Back to Journals » International Journal of General Medicine » Volume 16
Expression and Clinical Significance of TIGIT in Primary Breast Cancer
Authors Tang L, Sha M, Guo T, Lu H, Qian J, Shao Q, Ye J
Received 8 February 2023
Accepted for publication 4 May 2023
Published 12 June 2023 Volume 2023:16 Pages 2405—2417
DOI https://doi.org/10.2147/IJGM.S407725
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Limin Tang,1 Min Sha,2 Ting Guo,2 Huimin Lu,2 Jingyu Qian,2 Qixiang Shao,1 Jun Ye2
1Department of Immunology, School of Medicine, Jiangsu University, Zhenjiang, Jiangsu, People’s Republic of China; 2Department of Central Laboratory, The Affiliated Taizhou People’s Hospital of Nanjing Medical University, Taizhou School of Clinical Medicine, Nanjing Medical University, Taizhou, Jiangsu, People’s Republic of China
Correspondence: Jun Ye, Department of Central Laboratory, The Affiliated Taizhou People’s Hospital of Nanjing Medical University, Taizhou School of Clinical Medicine, Nanjing Medical University, 399 Hailing Road, Taizhou, Jiangsu, 225300, People’s Republic of China, Tel +86-13625171902, Email [email protected]
Purpose: The roles of T cell immunoreceptor with Ig and ITIM domains (TIGIT) in the diagnosis of primary breast cancer (PBC) are still unclear. This study was designed to investigate the expression of TIGIT in PBC patients, with an aim to analyze its diagnostic value in PBC.
Patients and Methods: We first explore the expression of TIGIT in cancer patients based on TCGA database, and then we analyzed its correlation with clinicopathological features. Afterwards, we compared the protein and mRNA expressions of TIGIT in two BC cell lines (MCF-7 and MDA-MB-231) and normal breast epithelial cell line (MCF-10A). Subsequently, 56 PBC female patients admitted to the Taizhou People’s Hospital from October 2018 to June 2021 were included in this study. Flow cytometry was used to detect TIGIT level on peripheral blood CD3+ T cells of PBC patients and healthy controls. TIGIT expression in PBC tissues was detected by immunohistochemistry (IHC) and immunofluorescence staining.
Results: TCGA database showed that compared with adjacent tissues, TIGIT was significantly upregulated in tumor tissues. High TIGIT expression was positively correlated with tumor stage and negatively correlated with recurrence free survival (RFS) and overall survival (OS). TIGIT level in BC cell lines, peripheral blood and tumor tissues of PBC patients was significantly higher than that of control (P < 0.05). TIGIT level was correlated with age (P < 0.05), rather than tumor size, pathological type, lymph node metastasis, ER, PR, HER-2, and P53. ROC curve showed that the optimal critical value of peripheral blood TIGIT for BC screening was 23.38%. Postoperative TIGIT level in peripheral blood was significantly decreased compared to the preoperative TIGIT level (P < 0.05).
Conclusion: TIGIT was upregulated in PBC and was correlated with age. It may be a potential target for the diagnosis and immunotherapy of PBC.
Keywords: TIGIT, primary breast cancer, immune checkpoint, tumor immunity, bioinformatics analysis
Introduction
According to GLOBOCAN estimates of cancer incidence and mortality produced by IARC, female breast cancer (BC) has surpassed lung cancer as the most diagnosed cancer in 2020 worldwide, with an estimated 2.3 million new cases (11.7%).1 Current major treatment methods for early BC include surgical resection, adjuvant therapy, radiotherapy, chemotherapy, and hormone therapy.2 Unfortunately, the therapeutic effects of these methods have been weakened due to early metastasis of tumors, side effects of drugs and drug resistance of tumors.
BC is a highly heterogeneous disease at the molecular level.3 Molecular targeted therapy is a method that specifically kills tumor cells without damaging normal tissues by delivering drugs that can bind to specific cancer-causing sites at the molecular level.4 It is expected to be a more effective and less toxic therapeutic strategy for BC. Tumor cells usually escape from immune surveillance by activating various immunosuppressive pathways, including the activation of inhibitory receptors on tumor-infiltrating T cells.5 Under normal circumstances, immune checkpoint molecules mainly maintain their own immune tolerance and protect normal tissues from the damage of the immune system.6 However, lymphocytes will abnormally express co-inhibitory molecules due to long-term exposure to tumor antigens, which will eventually lead to the exhaustion of T cells in the tumor microenvironment.7 One of the important manifestations of T cell exhaustion is high expression of immune checkpoint molecules such as programmed cell death protein 1 (PD-1), cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and T cell immunoglobulin-3 (Tim-3).8,9 Nowadays, monoclonal antibodies (mAbs) blocking PD-1, PD-L1, and CTLA-4 have been approved for multiple cancer indications,10–15 however, only a minority of patients benefit from them. Therefore, increasing attention has been paid to the recognition of new inhibitory receptors.
T cell immunoreceptor with Ig and ITIM domains (TIGIT), also known as VSig9, Vstm3 or WUCAM, is an inhibitory receptor expressed on the surface of T cells and NK cells, which is overexpressed in the presence of naive CD4+ T cell activation.16 TIGIT shows a high affinity to CD155 rather than CD112. In a previous study, the expression of TIGIT on NK cells was highly correlated with its effectiveness in suppressing cytotoxicity.17 It has also been shown that TIGIT could negatively regulate the anti-tumor response as its deficiency would lead to significant suppression of tumor growth.18 In addition, increased TIGIT was correlated with parameters of HIV progression, and ex-vivo single or combinational antibody blockade of TIGIT and/or PD-L1 restored viral-specific CD8+ T cell effector responses.19 Besides, TIGIT has been reported to be highly expressed in various tumor diseases such as ovarian cancer, liver cancer, and BC, which may be involved in the process of anti-tumor immune escape.20,21 However, little is known on the diagnostic values of TIGIT in BC patients. This study was designed to investigate the expression of TIGIT in BC tumor cells, as well as tissues and T cells of peripheral blood in BC patients, which may provide helpful information for the diagnosis and immunotherapy of BC.
Materials and Methods
TCGA Data Collection
The expression of TIGIT in 33 types of pan-cancer samples and 1376 invasive BC samples (291 adjacent normal tissues and 1085 tumor tissues) was obtained from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.com). We divided the samples into high- and low-expression groups using the median of TIGIT expression in the TCGA-BC dataset and performed differential analysis between the two groups to determine the expression cutoff. The data were used to analyze the expression of TIGIT in various cancers and the relationship between TIGIT expression and overall survival (OS) and recurrence-free survival (RFS) in patients with invasive BC.
Cell Culture
Two human BC cell lines (ie MCF-7 and MDA-MB-231) and one human normal breast epithelial cell line (MCF-10A) were cultured in RPMI 1640 medium containing 10% fetal bovine serum (Gibco). All the cells were cultured in a humidified 37°C incubator supplemented with 5% CO2 and collected or passaged when their growth reached about 80% confluency. All cell lines were purchased from Procell Life Science & Technology Co., Ltd. (Wuhan, China).
Western Blot
Total protein was extracted from the cell lines using RIPA lysis buffer (Beyotime, Shanghai, China). The protein content was evaluated by BCA method using a commercial kit purchased from Bioteke (Beijing, China). Protein (20 μg) was separated on 10% SDS-PAGE gels, and then was transferred onto PVDF membranes (Millipore, Bedford, USA) and blocked with 5% skimmed milk under room temperature. Subsequently, rabbit monoclonal primary antibodies against TIGIT (ab243903; 1:4000; Abcam) and against α-tubulin (66,031; 1:10,000; Proteintech, China) were added and incubated overnight at 4°C. PVDF membrane was washed four times with TBST for 5 min each time and then incubated with goat anti-mouse or anti-rabbit IgG horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. After washing PVDF membrane for four times, immunoreactive bands were displayed by enhanced chemiluminescence (ECL), photographed in grayscale and quantified using the Image J program (NIH, Bethesda, MD, USA). The α-tubulin was used as an internal reference to normalize bands. The ratio of IOD TIGIT/IOD α-tubulin indicated the relative expression of TIGIT protein.
Quantitative Real Time PCR (qRT-PCR)
The qRT-PCR was carried out with reference to the previous method.22 Briefly, TRIzol reagent (Takara, Dalian, China) was used to isolate total RNA from the cells. Reverse transcription of RNA into first-strand cDNA was performed using Rever Tra Ace® qPCR RT Master Mix (FSQ-201; TOYOBO, Japan) according to the manufacturer’s instructions. Then TIGIT mRNA level was measured by Power SYBR Green PCR Master Mix (Thermo Fisher Scientific, Woolston Warrington, UK) using the Light Cycler 480 II system (Roche diagnostics, Mannheim, Germany), with β-actin as an internal control. The TIGIT sequences were as follows: forward, 5’-TCTGCATCTATCACACCTACCC-3’; reverse, 5’-CCACCACGATGACTGCTGT-3’. The PCR reaction was initiated with a 10-min denaturation at 95°C. Amplification was carried out for 40 cycles of 30 s at 95°C, 30 s at 60°C and 30 s at 72°C. The 2−ΔΔCt method was used to analyze the relative expression of TIGIT gene.
Patients
We included 56 females with pathologically diagnosed primary breast cancer (PBC) admitted to the Taizhou People’s Hospital (Taizhou, China) from October 2018 to June 2021. The diagnostic criteria were in line with the guideline for BC (2017 edition). The inclusion criteria were as follows: patients with pathologically diagnosed PBC underwent surgical resection for the first time, and had not previously received antitumor therapy. Patients with chronic infections, infectious diseases, immune system diseases, or a history of cancer or receiving any antitumor therapy were excluded. Thirty-four healthy female individuals underwent regular physical examination with normal blood routine, lymphocytes, and tumor markers after flow cytometry, served as a control group. The study protocols were approved by the Medical Ethics Committee of Taizhou People’s Hospital (No. KY201804801). Written informed consent was obtained from all subjects before the study.
IHC
TIGIT expression in tumor tissue and adjacent normal tissue was detected by IHC. Briefly, tissue sections were deparaffinized, hydrated, and antigen retrieved with a citrate buffer (pH 6.0). After washing with PBS buffer and blocking with 10% goat serum, 70 μL anti-TIGIT primary antibody (ab243903; 1:200; Abcam) was added, and the mixture was incubated at 37°C for 1 h. Then, a secondary antibody included in the Dako K5007 kit was added. The mixture was incubated at room temperature for 30 min and rinsed with PBS buffer. Afterwards, the sections were incubated with 3.3’-diaminobenzidine (DAB), counterstained with hematoxylin, rinsed with running water and differentiated with 0.5% hydrochloric acid alcohol for 3–5 sec. Then, the sections were washed again with running water for 15 min, dehydrated, and mounted, and finally, were observed under an inverted optical microscope (Olympus, XDS-1A).
Immunofluorescent Staining
Immunofluorescent staining was performed to further determine the location of TIGIT expression in BC tissues. Briefly, tissue sections were deparaffinized, hydrated, and antigen retrieved with a citrate buffer (pH 6.0). After washing with PBS buffer and blocking with 10% goat serum, anti-TIGIT primary antibody (E5Y1W#99567; CST) was added, and the mixture was incubated overnight at 4°C. Then, the TIGIT fluorescent secondary antibody (Thermo Fisher Scientific, Waltham, MA) was added to give a red reaction product. Afterwards, the mixture was incubated with mouse monoclonal primary antibodies against CD3 (17A2#24,265; CST) and CD56 (123C3#3576; CST) overnight at 4°C. Then, the CD3 (A11008) or CD56 (A11012) fluorescent secondary antibody (Thermo Fisher Scientific, Waltham, MA) was added to give a green reaction product. After washing with PBS buffer, the nuclei were counterstained with DAPI for 10 min in a dark environment. Fluorescence images were visualized with a fluorescence microscope (Leica Microsystems, Wetzlar, Germany).
Flow Cytometry
EDTA-K3 anticoagulated peripheral blood (5 mL) was collected. Briefly, 5 μL anti-CD3-FITC mAb (BD company), 2 μL anti-TIGIT-PE mAb (BioLegend company), and 50 μL EDTA-K3 anticoagulated blood were added to the bottom of flow tube. The mixture was shaken vigorously and left in the dark conditions for 15 min. Then, 500 μL hemolysin was added, and the mixture was vortexed and left in the dark for 10 min. Upon centrifugation at 1500 rpm for 5 min, the supernatant was discarded, followed by adding 500 μL normal saline. TIGIT expression on the T cell surface was detected using FACS Calibur flow cytometer (BD Biosciences). Lymphocyte populations were identified by forward and side scatter, gated on CD3-positive cells. Isotype control (negative control) was used to define the background staining. FlowJo software (Tree Star Inc.) was utilized to analyze the generated data.
Statistical Analysis
Normally distributed measurement data were expressed as mean ± standard deviation, and Student’s t-test was used for comparison between two groups. The measurement data with skewed distribution were expressed as median (interquartile range), and nonparametric test (U-test) was used for comparison. Qualitative data were expressed in frequency, and a chi-square test was used for comparison. Receiver operating characteristic (ROC) curves were used to determine the optimal cut-off value for BC diagnosis. A p-value of <0.05 was statistically significant.
Results
TIGIT Was Upregulated in Invasive BC and Associated with Prognosis Based on TCGA Database
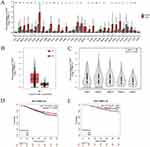
TCGA database showed that TIGIT was significantly upregulated in 33 types of tumor tissues than those in normal tissues (P < 0.05, Figure 1A). The expression of TIGIT in BC tissues was significantly higher than that in adjacent normal tissues (Figure 1B). TIGIT level in patients with stage II and stage III BC was significantly higher than those in patients with stage I BC (Figure 1C). Kaplan–Meier survival plots revealed that patients with high expression of TIGIT exhibited better recurrence-free survival (RFS) and overall survival (OS) (P < 0.05, Figure 1D and Figure 1).
TIGIT Was Upregulated in BC Cell Lines and Tumor Tissues
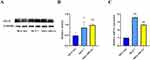
Compared with the normal MCF-10A cells, the protein and mRNA levels of TIGIT were significantly upregulated in the MCF-7 and MDA-MB-231 BC cells (P < 0.05, Figure 2). TIGIT expressions in tumor tissues and adjacent tissues are shown in Figure 3 and Table 1. Compared with the adjacent tissues, the positive expression rate of TIGIT in 56 tumor tissues was significantly increased (67.86% vs 5.88%, P < 0.0001). In addition, the results of immunofluorescence staining showed that TIGIT expression was mainly localized in cancer cells rather than tumor infiltrating cells (Figure 4).
 |
Table 1 TIGIT Expression in Tumor Tissues and Adjacent Tissues |
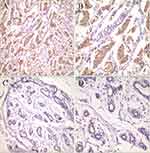 |
Figure 3 TIGIT expression in tumor tissues based on IHC (A, 200× and B, 400×) versus adjacent tissues (C, 200× and D, 400×). |
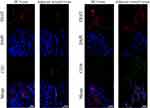 |
Figure 4 Representative immunofluorescence microscopic images of TIGIT, CD3, and CD56 in BC tissues and adjacent tissues. |
Up-Regulated TIGIT in Peripheral Blood of PBC Patients Exhibited Diagnostic Value
TIGIT level on peripheral blood T cell surface of PBC patients was significantly higher than that of healthy controls (27.56% vs 19.87%, u=318, P < 0.0001, Figures 5A–D and 6A). The ROC curve was drawn based on the percentage of TIGIT in peripheral blood T cells of PBC patients, and the area under the ROC curve was calculated as 0.833 (95% CI, 0.752–0.914, Figure 6B). The critical value for both optimum sensitivity (94.12%) and optimum specificity (69.64%) was 23.38%. This indicated that TIGIT in peripheral blood could distinguish healthy individuals from BC patients. Taken together, our data indicated that TIGIT showed diagnostic value for PBC.
 |
Figure 5 Flow cytometry histograms of CD3+ and TIGIT+ T cells in PBC patients (A and B) versus healthy controls (C and D). |
TIGIT on Peripheral Blood T Cells Was Downregulated in Postoperative PBC Patients
The changes of TIGIT level on peripheral blood T cells in 12 PBC patients before and after operation were analyzed. We found that compared with preoperative patients, TIGIT level on peripheral blood T cells was decreased significantly in postoperative patients (36.96±11.07% vs 31.83±10.54%, P < 0.05, Figures 7 and 8).
 |
Figure 7 Flow cytometry histograms of TIGIT level in peripheral blood of preoperative (A and B) versus postoperative (C and D) PBC patients. |
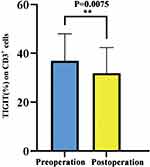 |
Figure 8 TIGIT level in peripheral blood of preoperative versus postoperative PBC patients. |
Correlation Between TIGIT Expression on Peripheral Blood T Cells and Clinicopathological Features
We analyzed the correlation of TIGIT expression level on peripheral blood T cells based on age, tumor size, pathological type, lymph node metastasis, estrogen receptor (ER), progesterone receptor (PR), HER-2, P53 and Ki-67 (Table 2). The results showed that TIGIT level in PBC patients aged >58 years was significantly higher than that in those aged ≤58 yrs (P = 0.0067). No significant correlation was observed between TIGIT level and other clinicopathological features. In this study, the correlation between TIGIT level and Ki-67 level was not shown to be attributed to the fact that the sample size with Ki-67 ≤14% (14% was used as a cut-off value) was too small (only 3) to perform a systematic analysis.
 |
Table 2 Correlation Between TIGIT Level on Peripheral Blood T Cells and Clinicopathological Features in PBC Patients |
Discussion
BC is the most common cancer in females worldwide with complex etiologies, involving genetic inheritance, lifestyle and environmental factors.23 Immune checkpoint molecules are a class of glycoproteins that exist on the cellular surface. The activated T cells can constitutively express positive costimulatory signaling molecules or negative coinhibitory signaling molecules. The activation, proliferation and differentiation of T cells can be regulated by promoting or inhibiting the activation signals of T cells.
TIGIT, a coinhibitory immune checkpoint molecule with immunosuppressive effects, was discovered in 2008 by researchers from Genentech Inc.24 It could bind to CD155/CD112 ligand and inhibit the activation and proliferation of T cells. Currently, it is generally believed that TIGIT is involved in the negative regulation of human immunity and is closely related to immune escape and carcinomas. Li et al25 showed that TIGIT expression on the T cellular surface in peripheral blood was upregulated in various cancers such as lung squamous cell carcinoma and colon cancer. Using flow cytometry, He et al26 found that the expression of TIGIT on peripheral blood T cells in 24 gastric cancer patients was significantly increased compared with 16 healthy controls. Tang et al27 analyzed the expression of TIGIT on peripheral blood T cells in 23 patients with locally advanced gastric cancer and 8 healthy controls, and their data showed that in addition to the significant increase in TIGIT expression on T cells in gastric cancer patients compared to healthy controls, TIGIT level was significantly reduced in patients who underwent D2 gastrectomy. Therefore, we speculated that TIGIT may play an important role in the immune escape during the pathogenesis of BC.
In this study, we compared the protein and mRNA expressions of TIGIT in two BC cell lines and normal breast epithelial cell lines. Besides, we detected the expression of TIGIT on the T cellular surface using flow cytometry, and TIGIT expression in tumor tissue and adjacent normal tissue was detected by IHC and immunofluorescent staining. Compared with the normal MCF-10A cells, the protein and mRNA levels of TIGIT were significantly upregulated in the MCF-7 and MDA-MB-231 BC cells. TIGIT on T cells in PBC patients was upregulated compared to that in normal controls, and the difference was statistically significant (P < 0.05). This may indicate depletion and dysfunction of T cells in the peripheral immune system of PBC patients, resulting in the inability of tumor cells to be effectively eliminated by the human immune system. In addition, compared with preoperative patients, TIGIT level on peripheral blood T cells decreased significantly in postoperative patients (P < 0.05). This suggested that TIGIT molecules may be involved in the pathogenesis and progression of BC. On the contrary, TIGIT was a safeguard molecule for the improvement of liver regeneration via negatively regulating NK-hepatocyte crosstalk.28 Database analysis also showed that TIGIT was associated with better prognosis in BC.29 These were consistent with the TCGA database results in this study showing that high expression of TIGIT was associated with a better prognosis in BC. Taken together, the role of TIGIT in human malignancies remains controversial. It may cooperate with different immune molecules to play completely opposite roles in tumor progression. Further studies are necessary for clarifying the function and underlying mechanism of TIGIT in BC.
In a previous study, Zhang et al30 found that age was one of the reasons for the different expression of TIGIT in tumors in their study on adult type B acute lymphoblastic leukemia; however, their study did not involve different age groups. Our study demonstrated that TIGIT expression in peripheral blood T cells of PBC patients was significantly correlated with age. Specifically, the expression of TIGIT on T cells in patients aged >58 (median age) was significantly higher than that in patients aged ≤58 (P < 0.05). This implied that TIGIT expression was positively correlated with age. Previous studies have shown that advanced age was a risk factor for thyroid cancer recurrence,31 and age was also a major risk factor for BC.32 Taken together, we suggested that TIGIT showed the potency to promote T-cellular depletion and apoptosis in the BC microenvironment with increasing age. In this study, the area under the ROC curve of TIGIT for PBC diagnosis was 0.833 (95% CI, 0.752–0.914), indicating moderate clinical diagnostic value of TIGIT. Besides, the critical value for both optimum sensitivity (94.12%) and optimum specificity (69.64%) was 23.38%, which effectively indicated the high sensitivity of peripheral blood TIGIT for PBC diagnosis. In the future, more studies are needed to confirm whether TIGIT can be used for early BC diagnosis, and whether it can improve the sensitivity and accuracy of diagnosis together with tumor marker CA153 that is considered to be the gold standard for diagnosis.
Recently, immunotherapy has been considered to play a pivotal role in treating malignancies, and immune checkpoint inhibitors have become one of the most effective methods for the treatment of malignant tumors. Using multiple immunohistochemical staining techniques, Li et al33 found that the expression of TIGIT was significantly different in 40 tumor tissues of Hodgkin’s lymphoma patients and in 2 normal adjacent tissues (P < 0.05). Tang et al27 reported that TIGIT level in 441 tumor tissues of patients with gastric cancer was significantly higher than that in adjacent tissues with IHC (P < 0.001). Besides, TIGIT level was significantly correlated with tumor size, histological type, clinical stage, and lymph node metastasis (P < 0.001). Similarly, the IHC results in this study showed that the positive expression rate of TIGIT in tumor tissues of PBC patients was significantly higher than that in adjacent tissues (P < 0.05). The mechanism by which TIGIT enhances immunotherapy sensitivity remains unclear. In a previous study, TIGIT exerted inhibitory functions in multiple processes of various tumor immune cycles.34 It could inhibit the function of NK cells and inhibit the apoptosis of tumor cells. TIGIT on T cells could inhibit the co-stimulatory ability of dendritic cells, increase the generation of anti-inflammatory cytokines such as IL-10 and induce PVR signals in tumor cells. Besides, myeloid cells stimulated by TIGIT+ Treg or PVR could suppress CD8+ T cell function and affect CD4+ T cell activation. Moreover, TIGIT could even directly suppress the roles of CD8+ T cells and protect cancer cells from elimination. Additionally, TIGIT was upregulated in activated T lymphocytes and showed a similar function to PD-1, which may suppress excessive immune responses.35,36 TIGIT could also negatively regulate the immune activity of T cells via downregulating the level of T cell receptors.35,37 Its blockade or deletion could promote NK cell-mediated anti-tumor responses and reduce the metastatic ability of tumor cells.38,39 These findings support ongoing clinical trial of PD-1/TIGIT-dual blockades in tumor progression, which may restore the process of tumor suppression.40,41 Overall, TIGIT showed a negative regulatory effect on tumor immunity, and immunotherapy targeting TIGIT will be a promising therapy for human malignancies.
Conclusion
In summary, TIGIT was significantly upregulated in BC tissues and peripheral blood T cells of BC patients. This suggested that TIGIT molecule may be involved in the regulation of immune response in tumor microenvironment, which may become a new target of immunotherapy for BC.
Data Sharing Statement
The data that support our findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was performed according to the convention of the Declaration of Helsinki. The study protocols were approved by the Medical Ethics Committee of Taizhou People’s Hospital (No. KY201804801). Written informed consent was obtained from all subjects before the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this article.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Burstein HJ, Curigliano G, Thürlimann B, et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen international consensus guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32(10):1216–1235. doi:10.1016/j.annonc.2021.06.023
3. Kashyap D, Pal D, Sharma R, et al. Global increase in breast cancer incidence: risk factors and preventive measures. Biomed Res Int. 2022;2022:9605439. doi:10.1155/2022/9605439
4. Zhang J, Da M, Yuan M, et al. Prevention and treatment measures related to peripheral neuropathy caused by paclitaxel-based chemotherapy drugs in breast cancer patients. Adv Clin Exp Med. 2022;12(7):6377–6383. doi:10.12677/ACM.2022.127919
5. Yeo J, Ko M, Lee DH, Park Y, Jin HS. TIGIT/CD226 Axis regulates anti-tumor immunity. Pharmaceuticals. 2021;14(3):200. doi:10.3390/ph14030200
6. Mocellin S, Benna C, Pilati P. Coinhibitory molecules in cancer biology and therapy. Cytokine Growth Factor Rev. 2013;24(2):147–161. doi:10.1016/j.cytogfr.2013.01.003
7. Anderson AC. Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol. 2012;24(2):213–216. doi:10.1016/j.coi.2011.12.005
8. Nair S, Elkord E. Immune checkpoint inhibitors in cancer therapy: a focus on T-regulatory cells, by Varun Sasidharan Nair and Eyad Elkord (Erratum). Immunol Cell Biol. 2018;96(2):236. doi:10.1111/imcb.12012
9. Tunger A, Kießler M, Wehner R, et al. Immune monitoring of cancer patients prior to and during CTLA-4 or PD-1/PD-L1 inhibitor treatment. Biomedicines. 2018;6(1):1. doi:10.3390/biomedicines6010026
10. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa1507643
11. Choueiri TK, Fishman MN, Escudier B, et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin Cancer Res. 2016;22(22):5461–5471. doi:10.1158/1078-0432.Ccr-15-2839
12. Huang R, Cui Y, Guo Y. Programmed cell death protein-1 predicts the recurrence of breast cancer in patients subjected to radiotherapy after breast-preserving surgery. Technol Cancer Res Treat. 2018;17:1533033818793425. doi:10.1177/1533033818793425
13. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. doi:10.3389/fonc.2018.00086
14. Dixon KO, Schorer M, Nevin J, et al. Functional Anti-TIGIT antibodies regulate development of autoimmunity and antitumor immunity. J Immunol. 2018;200(8):3000–3007. doi:10.4049/jimmunol.1700407
15. Gardiner D, Lalezari J, Lawitz E, et al. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS One. 2013;8(5):e63818. doi:10.1371/journal.pone.0063818
16. Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3--potential mechanisms of action. Nat Rev Immunol. 2015;15(1):45–56. doi:10.1038/nri3790
17. Sarhan D, Cichocki F, Zhang B, et al. Adaptive NK cells with low TIGIT expression are inherently resistant to myeloid-derived suppressor cells. Cancer Res. 2016;76(19):5696–5706. doi:10.1158/0008-5472.Can-16-0839
18. Kurtulus S, Sakuishi K, Ngiow SF, et al. TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest. 2015;125(11):4053–4062. doi:10.1172/jci81187
19. Chew GM, Fujita T, Webb GM, et al. TIGIT marks exhausted T cells, correlates with disease progression, and serves as a target for immune restoration in HIV and SIV Infection. PLoS Pathog. 2016;12(1):e1005349. doi:10.1371/journal.ppat.1005349
20. Gil Del Alcazar CR, Huh SJ, Ekram MB, et al. Immune escape in breast cancer during in situ to invasive carcinoma transition. Cancer Discov. 2017;7(10):1098–1115. doi:10.1158/2159-8290.Cd-17-0222
21. Cari L, Nocentini G, Migliorati G, Riccardi C. Potential effect of tumor-specific Treg-targeted antibodies in the treatment of human cancers: a bioinformatics analysis. Oncoimmunology. 2018;7(2):e1387705. doi:10.1080/2162402x.2017.1387705
22. Yuan L, Yuan P, Yuan H, et al. miR-542-3p inhibits colorectal cancer cell proliferation, migration and invasion by targeting OTUB1. Am J Cancer Res. 2017;7(1):159–172.
23. Mahoney MC, Bevers T, Linos E, Willett WC. Opportunities and strategies for breast cancer prevention through risk reduction. CA Cancer J Clin. 2008;58(6):347–371. doi:10.3322/ca.2008.0016
24. Pauken KE, Wherry EJ. TIGIT and CD226: tipping the balance between costimulatory and coinhibitory molecules to augment the cancer immunotherapy toolkit. Cancer Cell. 2014;26(6):785–787. doi:10.1016/j.ccell.2014.11.016
25. Li X, Wang R, Fan P, et al. A comprehensive analysis of key immune checkpoint receptors on tumor-infiltrating T cells from multiple types of cancer. Front Oncol. 2019;9:1066. doi:10.3389/fonc.2019.01066
26. He W, Zhang H, Han F, et al. CD155T/TIGIT signaling regulates CD8(+) T-cell metabolism and promotes tumor progression in human gastric cancer. Cancer Res. 2017;77(22):6375–6388. doi:10.1158/0008-5472.Can-17-0381
27. Tang W, Pan X, Han D, et al. Clinical significance of CD8(+) T cell immunoreceptor with Ig and ITIM domains(+) in locally advanced gastric cancer treated with SOX regimen after D2 gastrectomy. Oncoimmunology. 2019;8(6):e1593807. doi:10.1080/2162402x.2019.1593807
28. Bi J, Zheng X, Chen Y, Wei H, Sun R, Tian Z. TIGIT safeguards liver regeneration through regulating natural killer cell-hepatocyte crosstalk. Hepatology. 2014;60(4):1389–1398. doi:10.1002/hep.27245
29. Fang J, Chen F, Liu D, Gu F, Chen Z, Wang Y. Prognostic value of immune checkpoint molecules in breast cancer. Biosci Rep. 2020;40(7):Jul. doi:10.1042/bsr20201054
30. Zhang X, Zhang H, Chen L, Feng Z, Gao L, Li Q. TIGIT expression is upregulated in T cells and causes T cell dysfunction independent of PD-1 and Tim-3 in adult B lineage acute lymphoblastic leukemia. Cell Immunol. 2019;344:103958. doi:10.1016/j.cellimm.2019.103958
31. Wu J. Clinical significance of recurrence and metastasis index in patients with differentiated thyroid carcinoma and related risk factors. Pract J Cancer. 2017;32(4):606–608.
32. Konat-Bąska K, Matkowski R, Błaszczyk J, et al. Does breast cancer increasingly affect younger women? Int J Environ Res Public Health. 2020;17(13):4884. doi:10.3390/ijerph17134884
33. Li W, Blessin NC, Simon R, et al. Expression of the immune checkpoint receptor TIGIT in Hodgkin’s lymphoma. BMC Cancer. 2018;18(1):1209. doi:10.1186/s12885-018-5111-1
34. Im SJ, Hashimoto M, Gerner MY, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–421. doi:10.1038/nature19330
35. Zhang Q, Bi J, Zheng X, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19(7):723–732. doi:10.1038/s41590-018-0132-0
36. Zhang C, Wang Y, Xun X, et al. TIGIT can exert immunosuppressive effects on CD8+ T Cells by the CD155/TIGIT signaling pathway for hepatocellular carcinoma in vitro. J Immunother. 2020;43(8):236–243. doi:10.1097/cji.0000000000000330
37. Liu XG, Hou M, Liu Y. TIGIT, A novel therapeutic target for tumor immunotherapy. Immunol Invest. 2017;46(2):172–182. doi:10.1080/08820139.2016.1237524
38. Xu F, Sunderland A, Zhou Y, Schulick RD, Edil BH, Zhu Y. Blockade of CD112R and TIGIT signaling sensitizes human natural killer cell functions. Cancer Immunol Immunother. 2017;66(10):1367–1375. doi:10.1007/s00262-017-2031-x
39. Chan IS, Knútsdóttir H, Ramakrishnan G, et al. Cancer cells educate natural killer cells to a metastasis-promoting cell state. J Cell Biol. 2020;219(9):Sep. doi:10.1083/jcb.202001134
40. Chauvin JM, Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer. 2020;8(2):e000957. doi:10.1136/jitc-2020-000957
41. Ge Z, Peppelenbosch MP, Sprengers D, Kwekkeboom J. TIGIT, the next step towards successful combination immune checkpoint therapy in cancer. Front Immunol. 2021;12:699895. doi:10.3389/fimmu.2021.699895
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.