte homeostasis and prevention of sun-induced genotoxicity. Front Genet. 2016;7:146. doi:10.3389/fgene.2016.00146
28. Wong LS, Yen YT, Lin SH, Lee CH. IL-17A induces endothelin-1 expression through p38 pathway in prurigo nodularis. J Invest Dermatol. 2020;140(3):702–706.e2. doi:10.1016/j.jid.2019.08.438
29. Akio M, Hajime I, Hideo O, Norio K. Tyrosinase induction in normal human cultured melanocytes by endothelin-1. J Cardiovasc Pharmacol. 2004;44(1):S439–42. doi:10.1097/01.fjc.0000166321.76376.bb
30. Manaka L, Kadono S, Kawashima M, Kobayashi T, Imokawa G. The mechanism of hyperpigmentation in seborrhoeic keratosis involves the high expression of endothelin-converting enzyme-1alpha and TNF-alpha, which stimulate secretion of endothelin 1. Br J Dermatol. 2001;145(6):895–903. doi:10.1046/j.1365-2133.2001.04521.x
31. Hattori H, Kawashima M, Ichikawa Y, Imokawa G. The epidermal stem cell factor is over-expressed in lentigo senilis: implication for the mechanism of hyperpigmentation. J Invest Dermatol. 2004;122(5):1256–1265. doi:10.1111/j.0022-202X.2004.22503.x
32. Hara M, Yaar M, Gilchrest BA. Endothelin-1 of keratinocyte origin is a mediator of melanocyte dendricity. J Invest Dermatol. 1995;105(6):744–748. doi:10.1111/1523-1747.ep12325522
33. Dai X, Jin S, Xuan Y, et al. 590 nm LED irradiation improved erythema through inhibiting angiogenesis of human microvascular endothelial cells and ameliorated pigmentation in melasma. Cells. 2022;11(24):3949. doi:10.3390/cells11243949
34. Espósito ACC, Brianezi G, Miot LDB, Miot HA. Fibroblast morphology, growth rate and gene expression in facial melasma. An Bras Dermatol. 2022;97(5):575–582. doi:10.1016/j.abd.2021.09.012
35. Kim SJ, Park JY, Shibata T, Fujiwara R, Kang HY. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41(5):480–485. doi:10.1111/ced.12835
36. Wang J, Chen Z, Lu Y, et al. Soluble Pearl Extract provides effective skin lightening by antagonizing endothelin. J Cosmet Dermatol. 2021;20(8):2531–2537. doi:10.1111/jocd.13899
37. Yang S, Wang Z, Hu Y, et al. Hydrolyzed Conchiolin Protein (HCP) Extracted from Pearls Antagonizes both ET-1 and α-MSH for skin whitening. Int J Mol Sci. 2023;24(8):7471. doi:10.3390/ijms24087471
38. Flori E, Mastrofrancesco A, Mosca S, et al. Sebocytes contribute to melasma onset. iScience. 2022;25(3):103871. doi:10.1016/j.isci.2022.103871
39. Tamega AD, Miot HA, Moco NP, et al. Gene and protein expression of oestrogen- β and progesterone receptors in facial melasma and adjacent healthy skin in women. Int J Cosmet Sci. 2015;37(2):222–228. doi:10.1111/ics.12186
40. Xu S, Yu S, Dong D, Lee LTO. G Protein-coupled estrogen receptor: a potential therapeutic target in cancer. Front Endocrinol. 2019;10:725. doi:10.3389/fendo.2019.00725
41. Espósito ACC, de Souza NP, Miot LDB, Miot HA. Deficit in autophagy: a possible mechanism involved in melanocyte hyperfunction in melasma. Indian J Dermatol Venereol Leprol. 2021;1–3. doi:10.25259/IJDVL_927_20
42. Ceylan-Isik AF, Dong M, Zhang Y, et al. Cardiomyocyte-specific deletion of endothelin receptor A rescues aging-associated cardiac hypertrophy and contractile dysfunction: role of autophagy. Basic Res Cardiol. 2013;108(2):335. doi:10.1007/s00395-013-0335-3
43. Espósito ACC, Brianezi G, de Souza NP, Miot LDB, Miot HA. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32(2):101–108. doi:10.5021/ad.2020.32.2.101
44. Espósito ACC, Brianezi G, de Souza NP, Santos DC, Miot LDB, Miot HA. Ultrastructural characterization of damage in the basement membrane of facial melasma. Arch Dermatol Res. 2020r;312(3):223–227. doi:10.1007/s00403-019-01979-w
45. Espósito ACC, Cassiano DP, Bagatin E, Miot HA. Regarding the alterations in oxidative stress status induced by melasma treatments. Arch Dermatol Res. 2021;313(8):705–706. doi:10.1007/s00403-021-02205-2
46. Lagares D, García-Fernández RA, Jiménez CL, et al. Endothelin 1 contributes to the effect of transforming growth factor beta1 on wound repair and skin fibrosis. Arthritis Rheum. 2010;62(3):878–889. doi:10.1002/art.27307
47. Espósito ACC, Brianezi G, de Souza NP, Miot LDB, Marques MEA, Miot HA. Exploring pathways for sustained melanogenesis in facial melasma: an immunofluorescence study. Int J Cosmet Sci. 2018;40(4):420–424. doi:10.1111/ics.12468
48. Samaka RM, Bakry OA, Shoeib MA, Zaaza MM. Expression of iNOS and NF-κB in melasma: an immunohistochemical study. Anal Quant Cytopathol Histpathol. 2014;36(5):245–257.
49. Jo HY, Kim CK, Suh IB, et al. Co-localization of inducible nitric oxide synthase and phosphorylated Akt in the lesional skins of patients with melasma. J Dermatol. 2009;36(1):10–16. doi:10.1111/j.1346-8138.2008.00579.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.