Back to Journals » Journal of Inflammation Research » Volume 15
Expression and Clinical Significance of Th1/Th2/Th17 Cytokines and Lymphocyte Subsets in PCNSL
Authors Bian H, Wang L, Gao C, Liu Z, Sun Y, Hu M, Xiao Y, Hao F, Ma Y , Zhao X
Received 22 March 2022
Accepted for publication 29 June 2022
Published 7 July 2022 Volume 2022:15 Pages 3815—3828
DOI https://doi.org/10.2147/JIR.S366761
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Haiyan Bian,1 Lisheng Wang,2 Chengwen Gao,3 Zhihe Liu,1 Yang Sun,2 Minghui Hu,4 Yujing Xiao,5 Fengyun Hao,5 Yushuo Ma,1 Xia Zhao1
1Department of Hematology, the Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China; 2Laboratory of Molecular Diagnosis and Regenerative Medicine, the Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China; 3Laboratory of Medical Biology, the Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China; 4Clinical Laboratory, the Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China; 5Department of Pathology, the Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China
Correspondence: Xia Zhao, Department of Hematology, the Affiliated Hospital of Qingdao University, 16 Jiangsu Road, Shinan District, Qingdao, 266000, People’s Republic of China, Email [email protected]
Purpose: Primary central nervous system lymphoma (PCNSL) responds favorably to radiation, chemotherapy and targeted drug therapy. However survival is usually worse, the treatment-related drug resistance and recurrence are still clinical problems to be solved urgently. Studies have shown that cytokines are expressed in varying degrees in patients with lymphoma, which is significantly related to the progression, poor prognosis and drug resistance of lymphoma. We explore the expression and clinical significance of Th1/Th2/Th17 cytokines and lymphocyte subsets in patients with PCNSL to provide a more sufficient theoretical basis for its diagnosis and treatment.
Patients and Methods: We measured and analysed the levels of Th1/Th2/Th17 cytokines and the distribution of lymphocyte subsets (including Treg cells, CD3+, CD4+, CD8+, CD19+, and CD4+/CD8+) in 39 patients with PCNSL and 96 patients with diffuse large B-cell lymphoma (DLBCL) without central nervous system involvement. The cytokines of 13 healthy people and the lymphocyte subsets of 27 healthy people were measured as the control group.
Results: We found a significant difference in the level of Th1/Th2/Th17 cytokines and lymphocyte subsets between PCNSL and healthy controls, especially IL-2, after treatment, which was significantly higher than before treatment (p< 0.01). However, the level of CD19+ and CD4+/CD8+ decreased while CD8+ and CD3+ increased after treatment (regardless of whether the treatment was effective), and the difference was statistically significant. In addition, our analysis of different prognostic factors found that HD-MTX-based chemotherapy appears to have a longer progression-free survival and overall survival than osimertinib-based chemotherapy.
Conclusion: There are significant differences in Th1/Th2/Th17 cytokines and lymphocyte subsets among PCNSL, DLBCL, and healthy controls, and their detection is helpful for the diagnosis, treatment, and prognosis of PCNSL. HD-MTX-based chemotherapy may still be the first choice for PCNSL.
Keywords: PCNSL, Th1/Th2/Th17 cytokines, lymphocyte subsets, HD-MTX, osimertinib
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare aggressive non-Hodgkin’s lymphoma (NHL). The annual incidence rate is approximately 0.44/100,000, accounting for approximately 2% of all primary central nervous system tumours.1 As a special type of lymphoma, the diagnosis, treatment, and prognosis of PCNSL are different from those of other types of NHL. Approximately 95% of PCNSL cases are diffuse large B‐cell lymphoma (DLBCL), with the remainder consisting of T-cell lymphoma, Burkitt lymphoma, lymphoblastic lymphoma, and low-grade lymphoma.2,3 The lesions are usually limited to the brain, eyes, spinal cord or leptomeninges without systemic involvement.4 In recent years, the overall incidence of PCNSL has increased year by year. Elderly individuals are the main group, and the median age at diagnosis is 65 years old.5 Although high-dose methotrexate HD-MTX-based chemotherapy has made significant progress in PCNSL patients, the prognosis is still poor compared with lymphoma outside the central nervous system, with a 5-year survival rate of less than 30%.1 Studies have shown that cytokines are expressed to varying degrees in patients with lymphoma, which is significantly related to the progression, poor prognosis, chemotherapy response, and drug resistance of lymphoma. It has been confirmed that IL-6 and IL-10 are highly expressed in the cerebrospinal fluid of central nervous system lymphoma, and both suggest a poor prognosis.6,7 Plasma cytokines and lymphocyte subsets are currently easy to obtain as routine test indicators, but little research has been done. If the regularity of their expression changes in PCNSL patients can be found, this will help identify prognostic factors and new and safe treatment strategies. Therefore, this study aimed to explore the expression changes and clinical significance of cytokines and lymphocyte subsets in patients with PCNSL and to further analyze their prognostic factors to provide new ideas for the diagnosis, treatment, and prognosis of PCNSL.
Materials and Methods
Patients and Controls
A total of 39 inpatients with PCNSL in the Affiliated Hospital of Qingdao University from January 2020 to December 2021 were selected as study subjects. All patients with PCNSL were diagnosed by routine staining and immunohistochemical staining after operation or puncture biopsy. All patients received standard induction chemotherapy based on the latest National Comprehensive Cancer Network (NCCN) guidelines once a definite diagnosis was made. The study cohort included 14 males and 25 females. The median age of the patients was 59 years. Samples from 96 patients with DLBCL and the cytokines of 13 healthy people, and the lymphocyte subsets of 27 healthy people were collected as controls in this study. There was no significant difference in sex or age among there groups (P > 0.05).
This retrospective study protocol was approved by the Ethical Committee of the Affiliated Hospital of Qingdao University, China. The study complied with all of the provisions of the Helsinki Declaration. Informed consent was obtained from each participant.
Detection of Plasma Cytokines
The 12-in-1 cytokine detection kit (multiple microsphere flow immunofluorescence method) (LOT NO: 210918) and flow cytometry (Type: navios 3L 10c) were all purchased from Qingdao Raisecare Biological Technology Co., Ltd. The specific method was as follows: 3mL peripheral blood was collected by EDTA anticoagulant tube (no need for fasting).
Reagent Preparation
Recover all reagents to room temperature before use. (1) Preparation of microspheres: vortex the microspheres for 30s before the experiment, gently blow them with a pipette gun, beat them for about 30 times, and add samples immediately. (2) Preparation of washing buffer: restore 10x washing buffer to room temperature, wait for all salts to dissolve, and dilute to 1x with deionized water for use.
Experimental Operation
25 μL of experimental buffer, 25 μL of plasma and 25 μL of captured microsphere antibody to all flow tubes in turn, add 25 μL of detection antibody to mix well, and oscillate for 15 seconds. Incubate at room temperature for 2 hours in the dark (400 r/min), then add 25 μL phycoerythrin-labeled streptavidin (SA-PE) to each tube, and incubate in the dark for 0.5 hours at room temperature. Add 500 μ L washing buffer to each tube, 300 g centrifugal 5 min, discard the supernatant and add 200 μ L washing buffer, mix for about 10 seconds, and immediately go up flow cytometry to detect. Finally, the cytokine levels were performed using LEGENDplex™ data analysis software.8 The operation was performed thrice according to the manufacturer’s instructions. The unit of measurement is pg/mL.
Detection of Lymphocyte Subsets
The levels of T-cell subsets (CD3+ T cells, CD4+ T cells, CD8+ T cells, Treg cells) and CD19+ B cells in peripheral blood were detected by Beckman Navio flow cytometry and flow antibody (all purchased from Beckman Coulter, Inc.). The specific method was as follows: 5 mL peripheral blood of fasting patients was collected by EDTA-K2 anticoagulant tube in the morning, 5 μL CD4FITC/CD8PE/CD3PerCP was added into the flow tube, 40 μL peripheral blood anticoagulated with EDTA was added, vortex oscillation was mixed evenly, 15 min was incubated at room temperature in the dark, 200 μL hemolysin was added, and 15 min was incubated at room temperature again. After sufficient haemolysis, the percentage of cell subsets was detected by flow cytometry and analysed by Kaluza® (Beckman Coulter) data analysis software.9 CD3+ is total T cells, CD19+ is total B cells, CD4+ is helper T cells (Th), CD8 is suppressor T cells (Ts), and Tregs are regulatory T cells.
Chemotherapy Regimen
R-MDT: rituximab, HD-MTX, dexamethasone, temozolomide; R-BDT/BCT: rituximab, osimertinib, dexamethasone/semustine, temozolomide; R-ZDT: rituximab, zanubrutinib,
Efficacy Evaluation
Evaluation of the therapeutic effect of the study: According to the International Primary CNS Lymphoma Collaborative Group (IPCG),10 complete remission (CR), partial remission (PR), stable condition (SD) and disease progression (PD) were evaluated. We classified CR and PR as effective treatment and SD and PD as ineffective treatment.
Survival Analysis
Overall survival (OS)was measured from the date of diagnosis to the date of death or the last follow-up. Progression-free survival (PFS) was measured from diagnosis to the date of disease progression or death due to PCNSL. Survival functions were estimated using the Kaplan–Meier method and compared by the Log rank test.
Statistical Analysis
All statistical analyses were performed via SPSS Statistics 25.0 and GraphPad Prism 9.0. Continuous variables were expressed as the mean with medians and interquartile range (median, IQR), and categorical variables were presented as numbers and percentages (n, %). This is represented by a histogram or lattice diagram. The Kruskal–Wallis H-test was used to compare groups, and the Bonferroni test was used for pial comparisons. The comparison before and after treatment was conducted by paired nonparametric test (Wilcoxon signed-rank). P<0.05 indicates that the difference is statistically significant.
Results
The Base Information of Patients with PCNSL in This Study
A total of 39 patients were included in this study, including 14 males (35.9%) and 25 females (64.1%). The median age of diagnosis was 59 (51–67) years. The LDH level of most patients was normal (30/39), and the positive rate of Ki-67 in 79.49% of patients was greater than or equal to 80% (31/39). Approximately 25.64% (10/39) of patients were EBV positive and 33.33% (14/39) were C-Myc/Bcl-2 positive. Most of the patients have headaches, dizziness, limb numbness, unclear speech, and blurred vision as the first symptoms. The lesions are mostly limited to the frontal lobe, temporal lobe, parietal lobe, and medulla oblongata, cerebellum, and so on. Among the 39 patients, 37 patients received chemotherapy-based therapy, including 21 patients with high-dose methotrexate (R-MDT), 13 patients with osimertinib-based chemotherapy (R-BDT, R-BCT), and 3 patients with zanubrutinib-based chemotherapy (R-ZDT). The other 2 patients generally in poor condition, gave up chemotherapy and only received symptomatic support treatment (Table 1).
 |
Table 1 The Base Information of Patients with PCNSL in This Study |
Comparison of Th1/Th2/Th17 Cytokine Levels
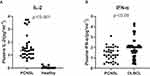
There were significant differences in the levels of plasma IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-17, TNF-α, and IFN-α among the PCNSL group, DLBCL group, and healthy control group at the first visit (P < 0.05) (Table 2). Pairwise comparison showed that the plasma levels of IL-1β, IL-2 (Figure 1A), IL-4, IL-5, IL-6, IL-10, IL-12, IL-17, TNF-α and IFN-α in the PCNSL group were significantly higher than those in the healthy control group, IFN-α was significantly lower than that in the DLBCL group (Figure 1B). In addition, the IL-2 of patients with effective treatment after treatment was significantly higher than that before treatment (Figure 2A), and there was no significant difference in other cytokines before and after treatment (whether the treatment was effective or not) (Tables 3 and 4, Figure 2B).
 |
Table 2 Plasma Th1/Th2/Th17 Cytokine Levels [Pg/mL, M (Range)] |
 |
Table 3 Plasma Th1/Th2/Th17 Cytokine Levels in 22 Patients with PCNSL in Effective Chemotherapy [Pg/mL, M (Range)] |
 |
Table 4 Plasma Th1/Th2/Th17 Cytokine Levels in 17 Patients with PCNSL in Ineffective Chemotherapy [Pg/mL, M (Range)] |
Comparison of Lymphocyte Subsets
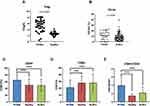
There were significant differences in the levels of Treg cells, CD3+, CD4+, CD8+, CD4/CD8, and CD19+ among the PCNSL group, DLBCL group, and healthy control group at the first visit (P < 0.05) (Table 5). Pairwise comparison showed that Treg cells in patients with PCNSL were significantly higher than those in the healthy control group, but CD19+ was higher than that in patients with DLBCL, which was not significantly different from those in the healthy control group (Figure 3A and B). At the first visit, Treg and CD8+ in the PCNSL group were significantly lower than those in the DLBCL group and healthy control group, while CD4+ and CD4+/CD8+ were significantly higher than those in DLBCL group and healthy control group (Figure 3C-E). In PCNSL group, CD19+ and CD4+/CD8+ decreased and CD8+ and CD3+increased after treatment (regardless of whether the treatment was effective), and the difference was statistically significant (P < 0.05) (Tables 6, 7) (Figure 4).
 |
Table 5 Levels of Lymphocyte Subsets at Initial Treatment [%, M (Range)] |
 |
Table 6 Levels of Lymphocyte Subsets in 22 Patients with PCNSL in Effective Chemotherapy [%, M(Range)] |
 |
Table 7 Levels of Lymphocyte Subsets in 17 Patients with PCNSL in Ineffective Chemotherapy [%, M(Range)] |
Clinical Prognostic Determinants in PCNSL
The median follow-up time of all PCNSL patients was 9 months. After treatment, 22 patients were effective (CR+PR, 56.41%), and 18 patients progressed (PD+SD, 43.59%). As of the follow-up date, 12 patients died, and 18 patients progressed. The overall survival curve of the patients is shown in Figure 5. The patient’s PFS rate and OS rate at 18 months were 39% and 57%, respectively. Because some patients are still in treatment, the follow-up time is short, and the overall median OS and PFS are not reached. Univariate analysis showed that the disease progression of the patients was not significantly related to sex, age, IL-2, CD19+, CD8+, CD3+, LDH, Ki67, EBV, or C-Myc/Bcl-2, but was significantly correlated with treatment regimen (p < 0.001) (Table 7). Among them, the patients with symptomatic support therapy (2 cases) had a median PFS of 1 month, and 13 patients with osimertinib-based chemotherapy regimens had a median PFS of 3 months (95% CI:1.74–4.26). Among them, 3 patients achieved a PR, an ORR (including CR and PR) of 25%, 10 patients were evaluated as PD, and 7 patients died. Patients treated with the HD-MTX-based chemotherapy regimen (21 cases) and patients treated with the zanubrutinib-based chemotherapy regimen (3 cases) did not reach the median OS and PFS as of the follow-up date (Figure 6) (Table 8). Because other related parameters of PFS in the univariate analysis did not reach the predetermined significance threshold, multivariate analysis was not carried out.
 |
Table 8 Analysis of Prognostic Factors |
 |
Figure 5 Kaplan–Meier curves for progression-free and overall survival of all patients with PCNSL. Notes: (A) progression-free survival curve; (B) overall survival curve. |
Discussion
Primary central nervous system lymphoma (PCNSL) is a highly invasive malignant tumour with different clinical manifestations and poor prognosis, although at present, with a better understanding of the molecular characteristics of PCNSL, individualized therapy based on standard induction of HD-MTX combined with targeted drugs has improved the survival rate of this rare invasive central nervous system lymphoma. However, recurrence is common, progression is rapid, and the long-term survival rate is still very low. Therefore, a better understanding of the biological and immunological characteristics of PCNSL is still necessary, which will provide a sufficient theoretical basis for its diagnosis and treatment.
Cytokines play an important role in the regulation of the immune system. This study analysed Th1/Th2/Th17 cytokines in 39 patients with primary central nervous system lymphoma. The results showed that the plasma levels of IL-2, IL-4, IL-12, IL-17, TNF, TNF-α, and IFN-α in patients with PCNSL were significantly higher than those in healthy controls, and IFN-α was significantly lower than that in DLBCL group. The IL-2 level in patients with effective treatment was significantly higher than before treatment. It is suggested that plasma cytokines have a certain guiding significance in the diagnosis and prognosis of PCNSL. For a long time, IL-2 has been regarded as a T-cell growth factor, that can act on lymphocytes by binding to the polymer IL2 receptor (IL-2R) and enter the cycle in the form of plasma soluble interleukin-2 receptor (sIL-2R). It is necessary for the proliferation and survival of effector T cells.11,12 Yuying Liu and colleagues found that sustained high levels of IL-2 in mouse tumour models and cancer patients can induce CD8+ T-cell depletion in the tumour microenvironment by activating the STAT5-5-HTP-AhR pathway, which in turn suppresses antitumor immunity.13 Before this, Beltra JC et al found that IL-2 can promote the apoptosis of effector T cells through activation-induced cell death, thus hindering the immune response.14 However, some studies have shown that IL-2 promotes the growth and differentiation of CD8+ T cells in vivo, is essential for the development and function of Treg cells, and enhances the antitumor immunity of the body.15,16 In recent years, IL-2 immunotherapy has been proven to have a good clinical response to the treatment of melanoma and renal cell carcinoma.17–19 In our study, the high IL-2 in newly diagnosed PCNSL patients may be because malignant lymphoma itself is an immune cell tumour. The abnormal proliferation of immune cells makes the patient’s immune system in a state of high response, which in turn stimulates the immune response. After the improvement of follow-up treatment, IL-2 continued to increase, in line with the antitumor characteristics of IL-2. IFN- γ is derived from activated T cells and NK cells and plays an immunomodulatory role. After many reports on IFN- γ as the most important antitumor cytokine member,20 Markovic et al21 showed that IFN- γ played a promoting role in the occurrence and development of tumours. In children with ALK-positive anaplastic large cell lymphoma (ALCL), high levels of IFN-γ are significantly correlated with high tumour stage, poor initial general condition and low 3-year PFS rate.22 However, in this study, we did not find significant differences in the levels of IFN-γ between PCNSL patients and healthy people. IFN- α protein is produced by white blood cells and is mainly involved in innate immunity in response to viral infection. Studies have shown that enhancing the expression of IFN-α in tumour infiltrating macrophages can induce more effective dendritic cell activation and immune cytotoxicity.23 TNF-α can specifically bind to tumour cell receptors, inhibit the expression of p53 protein itself, enhance cytotoxicity, and accelerate apoptosis. High levels of TNF-α will increase the risk of NHL in the future.24 Our results show that the levels of other cytokines except IL-8 and IFN-γ in patients with PCNSL are significantly higher than those in healthy controls, which may be related to the heavy tumour load and enhanced antitumor immune response in patients with PCNSL.
In addition, because malignant lymphoma is an immune cell tumour, the body’s immune function is abnormal. When the body is in a state of immunosuppression, the body’s ability to recognize and kill mutant cells decreases, and T lymphocyte subsets, as the most important cellular immunity, play an immunomodulatory role. The results showed that the levels of Treg cells in patients with PCNSL were significantly higher than those in the healthy control group. However, CD19+ cells were only higher than those in patients with DLBCL. At the first visit, the levels of CD8+ in the PCNSL group were significantly lower than those in the DLBCL group and healthy control group. At the same time CD4+ and CD4/CD8 were significantly higher than those in the DLBCL group and healthy control group, suggesting that T lymphocyte subsets were abnormally expressed in PCNSL patients. CD3 is a T-cell coreceptor that reflects the changes in the total level of T lymphocytes and helps to activate cytotoxic CD8+ T cells and CD4+ T helper cells.25 A decrease in CD3+ indicates a decrease in immunity, while if it is too high, it indicates that the tumour load is heavy and the immune response is strong. Batorov et al found that the increase in CD3+ T cells was associated with early recurrence or progression after autologous haematopoietic stem cell transplantation in patients with classical Hodgkin’s lymphoma.26 However, from our data, we can see that the percentage of CD3+ cells in patients with PCNSL and DLBCL is higher than that in healthy people, but the difference is not significant. At present, it is known that tumour-specific CD4+T cells play a role in the anticancer immune response because CD4+ T cells can induce immune cells in vivo and participate in the activation of B lymphocytes, macrophages and cytotoxic CD8+ T cells, while CD8+T cells have limited antitumor effect in the absence of CD4+ T cells.27,28 A decrease in CD4+ level indicates a decline in immune function, which affect the antitumor effect and leads to the continuous progression of tumour. Kusano and colleagues found that lower CD4+ at diagnosis had a significant negative impact on the survival of DLBCL patients treated with R-CHOP. Various adverse prognostic factors were associated with a significant decrease in CD4 in DLBCL patients.29 Because CD8+ can inhibit the function of B lymphocytes and CD4+, and then inhibit antibody formation and the cellular immune response.30 The increase in CD8+ levels will affect the antitumor effect of B lymphocytes and macrophages, resulting in the continuous proliferation of tumour cells and exacerbating the disease.31 Treg cells are recently recognized immunomodulatory cells that originate from the thymus and play a negative immunomodulatory role. They have two characteristics: immunosuppression and immune anergy. They have potential application value treating autoimmune diseases, immunotherapy of tumours and induction of transplant tolerance.32 In summary, considering that PCNSL is a highly invasive malignant tumour, the results of this study seem to contradict the above conclusions. It may be related to the fact that PCNSL is a highly invasive immune disease and the body is in a high response state. Therefore, we further analysed the changes in lymphocyte subsets in patients with PCNSL before and after treatment. The results showed that CD8+ and CD4+/CD8+ in patients with PCNSL after treatment were significantly higher than before treatment regardless of whether the treatment was effective. CD19 is a surface antigen of B lymphocytes and an important immune cell of the body, which is related to antitumor, antiviral and immune regulation. The low level of CD19+, the increase in CD8+ and the decrease in CD4+/CD8+ showed that the patients’ immunity decreased significantly after chemotherapy. Chemotherapy can regulate the level of lymphocyte subsets, and CD19+ is one of the main target point of B cells. Its level is related to the therapeutic effect of targeted drugs, which can provide a scientific basis for clinical adjustment and treatment.
In addition, we evaluated the efficacy of different chemotherapy regimens and found that sex, age, IL-2, CD19+, CD8+, CD3+, LDH, Ki67, EBV, or C-Myc/Bcl-2 were not related to the prognosis of PCNSL. Patients treated with methotrexate and zanubrutinib-based chemotherapy regimens had longer overall survival times and progression-free survival than those treated with Osimertinib. In many studies in the past, individualized therapy based on standard induction combined with targeted drugs based on HD-MTX has been confirmed. With in-depth study of the pathophysiology of PCNSL, it was found that the BCR pathway is the key mechanism of PCNSL. Anew drug targeting BCR pathway component, Bruton tyrosine kinase (BTK) inhibitors, has greatly improved the treatment of B-cell malignant tumours since the first generation of drugs ibrutinib.33 As second-generation BTK inhibitors, osimertinib and zanubrutinib have the characteristics of stronger selectivity, fewer adverse reactions, higher tolerability, higher target share and more lasting inhibition than first-generation drugs.34–36 A retrospective study of 23 patients with CNSL found that the 6-month PFS rates of patients with newly diagnosed CNSL and patients with relapsed/refractory CNSL were 100% and 67.70%, respectively, with the treatment regimen based on osimertinib.37 This is inconsistent with our research results, and the reason for this may be the small sample size and the short follow-up period. In particular, as a new drug just approved on the market on December 25, 2020, there are few clinical data for reference. The chemotherapy cycle of individual cases we included in the study is too short; therefore, the efficacy cannot be well evaluated. We will increase the sample size and follow-up time for further study in the follow-up.
Conclusion
Cytokines may provide an important reference for the auxiliary diagnosis, medication and prognostic intervention of PCNSL. Particularly, the increase of IL-2 may be related to the higher remission rate of the disease, suggesting that monitoring its level is helpful for the diagnosis and prognosis of PCNSL. Lymphocyte subsets are abnormally expressed in PCNSL patients and are related to different lymphoma types and curative effects. Lymphocyte subset is an important index to understand the function of cellular immunity and humoral immunity, and it is very important to evaluate the immune function of the body. Our findings suggest that monitoring the changes in lymphocyte subset levels can assess the severity of the patient’s disease and disease progression and guide the clinical formulation of optimal chemotherapy regimens to improve patients’ treatment effect and prognosis. Clinical studies of BTK inhibitors, especially Osimertinib, in patients with PCNSL are few, and the specific treatment efficacy is unclear. Therefore, prospective experimental studies are necessary. In short, our results suggest that HD-MTX based chemotherapy may still be the first choice for the clinical treatment of PCNSL.
Finally, this study has some limitations: this study was a single-centre study, the sample size of patients was relatively small, the disease progressed rapidly, the patients’ treatment compliance was poor, and some data were lost. In the future, a multicentre study with expanded sample size is needed to supplement more clinical data to further clarify the changes and clinical significance of cytokines and lymphocyte subsets in the treatment of patients with PCNSL, and the therapeutic role of osimertinib in primary central nervous system lymphoma.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the China Postdoctoral (No. 2020M682128) and 2020 Qingdao West Coast New Area Science and Technology Project (No. 2020-47).
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
1. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS Statistical Report: primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro-Oncology. 2015;17(Suppl4):iv1–iv62.
2. Deckert M, Engert A, Brück W, et al. Modern concepts in the biology, diagnosis, differential diagnosis and treatment of primary central nervous system lymphoma. Leukemia. 2011;25(12):1797–1807.
3. King RL, Goodlad JR, Calaminici M, et al. Lymphomas arising in immune-privileged sites: insights into biology, diagnosis, and pathogenesis. Virchows Archiv. 2020;476(5):647–665.
4. Jellinger KA. Lymphoma and Leukemia of the Nervous System. Eur J Neurol. 2012;19(5):e53–e53.
5. Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105(9):1414–1418.
6. Song Y, Zhang W, Zhang L, et al. Cerebrospinal Fluid IL-10 and IL-10/IL-6 as Accurate Diagnostic Biomarkers for Primary Central Nervous System Large B-cell Lymphoma. Sci Rep. 2016;6:38671.
7. Shao J, Chen K, Li Q, et al. High Level of IL-10 in Cerebrospinal Fluid is Specific for Diagnosis of Primary Central Nervous System Lymphoma. Cancer Manag Res. 2020;12:6261–6268.
8. Yadahalli R, Sarode GS, Sarode SC, et al. CC group of chemokines and associated gene expression of transcription factors: deciphering immuno-pathogenetic aspect of oral submucous fibrosis. Disease Month. 2022;1:101351.
9. Béné MC, Lacombe F, Porwit A. Unsupervised flow cytometry analysis in hematological malignancies: a new paradigm. Int J Lab Hematol. 2021;43(Suppl 1):54–64.
10. Barajas RF, Politi LS, Anzalone N, et al. Consensus recommendations for MRI and PET imaging of primary central nervous system lymphoma: guideline statement from the International Primary CNS Lymphoma Collaborative Group (IPCG). Neuro-Oncology. 2021;23(7):1056–1071.
11. Kalia V, Sarkar S. Regulation of Effector and Memory CD8 T Cell Differentiation by IL-2-A Balancing Act. Front Immunol. 2018;9:2987.
12. Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240(4856):1169–1176.
13. Liu Y, Zhou N, Zhou L, et al. IL-2 regulates tumor-reactive CD8(+) T cell exhaustion by activating the aryl hydrocarbon receptor. Nat Immunol. 2021;22(3):358–369.
14. Beltra JC, Bourbonnais S, Bédard N, et al. IL2Rβ-dependent signals drive terminal exhaustion and suppress memory development during chronic viral infection. Proc Natl Acad Sci U S A. 2016;113(37):E5444–5453.
15. Shevach EM. Application of IL-2 therapy to target T regulatory cell function. Trends Immunol. 2012;33(12):626–632.
16. Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12(3):180–190.
17. Fishman M, Dutcher JP, Clark JI, et al. Overall survival by clinical risk category for high dose interleukin-2 (HD IL-2) treated patients with metastatic renal cell cancer (mRCC): data from the PROCLAIM(SM) registry. J Immunother Cancer. 2019;7(1):84.
18. Davar D, Ding F, Saul M, et al. High-dose interleukin-2 (HD IL-2) for advanced melanoma: a single center experience from the University of Pittsburgh Cancer Institute. J Immunother Cancer. 2017;5(1):74.
19. Buchbinder EI, Dutcher JP, Daniels GA, et al. Therapy with high-dose Interleukin-2 (HD IL-2) in metastatic melanoma and renal cell carcinoma following PD1 or PDL1 inhibition. J Immunother Cancer. 2019;7(1):49.
20. Glasner A, Levi A, Enk J, et al. NKp46 Receptor-Mediated Interferon-γ Production by Natural Killer Cells Increases Fibronectin 1 to Alter Tumor Architecture and Control Metastasis. Immunity. 2018;48(2):396–398.
21. Markovic O, Popovic L, Marisavljevic D, et al. Comparison of prognostic impact of absolute lymphocyte count, absolute monocyte count, absolute lymphocyte count/absolute monocyte count prognostic score and ratio in patients with diffuse large B cell lymphoma. Eur J Intern Med. 2014;25(3):296–302.
22. Knörr F, Damm-Welk C, Ruf S, et al. Blood cytokine concentrations in pediatric patients with anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Haematologica. 2018;103(3):477–485.
23. Capobianchi MR, Uleri E, Caglioti C, Dolei A. Type I IFN family members: similarity, differences and interaction. Cytokine Growth Factor Rev. 2015;26(2):103–111.
24. Purdue MP, Lan Q, Bagni R, et al. Prediagnostic serum levels of cytokines and other immune markers and risk of non-hodgkin lymphoma. Cancer Res. 2011;71(14):4898–4907.
25. Ngoenkam J, Schamel WW, Pongcharoen S. Selected signalling proteins recruited to the T-cell receptor-CD3 complex. Immunology. 2018;153(1):42–50.
26. Batorov EV, Pronkina NV, Tikhonova MA, et al. Increased circulating CD3(+) T cells are associated with early relapse following autologous hematopoietic stem cell transplantation in patients with classical Hodgkin lymphoma. Leuk Lymphoma. 2019;60(10):2488–2497.
27. Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854.
28. Peterson AC, Harlin H, Gajewski TF. Immunization with Melan-A peptide-pulsed peripheral blood mononuclear cells plus recombinant human interleukin-12 induces clinical activity and T-cell responses in advanced melanoma. J clin oncol. 2003;21(12):2342–2348.
29. Kusano Y, Yokoyama M, Terui Y, et al. Low absolute peripheral blood CD4+ T-cell count predicts poor prognosis in R-CHOP-treated patients with diffuse large B-cell lymphoma. Blood Cancer J. 2017;7(4):e558.
30. Saligrama N, Zhao F, Sikora MJ, et al. Opposing T cell responses in experimental autoimmune encephalomyelitis. Nature. 2019;572(7770):481–487.
31. Peng M, Xiao-Long LU, Zhang L, Hong XU, Laboratory DO. Significance of T lymphocyte subsets and NK cells in the treatment of diffuse large B-cell lymphoma patients. Practical J Clin Med. 2018;1:548.
32. Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16(6):356–371.
33. Shirley M. Bruton Tyrosine Kinase Inhibitors in B-Cell Malignancies: their Use and Differential Features. Target Oncol. 2022;17(1):69–84.
34. Yu H, Wang X, Li J, et al. Addition of BTK inhibitor orelabrutinib to rituximab improved anti-tumor effects in B cell lymphoma. Mol Therapy Oncol. 2021;21:158–170.
35. Guo Y, Liu Y, Hu N, et al. Discovery of Zanubrutinib (BGB-3111), a Novel, Potent, and Selective Covalent Inhibitor of Bruton’s Tyrosine Kinase. J Med Chem. 2019;62(17):7923–7940.
36. Tam CS, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851–859.
37. Wu JJ, Wang WH, Dong M, et al. Orelabrutinib-bruton tyrosine kinase inhibitor-based regimens in the treatment of central nervous system lymphoma: a retrospective study. Invest New Drugs. 2022;40(3):650–659.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.