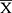Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Expression and Significance of TNF-α and NF-κB/p65 in Cutaneous Lichen Planus
Authors Chen JF, Zhang XM, Sanjel K, Zhang J, Ma C
Received 28 April 2022
Accepted for publication 28 July 2022
Published 2 August 2022 Volume 2022:15 Pages 1509—1516
DOI https://doi.org/10.2147/CCID.S372662
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Jun Fang Chen,1 Xue Mei Zhang,1,2 Kiran Sanjel,1,2 Juan Zhang,2 Cong Ma1
1The Dermatology and Venereology Department, The Affiliated Hospital of Inner Mongolia Minzu University, Tongliao, People’s Republic of China; 2Clinical Medical School, Inner Mongolia Minzu University, Tongliao, People’s Republic of China
Correspondence: Xue Mei Zhang, The Dermatology and Venereology Department, The Affiliated Hospital of Inner Mongolia Minzu University, Tongliao, Inner Mongolia, 028000, People’s Republic of China, Tel +8618504757225, Email [email protected]
Purpose: The purpose of this study was to explore the expression of TNF-α and NF-κB/p65 in Lichen planus skin lesions and their correlation with the pathogenesis of Lichen planus.
Patients and Methods: The case group consisted of 30 individuals diagnosed with LP based on clinical and histopathologic examination. The control group consisted of 10 individuals from an Orthopedic Department with normal skin. TNF-α and NF-κB/p65 expression in skin tissue samples was detected by immunohistochemistry.
Results: The immunohistochemical results showed that TNF-α and NF-κB/p65 expression levels were significantly higher in LP skin lesions than normal skin tissues (P ≤ 0.05). Positive TNF-α staining mainly occurred in the cytoplasm of keratinocytes of the stratum granulosum, stratum spinosum, and stratum basale in the epidermis and lymphocytes in the superficial dermis. Positive NF-κB/p65 staining mainly occurred in the nucleus and cytoplasm of keratinocytes of the stratum spinosum and stratum basale in the epidermis and lymphocytes in the superficial dermis.
Conclusion: TNF-α and NF-κB/p65 are overexpressed in cutaneous LP. The two are positively correlated in LP, suggesting that they both play essential roles in the pathogenesis of LP.
Keywords: lichen planus, TNF-α, NF-κB/p65, staining, immunohistochemistry
Introduction
Lichen planus (LP) is a chronic inflammatory disease affecting the skin, mucosa, and appendages. Its etiology is not fully known. Studies have shown that T cell-mediated immune responses are closely related to the disease,1,2 which eventually leads to the apoptosis of keratinocytes.
Nuclear factor kappaB/p65 (NF-κB/p65) is a multidirectional transcription factor that can influence the transcription of many genes. Nuclear factor kappaB/p65 (NF-κB/p65) is a multidirectional transcription factor that can influence the transcription of many genes. It plays an essential role in physiological and pathological processes such as the immune response, the inflammatory response, cell differentiation, and apoptosis. It also plays a vital role in the inflammatory process of oral LP like proinflammatory, proangiogenic, and immunoregulatory activity.3 At rest, it is bound to its inhibitory protein and is expressed in the cytoplasm in the form of a trimer. When cells are stimulated, it can be activated, enter the nucleus, bind to binding sites on its target genes, and produce an inflammatory response,4 and it plays an essential role in various autoimmune diseases (systemic lupus erythematosus, multiple sclerosis, inflammatory bowel disease).5
Tumor necrosis factor-alpha (TNF-α) is an inflammatory cytokine that can be produced by various cells, including monocytes, macrophages, keratinocytes, and activated T lymphocytes, and it has extensive biological activity.6 In particular, it is related to the proliferation and differentiation of various cells.
There are few studies on the relationship between TNF-α and NF-κB/p65 in the pathogenesis of LP. In this study, we aimed to explore the expression of TNF-α and NF-κB/p65 in LP skin lesions and their association with the pathogenesis of LP.
Tumor necrosis factor-alpha (TNF-α) is an inflammatory cytokine that can be produced by various cells, including monocytes, macrophages, keratinocytes, and activated T lymphocytes, and it has extensive biological activity.6 In particular, it is related to the proliferation and differentiation of various cells.
There are few studies on the relationship between TNF-α and NF-κB/p65 in the pathogenesis of LP. In this study, we aimed to explore the expression of TNF-α and NF-κB/p65 in LP skin lesions and their association with the pathogenesis of LP.
Materials and Methods
Participants
The case group consisted of 30 randomly selected LP patients who were treated at the Dermatology Department of the Affiliated Hospital of Inner Mongolia Minzu University from December 2017 to December 2019. The control group consisted of 10 individuals randomly selected from the Orthopedic Department who had no significant dermatological or systemic diseases.
The patients in the case group were clinically diagnosed with LP by a dermatologist (based on dermoscopy, involving the presence of prominent Wickham striae) and histopathologically diagnosed by a pathologist (Figure 1). The patients had not received any LP-causing medications (beta-blockers, methyldopa, penicillamine, quinidine), glucocorticoids, immunosuppressants, or other treatments in the prior 6 months. There was no significant history of other comorbidities, including other dermatological diseases. A serological test was also performed to rule out hepatitis C, which can cause LP-like cutaneous eruptions.
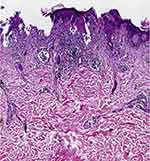 |
Figure 1 HE staining of LP (case group) showing hyperkeratosis of the epidermis, band like infiltration of lymphocytes.(HE x 100). |
Ethics Approval
The Medical Ethics Committee of the Affiliated Hospital of Inner Mongolia Minzu University reviewed and approved the research, and the participants gave written informed consent. The research was carried out following the Helsinki Declaration.
Tissue Sample Collection
After using local anesthetic (2% lignocaine), a 5-mm skin punch biopsy was taken from a new LP lesion of each of the individuals in the case group and from the back of each individual in the control group by a dermatologist (Xue Mei) at the Dermatology Department of our hospital. The tissue samples were then transported to the Pathology Department for hematoxylin and eosin staining followed by immunohistochemical analysis.
Immunohistochemical Analysis
A two-step immunohistochemical analysis, using an immunohistochemical series working solution kit (Wuhan Beiyinlai Biotechnology Co., Ltd, Wuhan, China), was performed to detect the expression of TNF-α and NF-κB/p65 in the tissues. First, the tissues were fixed with 10% neutral buffer formaldehyde, dehydrated with conventional ethanol, embedded in paraffin, cut into 4-µm sections, and subjected to dewaxing and hydration. Diluted 3% H2O2 solution was used to block endogenous peroxidase activity. The sections were then rinsed for 2 min with phosphate-buffered saline (PBS) three times. Heat-induced antigen retrieval was conducted using a pressure cooker method.
Rat anti-human TNF-α polyclonal antibody (dilution: 1:50) or rat anti-human NF-κB/p65 polyclonal antibody (dilution: 1:50) (Wuhan Beiyinlai Biotechnology Co., Ltd, Wuhan, China) were then added dropwise and the sections were incubated at 4°C overnight. The sections were then rinsed for 2 min with PBS three times. Next, 3.3’-diaminobenzidine (DAB) color development using DAB chromogenic solution (Wuhan Beiyinlai Biotechnology Co., Ltd, Wuhan, China) was conducted. Lastly, hematoxylin counterstaining, dehydration, and sealing of the slides were performed.
The positive control for TNF-α staining was human stomach tissue and the positive control for NF-κB/p65 staining was human colon cancer tissue with lymphocytic infiltration. As a negative control, PBS was used instead of primary antibody.
The immunohistochemical results were independently analyzed by two researchers who were unaware of the participants’ clinical data. Brownish-yellow particles in the cytoplasm or nucleus were considered to indicate positive staining for TNF-α or NF-κB/p65. To semi-quantify the results, a slide was randomly selected for each participant and observed under a microscope’s high-power field. Five fields were randomly selected for each slide. The results were classed as negative, weakly positive, moderately positive and strongly positive if ≤10% (-), 11–25% (+), 26–50% (++), and >50% (+++), respectively, of the cells were positively stained. The two researchers collected data and analyzed the immunohistochemical staining results separately.
Statistical Analysis
The data were analyzed using SPSS 25.0 software. The normally distributed data are expressed as mean ± standard deviation (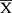 ± s) and the categorical variables are expressed as frequency (percentage). The groups were compared by independent-samples t-tests (t) and chi-square tests (χ2). A Spearman rank correlation analysis between TNF-α and NF-κB/p65 expression was also performed. P ≤ 0.05 was considered statistically significant.
± s) and the categorical variables are expressed as frequency (percentage). The groups were compared by independent-samples t-tests (t) and chi-square tests (χ2). A Spearman rank correlation analysis between TNF-α and NF-κB/p65 expression was also performed. P ≤ 0.05 was considered statistically significant.
Results
Among the 30 individuals with LP, 17 were male (56.6%), and 13 were female (43.33%). Their ages ranged from 21 to 62 years (mean age 40.43±9.96). Among the ten individuals in the control group, 5 were male (50%), and 5 were female (50%). Their ages ranged from 20 to 66 years (mean age 43.80±11.93). There were no significant differences in age, and gender distribution between the two groups (Table 1). In the case group the LP lesions were mostly on the forearm, waist, and lower limbs, with the largest lesion being 5×5 cm. Some cases presented with oral mucosal involvement.
 |
Table 1 Comparison of Demographic Details of Two Groups |
TNF-α Staining Results
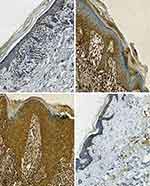
Among the 30 LP patients in the case group, 5 were strongly positive, 10 were moderately positive, 9 were weakly positive, and 6 were negative. Among the 10 controls in the control group, 1 was weakly positive and 9 were negative. The positive TNF-α staining was significantly higher in the LP group than the control group (χ2 = 4.02, P ≤ 0.05) (Table 2). In LP lesions (case group), the TNF-α staining was mainly in the cytoplasm of keratinocytes in the epidermal granular layer, spinous layer, and basal layer, and lymphocytes in the superficial dermis (Figure 2A–C). In normal skin tissue (control group), the same areas were negative or weakly positive (Figure 2D). TNF-α expression was significantly higher in the LP group than the control group (P ≤ 0.05) (Table 3).
 |
Table 2 Positive Expression Rates of TNF-α and NF-κB/p65 (%) |
 |
Table 3 Comparison of TNF-α and NF-κB/p65 Expression Levels in Tissue of LP Group and Control Group ( |
NF-κB/p65 Staining Results
Among the 30 LP patients in the case group, 4 were strongly positive, 10 were moderately positive, 8 were weakly positive, and 8 were negative. Among the 10 controls in the control group, 1 was weakly positive and 9 were negative. The positive NF-κB/p65 staining was significantly higher in the LP group than the control group (χ2 = 3.17, P ≤ 0.05) (Table 2). In LP lesions (case group), the NF-κB/p65 staining was mainly in the nucleus and cytoplasm of keratinocytes in the spinous layer and basal layer of epidermis, and lymphocytes in the superficial dermis (Figure 3A–C). In normal skin tissue (control group), the nucleus and cytoplasm of keratinocytes in the spinous layer and basal layer and lymphocytes in the superficial dermis was negative or weakly positive (Figure 3D). NF-κB/p65 expression was significantly higher in the LP group than the control group (P ≤ 0.05) (Table 3).
Correlation Between TNF-α and NF-κB/p65 Expression in LP Tissues
Spearman rank correlation analysis showed that TNF-α expression was positively correlated with NF-κB/p65 expression (rs = 0.627, P < 0.001).
Discussion
The histopathological characteristics of LP include hyperkeratosis of the epidermis, focal wedge-shaped thickening of the granular layer, irregular thickening of the spinous layer, liquefaction degeneration of basal cells, banded infiltration of lymphocytes, and the appearance of colloidal bodies in the superficial dermis.1 However, the precise mechanism underlying these changes is not known.
In recent years, it has been found that T lymphocyte-mediated autoimmune response is closely related to LP.2,7 Local TNF-α production and release in LP lesions are critical in immune system. In LP, TNF-α binding to tumor necrosis factor receptor-1 (TNFR-1) can promote apoptosis.8 The increased release of TNF-α in LP damages the mucosa and interferes with the tissue repair program via induction of a soluble natural antagonist of IL-22, decreases the collagen content, aggregates with adhesion molecules (integrins, selectins, cadherins) and aggravates the inflammatory response.9,10 Some researchers have found a correlation between TNF-α and the number of lymphocytes infiltrating LP lesions.11 Additionally, there is a significant increase in TNF-α in epidermal keratinocytes and superficial dermal lymphocytes at the liquefaction site of basal cells in LP.12 Thus, it can be seen that TNF-α plays an essential role in the pathogenesis of LP. Studies of oral LP have shown that after TNF-α binds to its receptors, it activates intracellular signaling pathways, regulates the expression of adhesion factors and chemokines, mediates the inflammatory response, increases the expression of NF-κB/p65, and thereby participates in the pathogenesis of oral LP.13–15 In addition, the promoter of the TNF-α gene contains an NF-κB/p65-binding site.13–15 Thus, TNF-α can act as a downstream factor of the NF-κB/p65 signaling pathway to regulate the inflammatory response. In psoriasis, the activity of NF-κB/p65 plays a vital role in TNF-α-mediated apoptosis.15 Up-regulation of NF-κB/p65 can promote the transcription of TNF-α, and the release of TNF-α can further increase the activity of NF-κB/p65 and thereby mediate the inflammatory response, forming a repeated regulatory feedback loop and participating in the pathogenesis of psoriasis.15
Therefore, we speculated that TNF-α and NF-κB/p65 may be simultaneously involved in the pathogenesis of LP, with their expression being positively correlated. In this study, TNF-α and NF-κB/p65 expression in LP skin lesions was detected by immunohistochemistry. It was found that the positive staining of TNF-α and NF-κB/p65 were significantly higher in LP skin lesions than in the control group. Additionally, Spearman rank correlation analysis showed that TNF-α and NF-κB/p65 expression levels were positively correlated. Among the LP patients, 4 patients with extensive lesions had strongly positive staining for both TNF-α and NF-κB/p65 and 1 patient (with bullous LP) had strongly positive staining for TNF-α and NF-κB/p65; in contrast, among the controls, none had strongly positive or moderately positive staining for either TNF-α or NF-κB/p65. These results indicate that TNF-α and NF-κB/p65 are involved in the pathogenesis of LP. We speculate that T cells are activated in LP lesions and release a large amount of TNF-α. TNF-α may further up-regulate NF-κB/p65, and NF-κB/p65 may promote TNF-α release, forming a vicious cycle in the inflammatory environment, leading to lymphocyte infiltration, and thereby mediating the chronic inflammatory process of LP.
Our study showed immunohistochemical staining in LP lesions for TNF-α and NF-κB/p65, which showed that they participate in the pathogenesis of LP, and both markers were positively correlated. The use of TNF-α inhibitors for the management of LP has already been reported in the literature. Yarom et al reported that etanercept was effective for treating oral LP, and Chao et al reported that adalimumab led to the remission of cutaneous and mucosal LP.16,17 Etanercept was also reported to be effective for the treatment of nail LP.18 However, there is also some evidence that TNF-α inhibitors can paradoxically cause LP-like eruptions.19 Some studies have hypothesized that lichenoid eruptions in genetically predisposed individuals develop due to cross-regulation between interferon I and TNF-α that causes these cytokines neutralize each other.19 In these individuals, drug-induced inhibition of TNF-α raises the levels of interferon I, activating T cells and dendritic cells and producing an inflammatory response that leads to the appearance of lesions.20 Thus, TNF-α inhibition can be considered to be both anti- or pro-inflammatory.20–22
Conclusion
We found that TNF-α and NF-κB/p65 may participate in the pathogenesis of LP and are positively correlated with each other in LP, which deepens the understanding of the pathogenesis of LP. Therefore, inhibiting both TNF-α and NF-κB/p65 may significantly reduce the occurrence and development of LP. This indicates that targeted therapy against both TNF-α and NF-κB/p65 could be used to treat LP.
Ethics Statement
The research involving human participants was reviewed and approved by the Medical Ethics Committee of The Affiliated Hospital of Inner Mongolia Minzu University. The participants provided their written informed consent to participate in this study. The research was carried out following the Helsinki Declaration.
Acknowledgments
We thank Dr. Jin (Director, Department of Orthopedics, The Affiliated Hospital of Inner Mongolia Minzu University) for helping us to recruit patients for the control group and Dr. Bai (Director, Department of Pathology, The Affiliated Hospital of Inner Mongolia Minzu University) for his help preparing the histopathology analysis and analyzing the results.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Inner Mongolia Natural Science Foundation (grant no. 2018MS08116).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sugerman PB, Satterwhite K, Bigby M. Autocytotoxic T-cell clones in lichen planus. Br J Dermatol. 2000;142(3):449–456. doi:10.1046/j.1365-2133.2000.03355.x
2. Amirchaghmaghi M, Pakfetrat A, Delavarian Z, et al. Evaluation of the efficacy of curcumin in the treatment of oral lichen planus: a randomized controlled trial. J Clin Diagn Res. 2016;10(5):134–137. doi:10.7860/JCDR/2016/16338.7870
3. Du J, Li R, Yu F, et al. Experimental study on 1,25(OH) 2D3 amelioration of oral lichen planus through regulating NF-κB signaling pathway. Oral Dis. 2017;23(6):770–778. doi:10.1111/odi.12659
4. Mozaffari HR, Ramezani M, Mahmoudiahmadabadi M, et al. Salivary and serum levels of tumor necrosis factor-alpha in oral lichen planus: a systematic review and meta-analysis study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(3):e183–9. doi:10.1016/j.oooo.2017.06.117
5. Carrozzo M. Understanding the pathobiology of oral lichen planus. Curr Oral Health Rep. 2014;1(3):173–179. doi:10.1007/s40496-014-0022-y
6. Jin X, Wang J, Zhu L, et al. Association between-308 G/A polymorphism in TNF-a gene and lichen planus: a meta-analysis. J Dermatol Sci. 2012;68(3):127–134. doi:10.1016/j.jdermsci.2012.09.003
7. Yang LH, Wang TY, Wang LS, et al. Expression of IL-6 IL-8 and TNF-a in serum of oral lichen planus. Mod Drug Appl China. 2016;10(12):27–29. doi:10.14164/j.cnki.cn11-5581/r.2016.12.017
8. Kara YA. The measurement of serum tumor necrosis factor-alpha levels in patients with lichen planus. Indian J Dermatol. 2018;63(4):297–300. doi:10.4103/ijd.IJD_474_17
9. Qi YH, Pang CC. Expression of TNF-a in lichen planus lesions and its relationship with infiltrating lymphocytes. Chin J Dermatol. 2011;44(10):727–728. doi:10.3760/cma.j.issn.0412-4030.2011.10.013
10. Ding ZY, Li X. Expression of TNF-a in lichen planus lesions. Chin J Dermatol. 2005;38(12):763. doi:10.3760/j.issn:0412-4030.2005.12.015
11. Man ZZ, Xu Y, Zhu LL, et al. Expression and significance of NF-kB TNF-a and IL-1β in lichen planus. Oral Med. 2013;33(7):461–464. doi:10.13591/j.cnki.kqyx.2013.07.009
12. Rusanen P, Marttila E, Uittamo J, et al. TLR1-10 NF-κB and p53 expression is increased in oral lichenoid disease. PLoS One. 2017;12(7):e0181361. doi:10.1371/journal.pone.0181361
13. Ge Y, Xu Y, Sun W, et al. The molecular mechanisms of the effect of dexamethasone and cyclosporin A on TLR4 /NF-κB signaling pathway activation in oral lichen planus. Gene. 2012;508(2):157–164. doi:10.1016/j.gene.2012.07.045
14. Babiuch K, Kusnierz-Cabala B, Kesek B, et al. Evaluation of proinflammatory, NF-kappaB dependent cytokines: IL-1α, IL-6, IL-8, and TNF-α in tissue specimens and saliva of patients with oral squamous cell carcinoma and oral potentially malignant disorders. J Clin Med. 2020;9(3):867. doi:10.3390/jcm9030867
15. Gesser B, Rasmussen MK, Iversen L. Dimethyl Fumarate Targets MSK1, RSK1, 2 and IKKα/β kinases and regulates NF-κB/p65 activation in psoriasis: a demonstration of the effect on peripheral blood mononuclear cells, drawn from two patients with severe psoriasis before and after treatment with dimethyl fumarate. Psoriasis. 2020;31(10):1–11. doi:10.2147/PTT
16. Yarom N. Etanercept for the management of oral lichen planus. Am J Clin Dermatol. 2007;8(2):121. PMID: 17428119. doi:10.2165/00128071-200708020-00010
17. Chao TJ. Adalimumab in the management of cutaneous and oral lichen planus. Cutis. 2009;84:352–358.
18. Irla N, Schneiter T, Haneke E, Yawalkar N. Nail lichen planus: successful treatment with etanercept. Case Rep Dermatol. 2010;2(3):173–176. doi:10.1159/000321419
19. Oliveira SC, Vasconcelos AHC, Magalhães EPB, Corrêa FJV, Rodrigues CEM. Clinical, histopathological and outcome analysis of five patients with lichenoid eruption following anti-tumor necrosis factor-alpha therapy for ankylosing spondylitis: report of one case and review of the literature. Cureus. 2020;12(9):e10598. doi:10.7759/cureus.10598
20. López de Padilla CM, Niewold TB. The type I interferons: basic concepts and clinical relevance in immune-mediated inflammatory diseases. Gene. 2016;576(1 Pt 1):14–21. doi:10.1016/j.gene.2015.09.058
21. Inoue A, Sawada Y, Yamaguchi T, et al. Lichenoid drug eruption caused by Adalimumab: a case report and literature review. Eur J Dermatol. 2017;27(1):69–70. doi:10.1684/ejd.2016.2898
22. McCarty M, Basile A, Bair B, Fivenson D. Lichenoid reactions in association with tumor necrosis factor alpha inhibitors: a review of the literature and addition of a fourth lichenoid reaction. J Clin Aesthet Dermatol. 2015;8(6):45–49. PMID: 26155327; PMCID: PMC4479369.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.