Back to Journals » International Journal of General Medicine » Volume 16
Exploring the Role of Modified Vascular Anatomical Molding (MVAM) in Prenatal Diagnosis Teaching and Prognosis Prediction of Fetal Complex Congenital Heart Disease (CCHD): A Preliminary Study
Authors An P , Song L, Song P, Zhang J, Lin Y, Feng G, Liu J
Received 26 May 2023
Accepted for publication 26 July 2023
Published 31 July 2023 Volume 2023:16 Pages 3229—3245
DOI https://doi.org/10.2147/IJGM.S421751
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yuriy Sirenko
Peng An,1,2,* Lina Song,1,* Ping Song,3,* Junyan Zhang,1,3 Yong Lin,1,3 Guoyan Feng,1 Junjie Liu3
1Department of Radiology, Xiangyang No.1 People’s Hospital, Hubei University of Medicine, Xiangyang, 441000, People’s Republic of China; 2Hubei Provincial Clinical Research Center for Accurate Fetus Malformation Diagnosis, Hubei University of Medicine, Xiangyang, Hubei Province, 441000, People’s Republic of China; 3Department of Obstetrics and Gynecology, Xiangyang No. 1 People’s Hospital, Hubei University of Medicine, Xiangyang, 441000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Junjie Liu; Guoyan Feng, Xiangyang NO.1 People’s Hospital, No. 15 Jiefang Road, Fancheng District, Xiangyang City, Hubei Province, People’s Republic of China, Email [email protected]; [email protected]
Objective: The present study aimed to explore the role of modified vascular anatomical molding (MVAM) in prenatal diagnosis teaching and prognosis prediction of fetal complex congenital heart disease (CCHD).
Methods: Step 1, MVAM method was used to cast the micro-blood vessels and trachea of 52 CCHD specimens. Subsequently, 52 MVAMs were analyzed and compared with the prenatal ultrasound to summarize their characteristics, misdiagnosis and MVAM’s teaching role. Step 2, the surgical and follow-up data of 206 CCHD cases were retrospectively analyzed. Cases that evolved into critical illnesses or died within 1– 3 years after surgery (poor prognosis) were classified into the study group (n = 77) and those with good prognosis into the control group (n = 129), which were split into the training set and the test set in the ratio 7:3 based on the time cut-off. In the training set, the prognosis of CCHD was predicted using the MVAM anatomical soft markers (distortion and narrowing of aorta/pulmonary artery, right ventricular infundibulum, etc.) and the decision curve analysis (DCA) performed. The model was validated using the test set, and a nomogram was finally established.
Results: It was observed that all 52 CCHD cases were confirmed using MVAM. A total of 91 cardiac malformations were recorded, among which 41 malformations were misdiagnosed, and 29 malformations were missed by the prenatal echocardiography. The MVAM method has a good teaching/feedback effect on prenatal diagnosis. The combined model exhibited a higher predictive performance in the training- and test-set. Its high clinical net benefit was proved by DCA. Additionally, the nomogram established using the combined model received a favorable response in clinical practice.
Conclusion: The research results indicated that MVAM improved the prenatal diagnosis teaching and training performance. The combined model established based on MVAM anatomical soft markers can offer a high clinical significance for prognosis prediction of CCHD.
Keywords: modified vascular anatomical molding, MVAM, complex cardiovascular malformation, CCHD, combined model, CM, interrupted aortic arch, IAA, coarctation of the aorta, COA, pulmonary valve stenosis, PVS, right aortic arch, RAA, transposition of the great arteries, TGA, right aortic arch with mirror-image branching, RAMI, double-outlet right ventricle, DORV
Background
The incidence of congenital heart disease (CHD) in Asia is about 8~12‰, which is significantly higher than that in other parts of the world. It is on a persistent rise due to the accelerated process of industrialization, food safety and environmental pollution.1,2 Complex congenital heart disease (CCHD) leads to a high disability rate, mortality and various postnatal complications, thereby requiring a complex perinatal detection and postpartum treatment. It is one of the most common congenital diseases with a poor prognosis. As a result, early prenatal diagnosis and postnatal treatment are particularly important in this disorder. Fetal echocardiography has become a first choice in the screening fetal CHD during pregnancy in many maternity and infant hospitals. It is widely known that the fetal echocardiography can be used in the diagnosis of congenital heart defects (CHD), and it holds true for all types of CHD under familiar conditions with the complex changes in cardiovascular anatomical structures. However, the display of small-micro-blood vessels is not good and complex. CCHD’s diagnostic accuracy also relies on the clinician’s anatomical knowledge and proficiency. Additionally, the CCHD has several vascular variations and is limited by the small volume of the fetal heart, thereby making the teaching of prenatal echocardiography very difficult.3–6 An accurate prenatal diagnosis of CHD is a very difficult task and has many implications, which may not be limited to surgical correction alone. It may also concern the possibility of terminating pregnancy, with a psychological impact on the prospective parents. In recent years, after the China’s “two-child policy” in 2016, a large number of elder and high-risk pregnant women gradually increased, while the domestic medical environment and doctor–patient relationship are very poor. Thousands of malignant criminal cases of medical injuries caused by the wrong diagnosis occur every year,7,8 and a misdiagnosis of prenatal diagnosis can lead to a huge financial medical compensation to the hospital. This has cast a “black veil” on the development of prenatal diagnosis in China. Fortunately, the modified vascular anatomical molding (MVAM) has brought an opportunity to fully observe the circuit of vascular branches in the state of the fetal physiological circulation and better reflect the three-dimensional structure of fetal cardiac microvessels. In this pursuit, we developed microvessel models on 52 cases of fetal CCHD and compared them with the prenatal fetal echocardiography, in order to analyze the characteristics of fetal CCHD and the causes of missed and misdiagnosis in fetal echocardiography, and further improve the application value of fetal echocardiography in the diagnosis of fetal CCHD. Another aim of the present study was to develop a prognosis prediction model of the fetal CCHD using the MVAM anatomical soft markers and prove its significance compared to the conventional prenatal diagnosis that offers sufficient heart details for Glenn, Fontan, atrioventricular septal reconstruction, plastic surgery and secondary surgery of CCHD. At present, our technology has been extended in many provinces and has received an excellent response. The MVAM technology is not only conducive to the prenatal echocardiography diagnosis teaching but also reduces unnecessary medical disputes and provides a strong support for the medical insurance system and prognosis for children with CHD. We reviewed literature reports on CCHD published in the past 30 years. The existing studies were generally focused on the treatment method and the molecular basis of CCHD (Figure 1). Few studies discussed the clinical data-MVAM anatomical soft markers combined model for predicting CCHD prognosis. Therefore, our innovative method may provide a new approach for the clinical evaluation of CCHD.6,7,9,10
 |
Figure 1 From the content of references retrieval from 1992 to 2022, CCHD has always been a research hotspot, with more research on molecular mechanism and CCHD management and less on prediction of CCHD by multimodal radiomics. Novelty of the work is a prediction model/nomograph tool established using the MVAM anatomical soft markers combined with clinical data, which has not been reported before. The specific method to obtain the figure: log in to https://www.citexs.com/website, select “AI literature big data analysis”, enter “congenital heart disease”, adjust the time node to “1992–2022”, and then confirm to “export the figure”. |
Materials and Methods
Data Collection
Fifty-two CCHD diagnosed by fetal echocardiography, final autopsy and microvascular casting from March 2014 to December 2022 in the Xiang Yang Hospital Group (includes Xiang Yang Maternal and Child Health Hospital, Xiangyang no.1 people hospital, Xiang Yang Hospital Maternal and Fetal Medical Consultation Centre, and Xiang Yang fetal heart disease database and specimen bank) were collected into this study, which included 30 males and 22 females. The pregnant women were 19–44 (32.5 ± 4.6) years old with 24–32 (26.2 ± 3.3) gestational weeks. The study was approved by the Xiangyang Hospital Group Ethics Committee (approval No.: 2013016x) and signed informed consent from the parents.
Prenatal Ultrasound Diagnostic Methods
We followed the methods of Han and Bhide et al9,10 and employed the GE Voluson E8 and Mindray Resona7 colour Doppler ultrasound machine with convex array transducers with a frequency of 3.0–5.5 MHz. The diagnostic standards of the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) and the British Journal of Obstetrics and Gynaecology (BJOG) were adopted with a close check on the structure of the cardiovascular system, valve morphology, and complex cardiovascular systemic circulation path.10–12 For suspected cases of the abnormal aortic arch and its branches, the three vessels-trachea view (3V+ T view), bilateral subclavian artery view and tracheal coronal view were combined along with observation of the specific circuit of the left ventricular outflow tract, ascending aorta, aortic branches and ductus arteriosus. All the 52 CCHD fetuses underwent a systemic ultrasound screening at the gestational age of 24–33 weeks. All resulting CCHD views/section images were stored in the Neusoft ultrasound system workstation and were screened and analyzed by two CCHD diagnostic qualified physicians having 20 years of work experience, who reached a final diagnosis with a mutual agreement.
Pathoanatomical Examination and Modified Vascular Anatomical Molding
Pathoanatomical examination: In the fetus diagnosed with CCHD using the fetal echocardiography, the family decided to terminate the pregnancy after multidisciplinary consultation, and autopsy and microvascular casting were implemented with the approval of the Science and Technology Ethics Committee of the hospital. Fetal modified vascular anatomical molding (MVAM) process: It was performed by a pathologist with a qualification of Deputy Chief Physician or above. The abdominal wall was cut, and the umbilical vein and abdominal aorta were separated. They were perfused with a cleaning agent, until the fluid flowing out of the heart and microvessels was clear with no blood clots. The abdominal aorta was ligated, the fixing agent was perfused into the umbilical vein at a uniform speed, fixed and corroded, and then washed until there was no residual tissue. The details of the improved casting method are as follows: 1. The chest wall was opened to expose the heart and lungs, the pericardium was retained, the abdominal wall was opened, and one side of the common iliac artery was disconnected. The umbilical vein was separated and punctured (1.0–1.5 mm scalp needle catheter was used for cannulation) and fixed, and the blood vessels were flushed with normal saline, until the clear fluid flowed out of the common iliac artery. 2. Epoxy resin, polyamide resin curing agent and ethyl acetate were used as the raw materials, and an appropriate amount of bright red pigment was added. After stirring and standing, the bubble-free mould agent was absorbed in the lower layer for use. 3. A 20 mL syringe was manually injected with a casting agent to make the heart and vascular cavity full, the pressure was maintained for 30s, and then the umbilical vein was then ligated. 4. After the perfusion specimen was fixed, the thoracic and abdominal organs were removed and subjected to potassium hydroxide solution for corrosion. After 48 hours, the alkaline solution was washed with running water and bleached with chlorine disinfectant. 5. The specimen was properly trimmed, and the casting results were recorded. 6. The inspection and diagnosis of the cast model was completed by pathologists, obstetricians, ultrasound doctors, radiologists and cardiac surgeons together to reduce the bias. An alternate filling material was used, viz. acrylonitrile-butadiene-styrene (ABS): The casting medium included a mixture of 60 g of ABS (Shanghai Chemical Plant Co. Ltd., China), 70 mL of acetone and dye. High-quality azo pigments such as permanent orange (RN C.I.5), bronze-red (C.I.21), benzidine yellow (GC.I.12), emerald green (XC.I.11), etc. were used. If the above pigments were not available, water-soluble painting pigments were employed. After the desired color was achieved by mixing multi-colors, it was added into ABS at a ratio of 1/120.13–15
Prenatal Diagnosis Teaching-Training and CCHD’s Predictive Model Construction Using MVAM
Step 1, Prenatal diagnosis teaching using MVAM: Forty-five physicians qualified for prenatal diagnosis at the Xiang Yang Hospital Group were randomly selected. They were randomly divided into two groups using the random number table as 23 physicians in the study group (MVAM teaching training + conventional CCHD teaching) and 22 physicians in the control group (conventional CCHD teaching). The two groups of physicians did not differ significantly in age, gender, experience, and qualification (P > 0.05). Each physician was required to review the ultrasound images and videos in PACS and diagnosed 10 cases of CCHD independently after training. The success rate of diagnosing malformations of the aorta, pulmonary artery, ductus arteriosus, and subclavian artery and the average time to diagnosis were compared between the two groups.
Step 2, CCHD’s predictive model construction using MVAM: The surgical and follow-up data of 266 CCHD cases diagnosed at the Xiang Yang Hospital Group from January 2015 to January 2022 were retrospectively analyzed (Figure 2). Cases who evolved into critical illnesses or died within 1–3 years after surgery (poor prognosis) were classified into the study group (n = 115). There were 151 cases in the control group (good prognosis), with a gestational age of 33 to 41 weeks with an average of 36.6 ± 3.3 weeks. Their birth weight was 2.1 to 5.2 kg, with an average of 3.0 ± 0.81 kg. The two groups did not significantly differ in the fetal gender or maternal situation (P > 0.05). Inclusion criteria: 1. CCHD lesions ≥2 conforming to the ISUOG practice guidelines and requiring corrective surgery or two-stage surgery, with complete follow-up data; 2. Simple CCHD not combined with lethal complex extracardiac malformations; 3. Not combined with diseases other than CCHD, such as severe pneumonia and urinary tract infections; 4. CCHD cases having received surgical treatment and having detailed clinical and follow-up data. Exclusion criteria: 1. CCHD combined with other complex extracardiac malformations; 2. Postpartum respiratory distress, severe pneumonia, and other life-threatening diseases; 3. CCHD cases who died after conservative treatment; 4. CCHD cases given up by their guardians; 5. Absence of follow-up. Finally, 206 CCHD cases were enrolled, including 129 cases achieving good prognosis and 77 cases with poor prognosis. They were split into the training set and the test set in a 7:3 ratio based on the time cut-off. In the training set, we predicted the prognosis of CCHD using the MVAM anatomical soft markers (distortion and narrowing of aorta/pulmonary artery and distortion and narrowing of right ventricular infundibulum) and performed the decision curve analysis. The model was further validated on the test set. A nomogram was finally established and tested clinically.
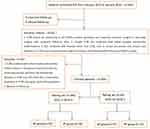 |
Figure 2 The simplified inclusion and exclusion criteria for CCHD patient enrollment in the present study. |
Statistics
All statistical analyses were conducted using jamovi 2.2.5. Counting data is expressed in frequency or percentage and is tested using chi square or Fisher exact test. If the measurement data obeyed the normality test, they were expressed as X ± s and compared by t-test; otherwise, the intergroup comparisons were conducted using the Mann–Whitney U-test. When P < 0.05, the finding was considered statistically significant. The models based on the CCHD patients’ general clinical data, prenatal echocardiography, and MVAM anatomical soft markers and the combined model were built using logistic regression, respectively. The predictive value of each model was analyzed by plotting ROC curves. P < 0.05 indicated a significant difference. The higher the AUC, the greater the predictive efficacy would be. The decision curves and the nomographs were generated using the R software (the R Project for Statistical Computing, version 3.4.1).15–17
Results
Step 1-Prenatal Diagnosis Teaching-Training Using MVAM
According to the MVAM, all 52 cases of fetuses were confirmed with CCHD, and no false-positive results were observed. In the 91 CCHD recorded, 41 malformations were misdiagnosed by prenatal echocardiography, including the interrupted aortic arch (IAA), double outlet right ventricle (DORV) with transposition of the great arteries (TGA), atrial isomerism, aberrant subclavian artery and anomalous pulmonary venous drainage; 29 malformations were missed by the prenatal echocardiography, including abnormal ductus arteriosus, infrahepatic interruption of the inferior vena cava (IIVC), the right aortic arch with mirror-image branching (RAMI), aberrant subclavian artery (ASA), and anomalous pulmonary venous drainage. Fifty-two CCHD were accurately confirmed based on combination with the prenatal echocardiography, MVAM and anatomy. The diagnostic accuracy of CCHD was significantly higher in the study group than in the control group, respectively (aortic malformation: Z = 4.694, p < 0.05, pulmonary artery malformation: Z = 5.346, p < 0.05, ductus arteriosus abnormalities: Z = 4.631, p < 0.05, aberrant subclavian artery: Z = 3.173, p = 0.002, lung lobe malformation: Z = 4.321, p < 0.05, atrial isomerism: Z = 4.592, p < 0.05), while the average time of diagnosis was much shorter in the former (7.87 ± 1.42 vs 14.1 ± 5.07, t = 5.659, p < 0.05) (Table 1) (Table 2) (Figures 3 and 4).
 |
Table 1 Fifty-two Cases with Prenatal Misdiagnosis or Missed Diagnosis Corrected by Postnatal MVAM |
 |
Table 2 Comparison of Diagnostic Accuracy and Operating Time Between Two Groups in MVAM Teaching Research |
Step 2-CCHD’s Predictive Model Construction Using MVAM
The univariate analysis identified the factors influencing the prognosis following the surgery for CCHD. The two groups of patients differed significantly in neonatal weight, Apgar score, premature birth, distortion or narrowing of the right ventricular infundibulum, lung lobe malformation, aberrant subclavian artery, abnormal ductus arteriosus, atrial isomerism, single atrium or single ventricle (P < 0.05) (Table 3 and Table 4). Multivariate analysis: neonatal weight, premature birth, distortion or narrowing of the right ventricular infundibulum, lung lobe malformation, aberrant subclavian artery, abnormal ductus arteriosus, single atrium or single ventricle were found to be the independent risk factors in the CCHD prognosis (P < 0.05) (Table 5).
 |
Table 3 Logistic Regression Analysis Results of Clinical Model Based on Clinical Features for Predicting the CCHD Prognosis |
 |
Table 4 Logistic Regression Analysis Results of MVAM Anatomical Soft Markers Model Based on Anatomical Soft Markers for Predicting the CCHD Prognosis |
 |
Table 5 Logistic Regression Analysis Results of Combined Model Based on Clinical Data-Anatomical Soft Markers for Predicting the CCHD Prognosis |
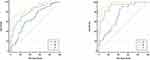
Predictive models and external validation: three predictive models (clinical model included the neonatal weight, Apgar score, premature birth; MVAM Anatomical soft markers model included distortion or narrowing of the right ventricular infundibulum, lung lobe malformation, aberrant subclavian artery, abnormal ductus arteriosus; and combined model that included all above) were built based on the above risk factors in the training set. The predictive efficacy of the three models was compared by Delong’s test using the MedCalc Software. It was found that the combined model had the highest predictive value [AUC: 0.872 (OR 0.0311, 95% CI: 0.807–0.922)]. The difference was statistically significant compared with the clinical model [AUC: 0.659 (OR 0.0462, 95% CI: 0.576–0.736), P < 0.05], and MVAM Anatomical soft markers model [AUC:0.802 (OR 0.0383, 95% CI: 0.728–0.863), P < 0.05]. The decision curve analysis also confirmed the higher net benefit of the combined model. The external validation on the testing set yielded the same result: The combined model [AUC: 0.961 (OR 0.0215, 95% CI: 0.878–0.994)] outperformed the clinical model [AUC:0.730 (OR 0.0641, 95% CI: 0.601–0.836), P < 0.05], and MVAM Anatomical soft markers model [AUC:0.911 (OR 0.0384, 95% CI: 0.810–0.969), P = 0.08]. The nomogram and the calibration curves developed using the R software had been used in clinical settings (Figures 5 and 6).
 |
Figure 6 In the training set (left) and the test set (right), it is confirmed that the combined model had the higher net benefit. |
Discussion
Congenital heart disease (CHD) is the most common congenital malformation in China, and about 360,000 children with CHD were born in China in 2018. Fetal echocardiography is a reliable means to diagnose the fetal CCHD. However, the cardiovascular malformations in the fetuses with CCHD are quite complicated, especially the abnormal circuit of the blood vessels and their branches face a difficulty to be fully visualized that affects the quality of diagnosis. Besides, limitations of the experience and knowledge of the anatomical structure of various types of CCHD lead to a different understanding of CCHD and relevant screening skills among the diagnostic medical sonographers. Thus, a detailed and accurate diagnosis of CCHD by prenatal echocardiography remains a huge challenge. In addition, the prenatal diagnostic legal disputes caused by CCHD in China are rising year by year, reaching 20%. Based on the social medical insurance model, if pregnant women do not buy an expensive congenital disability insurance before the neonatal delivery, once postpartum newborns are diagnosed with CCHD, huge treatment costs are needed to be borne by the families, which greatly hinders the development of prenatal ultrasound. The MVAM is especially suitable for the CCHD with abnormal vascular routing display and can be used as a control index to analyze the causes of missed or misdiagnosis of fetal echocardiography.16–18 Additionally, the developed method was easy, simple and cheap. It improved the prenatal diagnosis and provided supplementary information to patients for completing the postmortem diagnosis. The proposed method may be added to routine autopsies, and it does not preclude completely the use of other methods. More importantly, our updated CCHD prognosis model based on MVAM anatomical soft markers achieved a high predictive performance, which may provide support for CCHD surgical management.
The developed MVAM technique can stereoscopically display the abnormalities of the mutual positional relationship and the complex spatial structure of the large-microvessel malformations. Therefore, the detailed information provided by the casting technique can help to improve the accuracy of an echocardiography diagnosis of CCHD, thereby providing a virtual reality tool to conduct the simulated CCHD cognitive training for diagnostic medical sonographers. At the same time, the correctly restored CCHD cardiovascular models can also be used to establish a permanently stored database by computerized tomography technology in the future 3D printing restorations and replications. As a result, the rare CCHDs can be infinitely copied for the cardiac surgeons, obstetricians and gynecologists for future study. We successfully prepared 52 MVAMs and found a total of 91 abnormalities, of which 29 malformations were missed, and 41 malformations were misdiagnosed by prenatal echocardiography, resulting in an accurate ultrasound diagnosis rate of only 23.07%. Furthermore, these diagnostic results were found amongst the senior physicians/experts, which could be unimaginably low, if conducted by the general medical sonographers. The detailed information of the casts perfectly explained some of the doubts in prenatal ultrasound images.19–21
A traditional autopsy has always been regarded as the gold standard in verifying a prenatal diagnosis. However, in our 20 years extensive research and experience, there are many defects in the diagnosis of CCHD in regional anatomy. For example, CCHD specimens are usually small and weigh less than 3 kg, wherein the thoracic structure can be easily destroyed, and the variation of microvessels cannot be observed in detail. Each CCHD specimen is extremely precious. The traditional formalin immersion cannot perfectly reflect the cardiovascular morphology, which often leads to the shrinkage of the heart and eventually leads to damage. Our modified MVAM can more clearly, completely, and permanently display the spatial relationship of the aorta and its branches (including abnormal arteriovenous, trachea, and oesophagus).22,23 Fetuses with CCHD are often diagnosed with small for gestational age (particularly the head is small) and present a higher risk of prematurity.24,25 It is difficult to cast a small fetus model. Currently, we use a small intubation and small pressure or a micropump for perfusion to complete the casting as perfectly as possible. It should be noted that this method does not completely hinder the routine autopsy, because our perfusion is mainly carried out through the arteriovenous system and trachea, and we roughly check the cardiovascular structure and record the data after opening the chest. The experimental materials of this study included simply the ABS or denture base powder, pigment, syringe, catheter, sodium hydroxide solution and sulfuric acid. The cost of a mold is about 200 yuan ($ 31.4).
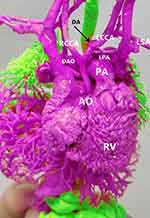
Abnormal ductus arteriosus: Our study suggests that the highest rate of prenatal misdiagnosis involves an abnormal ductus arteriosus, including abnormal ductus arteriosus connection, abnormal morphology with stenosis, and absence of ductus arteriosus. In severe right heart system obstructive congenital heart disease, the reverse blood flow in the arterial duct occurs due to the need of perfusion of the pulmonary artery from the aorta to support the growth and development of the lung. The changes in the blood flow direction and flow volume cause a distortion and narrowing of the ductus arteriosus. When the ductus arteriosus is distorted, the winding blood flow signal from the aorta to a pulmonary artery can be displayed. However, when it is too narrow, the blood flow is difficult to be displayed and may be easily misdiagnosed as an absence. For example, in Case 20, the prenatal ultrasound was misdiagnosed as coarctation of the aorta (CoA) with descending aorta originating from the right ventricle, while the cast confirmed it was an interrupted aortic arch (IAA) with a descending aorta originating from the pulmonary artery, the abnormal connection of ductus arteriosus (pulmonary artery–ductus arteriosus–left common carotid artery), and aberrant left subclavian artery (Figures 3 and 4). Also, in Case 13, only the aortic coarctation was diagnosed by prenatal ultrasound, while the ductus arteriosus abnormal connections (LCCA-DA-PA) were confirmed using the MVAM.24,25
Aberrant subclavian artery: Aberrant left subclavian artery is an abnormal branch of the aortic arch, which is common in the left aortic arch with aberrant right subclavian artery or right aortic arch with aberrant left subclavian artery. The diameter of the aortic branches in the fetal period is small. As a result, the examiner can easily miss the accurate diagnosis. Therefore, when the left ductus arteriosus with the right aortic arch appears in the examination, we should consider, whether there is an aberrant left subclavian artery, for example: In Case 10, the prenatal ultrasound only diagnosed DORV and PAS. However, DORV with TGA, RAA with ALSA was confirmed using the vascular casting. When the left ductus arteriosus with the left arch appears, attention should be paid to, whether there is an aberrant right subclavian artery. The examiner should pay attention to the scanning of the three vessels-trachea view (3V+ T view) and increase the use of spatio-temporal image correlation to evaluate the spatial position relationship of the micro-blood vessels, which is helpful for the clear and stereoscopic display of the aortic branches and their circuit that are not clearly displayed in the two-dimensional image view. In addition, RAA increases the risk of associated cardiac defects regardless of the laterality of the ductal arch, which should also be focused. Also, the concomitance of right ductus and right aortic arch is an object of a recent meta-analysis. Since, there were cases of RAA and ALSA in Table 1, we believe that the left ductal arch in RAA occurs frequently in cases with interrupted aortic arch with TGA or coarctation of the aorta with TGA by using the cast model re-analysis. The casting technique exactly showed the ductal path, which was obviously very easy to assist in the diagnosis of the ductal laterality. For example, Figures 3 and 4 present LDA. In recent years, postmortem MRI25,26 and micro CT serve as an alternative to the autopsy confirmation in the fetal post-mortem by providing more details.27–29 It is undeniable that these developments are much more advanced than the cast mold to some extent. However, our team (especially cardiologists and junior residents) believe that the MVAM technology still has certain advantages. Furthermore, the MRI and micro CT images need a proficient expert and are not easy to understand. The MVAM model can directly show the detailed path of large blood vessels and their branches, which is very convenient for medical teaching. A CCHD deformity model can bring a deep visual impression among the medical students. Moreover, the MVAM casting process can be easily performed after postmortem MRI or micro CT. Therefore, MVAM has advantages in both cost and effectiveness.
Our search of CCHD prognosis prediction that combined clinical and image data on PubMed and Scopus databases produced a very few reports. Moreover, no studies involving the use of MVAM Anatomical soft markers have been published yet. The incidence of poor prognosis in 1 to 5 years postoperative was 37.4% in CCHD patients in this study. This incidence was slightly higher than what has been reported earlier, which may be explained by population variation, limited sample size, and longer follow-up duration. Given the above reasons, postoperative long-term follow-up for prognostic monitoring of CCHD and developing early predictive tool for CCHD prognosis are highly important. To extract more predictive features for CCHD prognosis, we compared the clinical and anatomical features using logistic regression between the 2 groups. The univariate analysis showed that neonatal weight, Apgar score, premature birth, distortion or narrowing of the right ventricular infundibulum, lung lobe malformation, aberrant subclavian artery, abnormal ductus arteriosus, atrial isomerism, single atrium or single ventricle were statistically significant features (P<0.05). The multivariate regression analysis confirmed that neonatal weight, premature birth, distortion or narrowing of the right ventricular infundibulum, lung lobe malformation, aberrant subclavian artery, abnormal ductus arteriosus, single atrium or single ventricle were independent risk factors for CCHD prognosis. Neonatal weight, Apgar score, and premature birth are important clinical indicators of neonatal conditions have a direct bearing on the cardiopulmonary development, immunity, and surgical tolerance. These indicators are crucial for the prognosis of CCHD and deserve due attention from cardiac surgeons. Single atrium or single ventricle is an important type of CCHD and may be corrected by Glenn surgery, Fontan procedure, and reconstruction of the interventricular septum. However, nearly all of the available surgeries are difficult, and the prognosis is usually poor. Distortion or narrowing of the right ventricular infundibulum, malformation of the lung lobes, and distortion or narrowing of the right ventricular infundibulum, lung lobe malformation, aberrant subclavian artery, abnormal ductus arteriosus, atrial isomerism were newly discovered MVAM anatomical soft markers with prognostic significance. Lesions in the right ventricular infundibulum are usually complex. Distortion or narrowing of the right ventricular infundibulum is usually combined with ventricular septal defect or transposition of the great arteries, which increases the surgical difficulty. Malformation of the lung lobes refers to a deformity of bilateral lung lobes and morphological variations of human pulmonary fissures (Non conventional left lung with 8 lobes and right lung with 10 lobes). Atrial isomerism is a very rare condition featured by a symmetric development of the normally asymmetric atria or ventricles. For example, the left atrium and left ventricle are abnormally connected to the right auricle and pulmonary artery and their structures are similar to that of the right side of the heart. In other words, the left side of the heart fulfills the functions that are normally achieved by the right side of the heart, and vice versa. Malformation of the lung lobes usually occur concurrently with atrial isomerism. The combination of malformations is even more difficult to be managed by surgery, and the prognosis is poor. Aberrant subclavian artery and abnormalities of ductus arteriosus generally predict the conotruncal abnormality and coarctation and interruption of the aorta, respectively. However, the two conditions commonly occur together, and the pediatric patients require two- or three-stage surgeries, and the prognosis is also poor. The anatomical soft markers newly discovered in this study were conventionally ignored in prenatal diagnosis, which brings uncertainty to surgery. Observation of these anatomical soft markers can assist the CCHD surgery by offering more cardiac information to surgeons. The combination model based on the above factors exhibited a higher predictive performance, and its net clinical benefit was proved by the decision curve analysis. This model was also validated on the test set, and the nomogram established using this model received favorable response in clinical practice.16,30–32
Limitations
The casting of fetal cardiovascular model is indeed a novel and futuristic technology; however, the processing and preservation of smaller specimens are difficult, and the preparation process of this specimen is also complex. Further, our model only considered some common clinical indicators and the newly discovered MVAM anatomical soft markers. Other potentially significant factors might have been left out and need a further investigation in the future. The sample size was small, and only logistic regression was performed in this study. Other valuable factors might have been filtered out by setting the p-value at 0.05. In the future, we envisage constructing more effective predictive models based on multi-center studies and machine learning.
Conclusions
In the present study, we developed the MVAM technique for CCHD prenatal diagnosis teaching and prognostic prediction. Our study could accurately demonstrate the feasibility of diagnosing complex life-threatening congenital heart disease and permanent preservation. The combination model based on newly discovered MVAM anatomical soft markers can help with CCHD diagnosis teaching, prognostic prediction, and treatment management.
Data Sharing Statement
All relevant data supporting the conclusions of this article are included within the article from Xiang Yang Hospital Group (China Birth Defects Monitoring Center).
Ethics Approval and Consent to Participate
This study ethics has been approved by the Human Ethics Committee of Xiang Yang Hospital Group Ethics Committee (approval No.: 2013016x). Our present medical research was conducted according to the principles expressed in the Declaration of Helsinki. We also obtained, written consent from the patient’s family.
Consent for Publication
Written informed consent was obtained from the patient’s family for publication of this case and any accompanying images.
Acknowledgment
The authors gratefully acknowledge Wang. Y, W. P. Gu and NA. An for assistance with translating references and Applied improved anatomical technology.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The “323” Public Health Project of the Hubei health commission and the Xiangyang No.1 People’s Hospital (XYY2022-323).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Qu Y, Liu X, Zhuang J, et al. Incidence of congenital heart disease: the 9-year experience of the Guangdong Registry of Congenital Heart Disease, China. PLoS One. 2016;11(7):e0159257. doi:10.1371/journal.pone.0159257
2. Zhuang J. The continuing challenge of congenital heart disease in China. J Thorac Cardiovasc Surg. 2015;150(3):738. doi:10.1016/j.jtcvs.2015.04.036
3. Best S, Wou K, Vora N, Van der Veyver IB, Wapner R, Chitty LS. Promises, pitfalls and practicalities of prenatal whole exome sequencing. Prenat Diagn. 2018;38(1):10–19. doi:10.1002/pd.5102
4. Salomon LJ, Alfirevic Z, Berghella V, et al. ISUOG practice guidelines (updated): performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol. 2022;59(6):840–856. doi:10.1002/uog.24888
5. Abu-Rustum RS, Frangieh A, Fahed R, Soutou B, Abdelahad A. Limitations of 3-dimensional sonography in the prenatal evaluation of a skin denudation syndrome. J Ultrasound Med. 2013;32(7):1301–1303. doi:10.7863/ultra.32.7.1301
6. Quintero RA, Chmait RH. The cocoon sign: a potential sonographic pitfall in the diagnosis of twin - twin transfusion syndrome. Ultrasound Obstet Gynecol. 2004;23(1):38–41. PMID: 14970997. doi:10.1002/uog.945
7. Yang XP, Chen F, Liu X, Zhou XR. Analysis on forensic expertise of 49 medical disputes in prenatal examination. Fa Yi Xue Za Zhi. 2018;34(5):508–511. doi:10.12116/j.issn.1004-5619.2018.05.014
8. An P, Ye YJ, Li QX, et al. Medical disputes in relation to prenatal ultrasound in China. Ultrasound Obstet Gynecol. 2020;56(1):11–14. doi:10.1002/uog.22020
9. Han W, Xie M, Cheng TO, et al. The vital role the ductus arteriosus plays in the fetal diagnosis of congenital heart disease: evaluation by fetal echocardiography in combination with an innovative cardiovascular cast technology. Int J Cardiol. 2016;202:90–96. doi:10.1016/j.ijcard.2015.08.156
10. Bhide A, Acharya G, Bilardo CM, et al. ISUOG practice guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet Gynecol. 2013;41(2):233–239. doi:10.1002/uog.12371
11. Bhide A, Acharya G, Baschat A, et al. ISUOG Practice Guidelines (updated): use of Doppler velocimetry in obstetrics. Ultrasound Obstet Gynecol. 2021;58(2):331–339. doi:10.1002/uog.23698
12. Ioannou C, Talbot K, Ohuma E, et al. Systematic review of methodology used in ultrasound studies aimed at creating charts of fetal size. BJOG. 2012;119(12):1425–1439. doi:10.1111/j.1471-0528.2012.03451.x
13. Cicinelli E, Einer-Jensen N, Galantino P, Alfonso R, Nicoletti R. The vascular cast of the human uterus: from anatomy to physiology. Ann N Y Acad Sci. 2004;1034:19–26. doi:10.1196/annals.1335.002
14. Kilner PJ, Ho SY, Anderson RH. Cardiovascular cavities cast in silicone rubber as an adjunct to post-mortem examination of the heart. Int. J Cardiol. 1989;22(1):99–107. doi:10.1016/0167-5273(89)90141-1
15. Debbaut C, Monbaliu D, Casteleyn C, et al. From vascular corrosion cast to electrical analog model for the study of human liver hemodynamics and perfusion. IEEE Trans Biomed Eng. 2011;58(1):25–35. doi:10.1109/TBME.2010.2065229
16. Lin YJ, Jiao KL, Liu B, Fang L, Meng S. Antiplatelet and myocardial protective effect of Shexiang Tongxin Dropping Pill in patients undergoing percutaneous coronary intervention: a randomized controlled trial. J Integr Med. 2022;20(2):126–134. doi:10.1016/j.joim.2022.01.001
17. Giorgione V, Fesslova V, Boveri S, et al. Adverse perinatal outcome and placental abnormalities in pregnancies with major fetal congenital heart defects: a retrospective case-control study. Prenat Diagn. 2020;40(11):1390–1397. PMID: 32557693. doi:10.1002/pd.5770
18. Pioro M, Mykitiuk R, Nisker J. Wrongful birth litigation and prenatal screening. CMAJ. 2008;179:1027–1030. doi:10.1503/cmaj.080454
19. Hassan M, Chitty L, Reardon H. Wrongful birth: clinical settings and legal implications. Semin Fetal Neonatal Med. 2014;19(5):312–316. doi:10.1016/j.siny.2014.08.006
20. Alfirevic Z. DISQ 3: failure to diagnose a fetal anomaly on a routine ultrasound scan at 20 weeks. Ultrasound Obstet Gynecol. 2005;26(7):797–798. doi:10.1002/uog.2631
21. Salomon LJ, Alfirevic Z, Berghella V, et al. Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol. 2011;37(1):116–126. doi:10.1002/uog.8831
22. Tuchtan L, Lesieur E, Bartoli C, et al. Diagnosis of congenital abnormalities with post-mortem ultrasound in perinatal death. Diagn Interv Imaging. 2018;99(3):143–149. doi:10.1016/j.diii.2017.11.005
23. Johns N, Al-Salti W, Cox P, Kilby MD. A comparative study of prenatal ultrasound findings and post-mortem examination in a tertiary referral centre. Prenat Diagn. 2004;24(5):339–346. doi:10.1002/pd.871
24. Prayer D, Malinger G, De Catte L, et al. ISUOG Practice Guidelines (updated): performance of fetal magnetic resonance imaging. Ultrasound Obstet Gynecol. 2023;61(2):278–287. doi:10.1002/uog.26129
25. Cavoretto PI, Sotiriadis A, Girardelli S, et al. Postnatal outcome and associated anomalies of prenatally diagnosed right aortic arch with concomitant right ductal arch: a systematic review and meta-analysis. Diagnostics. 2020;10(10):831. doi:10.3390/diagnostics10100831
26. Kang X, Carlin A, Cannie MM, Sanchez TC, Jani JC. Fetal postmortem imaging: an overview of current techniques and future perspectives. Am J Obstet Gynecol. 2020;223(4):493–515. doi:10.1016/j.ajog.2020.04.034
27. Votino C, Jani J, Verhoye M, et al. Postmortem examination of human fetal hearts at or below 20 weeks’ gestation: a comparison of high-field MRI at 9.4 T with lower-field MRI magnets and stereomicroscopic autopsy. Ultrasound Obstet Gynecol. 2012;40(4):437–444. doi:10.1002/uog.11191
28. Sandrini C, Boito S, Lombardi CM, Lombardi S. Postmortem micro-CT of human fetal heart-A systematic literature review. J Clin Med. 2021;10(20):4726. doi:10.3390/jcm10204726
29. Lombardi CM, Zambelli V, Botta G, et al. Postmortem microcomputed tomography (micro-CT) of small fetuses and hearts. Ultrasound Obstet Gynecol. 2014;44(5):600–609. doi:10.1002/uog.13330
30. Hutchinson JC, Arthurs OJ, Ashworth MT, et al. Clinical utility of postmortem microcomputed tomography of the fetal heart: diagnostic imaging vs macroscopic dissection. Ultrasound Obstet Gynecol. 2016;47(1):58–64. doi:10.1002/uog.15764
31. Heibel J, Graham EM, Mahle WT, et al. Perioperative metabolites are associated with adverse neonatal congenital heart disease surgical outcomes. J Am Heart Assoc. 2022;11(16):e024996. doi:10.1161/JAHA.121.024996
32. Geenen LW, Opotowsky AR, Lachtrupp C, et al. Tuning and external validation of an adult congenital heart disease risk prediction model. Eur Heart J Qual Care Clin Outcomes. 2022;8(1):70–78. doi:10.1093/ehjqcco/qcaa090
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.