Back to Journals » Cancer Management and Research » Volume 15
Exploring the Expression and Prognosis of Mismatch Repair Proteins and PD-L1 in Colorectal Cancer in a Chinese Cohort
Authors Han L, Zhang Y, Li L, Zhang Q, Liu Z, Niu H, Hu J, Ding Z, Shi X, Qian X
Received 29 April 2023
Accepted for publication 14 July 2023
Published 7 August 2023 Volume 2023:15 Pages 791—801
DOI https://doi.org/10.2147/CMAR.S417470
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Lu Han,1,2,* Yaping Zhang,2– 4,* Li Li,2,* Qun Zhang,2 Zhihao Liu,2,3 Haiqing Niu,1,2 Jing Hu,2 Zhou Ding,2 Xiao Shi,2 Xiaoping Qian1,2
1Department of Oncology, Nanjing Drum Tower Hospital Clinical College of Nanjing University of Chinese Medicine, Nanjing, People’s Republic of China; 2The Comprehensive Cancer Centre of Drum Tower Hospital, Medical School of Nanjing University, Nanjing, People’s Republic of China; 3Department of Oncology, Nanjing Drum Tower Hospital Clinical College of Xuzhou Medical University, Nanjing, People’s Republic of China; 4Department of Pathology, The First People’s Hospital of Yangzhou, Yangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoping Qian, Department of Oncology, Nanjing Drum Tower Hospital Clinical College of Nanjing University of Chinese Medicine, 321 Zhongshan Road, Nanjing, Jiangsu, 210008, People’s Republic of China, Tel +86-13951743162, Fax +86-25-68182342, Email [email protected]
Purpose: Exploring the expression and prognosis of mismatch repair proteins and PD-L1 in colorectal cancer.
Patients and Methods: A total of 272 patients with surgically resected CRC were enrolled in the study from January 2018 to May 2022 at Nanjing Drum Tower Hospital (The Affiliated Hospital of Nanjing University Medical School). Surgically resected samples were collected from patients along with general, clinicopathological, and imaging data for each patient. Immunohistochemistry (IHC) was used to detect expression of MSH2, MSH6, MLH1, and PMS2 proteins in tumor tissue. X-squared (X2) testing was performed to investigate the correlation between expression of MMR proteins and PD-L1 in CRC tumor tissues and clinicopathological characteristics. Correlation analysis was also used to compare the deletion of four MMR proteins in CRC tumor tissues. A survival curve and Log rank test were used to investigate the relationship between the expression of MMR proteins and PD-L1 with regard to CRC patient prognosis and survival.
Results: MMR protein expression deletion was correlated with tumor location, the degree of tissue differentiation, and TNM stage (P< 0.05). PD-L1 expression was correlated with TNM stage (P< 0.05). Correlation analysis of deletion of MMR protein isoform expression found that PMS2 deletion was significantly correlated with MLH1 deletion (P< 0.05). Similarly, MSH2 deletion was significantly correlated with MSH6 deletion (P< 0.05). PMS2 deletion was also found to be correlated with PD-L1 expression (P< 0.05). Progression-free survival was found to be significantly longer in mismatch repair-proficient (pMMR) patients compared with mismatch repair-deficient (dMMR) patients.
Conclusion: Deletion of MMR proteins and expression of PD-L1 are closely related to clinicopathological characteristics and overall prognosis of CRC patients. This suggests the relevance of MMR and PD-L1 as potential biomarkers for treatment of CRC patients.
Keywords: MMR, pathological features, survival analysis, IHC
Introduction
There has been significant progress in cancer care over the last several decades. This progress has allowed scholars to make significant breakthroughs in understanding the tumor immune response. As a result, the application of immunotherapy and immune checkpoint inhibitors (ICPIs) has rapidly become a viable direction in cancer treatment for patients in the clinic.1,2 Germani et al recently demonstrated that patients with colorectal cancer who express high levels of microsatellite instability (MSI-H)/mismatch repair-deficient (dMMR) (dMMR-MSI-H CRC) in metastatic colorectal cancer (mCRC) have better outcomes with PD-L1 inhibitors.3,4 Several prospective clinical trials of ICPIs for chemotherapy-resistant dMMR-MSI-H CRC have demonstrated a high disease control rate (DCR) as well as progression-free survival (PFS); however, only 15% of patients were found to have MSI-H/dMMR pathology and subsequently benefit from a single treatment of PD-1 inhibitors. This suggests a high degree of intertumoral heterogeneity (ITH).2,3,5–7 In addition, outcomes of patients with CRC treated with adjuvant chemotherapy (ACT) were found to be highly variable. Recent studies have shown that patients with dMMR/MSI-H are better treated with chemotherapy than those containing mismatch repair-proficient (pMMR),8–10 however, a recent study by Ribic et al suggested that MSS (pMMR) patients are more likely to benefit when treated with ACT.11 The purpose of this paper is to summarize pathological and clinical data of CRC patients and analyze the correlation between MMR, PD-L1, clinicopathological characteristics, and therapeutic efficacy.
Materials and Methods
General Data
A retrospective descriptive analysis of 272 patients with CRC who visited Nanjing Drum Tower Hospital (The Affiliated Hospital of Nanjing University Medical School) from January 2018 to May 2022, all patients received standardized treatment regimens for CRC recommended by NCCN guidelines after surgery, briefly, patients with stage II CRC require four cycles of post-operative adjuvant regimens of XELOX, patients with stage III CRC require six to eight cycles of post-operative adjuvant regimens of XELOX, and patients with stage IV CRC receive cetuximab-targeted therapy in combination with FOLFOX intravenous chemotherapy if genetic testing shows wild-type RAS and BRAF and the lesion is located in the left hemicolectomy, otherwise they receive bevacizumab-targeted therapy in combination with FOLFOX intravenous chemotherapy. All patients were diagnosed pathologically and did not undergo chemotherapy or related treatment before surgery. All clinicopathological cancer tissues were collected.
Inclusion and Exclusion Criteria
Inclusion criteria: (1) all patients met the criteria for diagnosis of colorectal cancer; (2) all patients underwent radical surgery for colorectal cancer, confirmed by pathological examination. (3) None of the patients had previously undergone radiotherapy, chemotherapy, targeted immunotherapy or other related treatments before surgery. Exclusion criteria: (1) A history of disease that could affect tumor progression or important organs; (2) Incomplete clinical data.
Detection Method
Immunohistochemistry (IHC) Staining of MMR Proteins
We performed IHC analysis of 2μm the formalin-fixed, paraffin-embedded (FFPE) tumor tissues with MLH1 antibody (1:200, clone number ES05, Dako, Denmark), PMS2 antibody (1:200, clone number EP51, Dako, Denmark), MSH2 antibody (1:200, clone number FE11, Dako, Denmark) and MSH6 antibody (1:200, clone number Pu29, Dako, Denmark), according to the manufacturer’s instructions. For each test, phosphate buffer was used as a negative control and slice of clearly positive specimen was used as a positive control. The operation steps are performed strictly according to the kit instructions.
IHC Staining of PD-L1 Protein
Tissue was wax blocked, dewaxed, hydrated, antigen repaired and blocked using peroxidase. Primary antibody for PD-L1 (Dako, Denmark, monoclonal mouse anti-human, clone number 22C3, working solution dilution 1:500) was added to the tissue and the rest of the procedure was performed as described above. Staining was performed via Dako autostainer Link 48 IHC. Negative and positive controls were set up as described above for each test.
Interpretation of Results
Routinely, IHC-stained sections are interpreted under double-blind conditions.
dMMR Judgment Criteria
Background of the positive control group was used as internal control. The appearance of brownish-yellow granular staining in the nuclei of tumor cells was recorded as a positive test, while the absence of staining was recorded as negative (Figure 1). The absence of expression of 1 MMR protein was recorded as dMMR, with expression of all 4 proteins considered to be pMMR.
 |
Figure 2 PD-L1 expression in CRC resection samples (IHC, ×400). Notes: (A and B) show negative and positive expression of PD-L1, respectively. |
PD-L1 Judgment Criteria
PD-L1 staining results were determined using the combined positive score (CPS), which is the ratio of tumor cells containing membrane staining, as well as lymphocytes and macrophages with direct cytosolic staining associated with tumor cells. The surviving tumor cells are then multiplied by 100. PD-L1 positivity is defined as CPS ≥ 10, while PD-L1 negativity is defined as CPS <10 (Figure 2).12–15
Patient Follow-Up
Patients were tracked via telephone and outpatient review from the time they were discharged from the hospital post-surgery. Patients were checked once every 3 months within 2 years post-surgery, and once every 6 months after 2 years post-surgery. The endpoint of this study was June 20, 2022.
Statistical Methods
SPSS 22.0 statistical software was used for data analysis. Qualitative data (%) was analyzed via χ2 test, with correlation analysis of dichotomous data performed via column correlation χ2 test where column correlation coefficients were calculated. Kaplan–Meier plots were used to plot survival curves. Survival curve analysis was performed via Log rank test. (P value <0.05) was considered statistically significant.
Results
General Data
Among 272 CRC patients, 178 were male and 94 were female. Eighty-two patients had tumors in the left colon, whereas 149 patients had tumors in the ascending colon and 41 patients contained tumors in the rectum. Two hundred and seventeen tumors were ≤5 cm in diameter and 55 were >5 cm. Ninety-six tumors showed signs of nerve invasion, while 176 did not. Seventy tumors showed metastatic vascular invasion, while 202 were not shown to be invading the vasculature. One hundred and eighteen of the 272 tumors were associated with lymph node metastasis while 154 tumors were not. The pathological types of CRC recorded include: 269 cases of colorectal adenocarcinoma and 3 cases of mucinous adenocarcinoma of the colorectum, which were highly differentiated in 14 cases, moderately differentiated in 194 cases, and poorly differentiated in 64 cases. One hundred and seventeen CRC patients had TNM stages I or II, and 155 CRC patients had TNM stages III or IV. Fifteen CRC patients had dMMR and 257 CRC patients had pMMR. One hundred and forty-two patients were PD-L1 positive, and 130 patients were PD-L1 negative.
Relationship Between the Expression of MMR and PD-L1 and the Clinicopathological Features of CRC Primary Lesions
As shown in Table 1, expression deficiency in MMR proteins was significantly correlated with tumor location, TNM stage, and the degree of differentiation (P<0.05). The rate of dMMR was higher in patients with colon cancer located on the left side of the colon than that of patients with cancer in the ascending colon or rectum. The rate of dMMR was higher in patients with poor differentiation compared to patients with moderate differentiation and high differentiation. The rate of dMMR was found to be higher in patients with stage III and IV cancer than in patients with stage I and II cancer. In contrast, PD-L1 positivity was only associated with TNM stage (P<0.05). The rate of PD-L1 positivity was higher in stage III and IV cancer patients than in stage I and II cancer patients (P=0.045).
 |
Table 1 Chi-Square Test Between the Expression of MMR and PD-L1 and Clinicopathological Features of CRC Primary Lesions |
Relevance of MMR Protein Isoform Expression Deficiency
We show in Table 2 and Table 3 that CRC patients show a correlation between the deletion of MLH1 and PMS2 protein expression (X2=188.245, P<0.05, column correlation coefficient C = 0.640). Deletion of MSH2 expression was also found to correlate with deletion of MSH6 expression (X2=120.426, P<0.05, column correlation coefficient C = 0.554).
 |
Table 2 Chi-Square Test Between MLH1 Expression and PMS2 Expression |
 |
Table 3 Chi-Square Test Between MSH2 Expression and MSH6 Expression |
Correlation of MMR Protein Expression with PD-L1 Expression
As described in Table 2 and Table 3, we detected 7 cases of MLH1 negative expression, 5 cases of MSH2 negative expression, 4 cases of MSH6 negative expression, and 10 cases of PMS2 negative expression amongst 272 patients. One hundred and forty-two cases of positive PD-L1 expression and 130 cases of negative PD-L1 expression were found among all 272 patients. Positive PD-L1 expression was found to be significantly correlated with the absence of PMS2 expression (P<0.05) (Table 4).
 |
Table 4 Chi-Square Test Between MMR Protein Expression and PD-L1 Expression |
Survival Analysis
As shown in Figure 3, the follow-up period for this study lasted 16–42 months post-surgery. At the end of the study, 222 patients were progression-free, and 50 patients were found to progress or succumb to disease. Progression-free survival (PFS) was 12.75–24 months in patients with dMMR and 18–44 months in patients with pMMR. The difference in survival curves between the two groups was statistically significant (Log rank = 4.118, P=0.042).
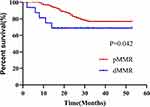 |
Figure 3 Log rank test between CRC patients in the dMMR group and those in the pMMR group. |
Discussion
Colorectal cancer (CRC) is one of the most common malignancies of the gastrointestinal tract. It is the third highest occurring cancer worldwide and the second leading cause of cancer-related death.16,17 As the etiology and mechanics of CRC have been investigated, scientists have concluded that genetic variants leading to the development of CRC are a key part of the mechanism driving this disease, such as the deletion of MMR protein expression.18 The main MMR proteins related to CRC include: MLH1, MSH2, MSH6, and PMS2, which are translated from a set of mismatch repair genes (MLH1, MSH2, MSH6, and PMS2). The role of these genes is to correct mismatched DNA base pairs in real time and restore the normal nucleotide sequence to ensure proper duplication of DNA content into the daughter cell.19,20
A recent study by Xu Yeet al that analyzed samples from 834 Chinese colorectal cancer patients suggested that the proportion of patients with dMMR is approximately 5.6%,21 the results of this present study, which statistically analyzed the expression of MMR protein in cancer tissues and its correlation with clinicopathology, showed that the proportion of dMMR was about 5.5%, and the results of the study confirmed the above view. Furthermore, previous studies have shown that MLH1 deficiency is often paired with PMS2 deficiency, and MSH2 deficiency is often paired with MSH6 deficiency.22–24 In our study, we found a correlation between MMR protein isoform deletions in 7 instances of MLH1, 5 instances of MSH2, 4 instances of MSH6, and 10 instances of PMS2. We found 7 instances of MLH1 and PMS2 co-deletion (100% of MLH1 expression deficiency), and 3 instances of MSH2 and MSH6 co-deletion (75% of MSH6 expression deficiency). Similarly, we found PMS2 deletion was significantly correlated with MLH1 deletion (P<0.05) and that MSH2 deletion was significantly correlated with MSH6 deletion (P<0.05). This is potentially due to the homology of MLH1 and PMS2 proteins, as well as the homology of the MSH2 and MSH6 proteins, which have the capacity to form functional dimers, thus leading to degradation if one of them is mutated or deleted.25,26 Previous studies have shown that PD-L1 expression was more common in patient tumors containing dMMR status than pMMR.27,28 In addition, Kiret al discovered that PMS2 deficiency was an independent risk factor for PD-L1 positivity through analysis of 176 resected endometrial cancer specimens.29 Further analysis in our study revealed that PMS2 expression deficiency did associate with PD-L1 expression, which was consistent with the above findings (P < 0.05), the reason for this may be due to the situation that, in this study, PMS2 deficiency was the most common type of dMMR (10 in 15), and the larger amount of data makes the association between PMS2 deficiency and PD-L1 positive expression easier to detect compared to the other three proteins. However, studies related to the relationship between PMS2 and PD-L1 in CRC tumors are few, thus more in-depth studies are needed to clarify the mechanisms. But to some extent, this study provides a mechanistic basis and research idea to build upon for future studies.
Correlation analysis of the present study regarding MMR expression status with clinicopathological characteristics of CRC patients showed that dMMR was mostly seen in patients with stage III or IV cancer, who with low differentiation left-sided colon cancers. These results regarding the degree of differentiation and tumor stage were consistent with the results reported in several previous studies.22,23,30 However, analyses regarding tumor location were found to be different from previous studies, for example, Chen et al showed that dMMR status was mostly seen in patients with right hemicolectomies, and Xue et al had the similar finding,31,32 which is possible as the right colon is farther away from the anus, and thus less likely to be susceptible to external factors and more susceptible to genetic factors. However, the results of this study suggest that dMMR is more common in the left hemicolectum, this once again proves the heterogeneity among different patients and the complexity of tumor pathogenesis to some extent, and we will continue to conduct in-depth research on this topic, expand the sample size, and conduct multi-center prospective research, hoping to get more discoveries.
Despite advances in medical care that have led to significant progress in early detection and treatment of CRC, prognosis for CRC patients remains poor.17,33,34 Approximately 40% of patients with localized lesions at the time of initial diagnosis develop in situ or distant recurrence post-treatment. It has also been shown that approximately 20% of patients have distant metastases at initial diagnosis.34,35 These patients have an average 5-year survival rate of less than 20%.36 The standard first-line treatment for CRC is a FOLOFX or XELOX regimen with 5-fluorouracil (5-FU) in combination with oxaliplatin (Oxa). However, studies have shown that the response rate of CRC patients to FOLFOX-based chemotherapy is approximately 10–15%. This is especially true for mCRC patients, whose 5-year survival rates is low.30,37–39 Advances in immunotherapy over the last decade have led to significant breakthroughs for the treatment of solid tumors, especially in lung and skin cancers.36 Since immune checkpoint therapy aimed at treating CRC patients with mutations in dMMR genes and MSI-H received regulatory approval in 2017, many countries have reported many applications of immune check point inhibitors (ICIs) in the treatment of CRC patients with dMMR/MSI-H, and achieved gratifying clinical research.40–42 However, due to the presence of multiple oncogenic mutations in CRC tumors, the disease typically manifests as a heterogeneous and multigene-driven tumor in most patients.43 PD-1 and its ligand PD-L1 both play a significant role in immune system recognition of tumor cells in the body, and as such have become a hot topic in the immunology field in recent years due to their ability to act as a negative immune co-stimulatory molecules.44 PD-L1, also known as CD274 or B7-H1, is widely expressed in antigen-presenting cells (APCs), malignant cancer cells, non-hematopoietic cells, and non-lymphoid cells known as ICPIs.45,46 It has been shown that PD-L1 overexpression by tumor cells binds to PD-1 molecules on the surface of tumor infiltrating T-cells thus allowing cancer cells to evade immune surveillance further driving tumor development.47,48 However, the role of PD-L1 with regards to CRC has not yet been shown to be significant yet. Furthermore, recent studies of the expression of PD-L1 in CRC and its correlation with MMR status and clinical prognosis of patients are considered controversial. For example, Caiet al analyzed 632 combined CRC samples from The Cancer Genome Atlas (TCGA) database and Chinese CRC patients, and found that higher the expression of PD-1 and PD-L1 led to a better the prognosis in CRC patients.49 However, Gaoet al noted that high PD-L1 expression represented poor overall survival (OS) and poor progression-free survival (PFS) in 240 patients suffering from liver cancer.50,51 Wanget al, through analysis of 8823 patient samples from publicly available databases such as PubMed, found that PD-L1 expression predicted a poor prognosis for CRC patients.52–54 Therefore, to further clarify the relationship between PD-L1 expression in CRC tumor tissues and patient prognosis, we performed IHC on 272 clinical samples using PD-L1 (clone number 22C3) antibody. We interpreted the results via CPS score, and the results from this experiment showed that PD-L1 expression was correlated with TNM stage. Essentially, as the tumor stage increased, so did the level of PD-L1 positivity (P<0.05). However, in terms of patient survival, the data of this study supported the difference in PFS produced by MMR status (ie, shorter survival time in dMMR patients compared with pMMR patients (P<0.05). Combined with the above analysis, we found that dMMR status representing poor prognosis is more common in patients with left side of the colon, poorly differentiated, stage III or IV, which could suggest that such patients should receive treatment intervention as soon as possible, and should be reviewed as soon as possible in the treatment process in order to find the progress of the disease as soon as possible and adjust the treatment plan.
There are many cases of MSI-H-CRC with obvious disease control after receiving immunotherapy. However, the drug resistance of MSS-CRC to PD-1/PD-L1 treatment is still a big problem in CRC treatment. How to transform “cold tumor” into “hot tumor” needs more programs to verify. Marion Thibaudin et al reactivated the immune response ability of tumor infiltrating lymphocytes (TIL) in MSS-CRC through the combined treatment of atelizumab and tiragolumab. This study shows that the combined immunotherapy can make MSS-CRC have greater benefits, which not only provides a breakthrough for the treatment of CRC with pMMR status, but also provides new evidence for us to study the relationship between PD-L1 and the prognosis of colorectal cancer.55 Recently, Hsieh RC and others found that radiotherapy combined with ICIs can promote the start of anti-tumor immune system, play an immune sensitization effect, and thus can lead to systemic tumor regression. Combined with the results of this study, we put forward a conjecture whether the expression of PD-L1 and MMR protein in patients with colorectal cancer receiving radiotherapy is related to their prognosis and whether radiotherapy will affect the expression of MMR or PD-L1, which provide a new direction for further clarifying the relationship between PD-L1 and MMR and the treatment scheme of colorectal cancer.56
Since this study is a retrospective study conducted by a single center, it aims to find indicators that are closely related to the clinical benefits in patients with different mutation types at the protein level. However, due to the small sample size of the study, there are certain limitations to some extent that need to be accounted for, in addition, this study only analyze dMMR status via IHC without microsatellite analysis as well as only mutations at the MMR protein level were analysed and not explored at the gene level. Therefore, further studies are needed to verify the conclusion of this experiment in the future, with a view to formulating more effective individualized treatment plan for CRC patients. In the next step, we will continue to expand the sample size and do a series of related studies in order to draw more conclusions to guide clinical treatment.
Conclusion
MMR and PD-L1 expression in tumor tissue of CRC patients is related to pathological features. MMR protein expression is related with patient prognosis. Specifically, pMMR patients had an overall better prognosis than dMMR patients. This data suggests that MMR has the potential to be used as a prognostic indicator for the treatment of CRC patients.
Data Sharing Statement
The data supporting the conclusion of this review have been included within the article.
Ethics Statement
This study conformed to the principles outlined in the Declaration of Helsinki (World Medical Association Declaration of Helsinki). The study’s protocol was approved by the ethics committee of the Nanjing Drum Tower Hospital (The Affiliated Hospital of Nanjing University Medical School), and informed consent was obtained from clinicians and patients (approval number: 2017-196-04).
Funding
This study was supported by grants from Nanjing health science and technology development key program (no ZKX21028), Provincial Natural Science Foundation of Jiangsu (no BK20211007), Jiangsu scientific and technological development of traditional Chinese medicine Key projects (no ZD202227).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kishore C, Bhadra P. Current advancements and future perspectives of immunotherapy in colorectal cancer research. Eur J Pharmacol. 2021;893:173819. doi:10.1016/j.ejphar.2020.173819
2. Ganesh K, Stadler ZK, Cercek A, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16(6):361–375. doi:10.1038/s41575-019-0126-x
3. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi:10.1126/science.aan6733
4. Germani M, Moretto R. Immune checkpoint inhibitors in mismatch repair proficient/microsatellite stable metastatic colorectal cancer patients: insights from the AtezoTRIBE and MAYA trials. Cancers. 2021;14(1):52. doi:10.3390/cancers14010052
5. Schrock AB, Ouyang C, Sandhu J, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol. 2019;30(7):1096–1103. doi:10.1093/annonc/mdz134
6. Boland CR, Thibodeau SN, Hamilton SR, et al. A national cancer institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58(22):5248–5257.
7. Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135(4):1079–1099. doi:10.1053/j.gastro.2008.07.076
8. Alwers E, Jansen L, Bläker H, et al. Microsatellite instability and survival after adjuvant chemotherapy among stage II and III colon cancer patients: results from a population-based study. Mol Oncol. 2020;14(2):363–372. doi:10.1002/1878-0261.12611
9. Koenig JL, Toesca DAS, Harris JP, et al. Microsatellite instability and adjuvant chemotherapy in stage II colon cancer. Am J Clin Oncol. 2019;42(7):573–580. doi:10.1097/COC.0000000000000554
10. Taieb J, Shi Q, Pederson L, et al. Prognosis of microsatellite instability and/or mismatch repair deficiency stage III colon cancer patients after disease recurrence following adjuvant treatment: results of an ACCENT pooled analysis of seven studies. Ann Oncol. 2019;30(9):1466–1471. doi:10.1093/annonc/mdz208
11. Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–257. doi:10.1056/NEJMoa022289
12. Wainberg Z, Fuchs C, Tabernero J, et al. Efficacy of pembrolizumab (pembro) monotherapy versus chemotherapy for PD-L1–positive (CPS ≥10) advanced G/GEJ cancer in the Phase II KEYNOTE-059 (cohort 1) and Phase III KEYNOTE-061 and KEYNOTE-062 studies. J Clin Oncol. 2020;38(4_suppl):427. doi:10.1200/JCO.2020.38.4_suppl.427
13. De Marchi P, Leal LF, Duval Da Silva V, et al. PD-L1 expression by Tumor Proportion Score (TPS) and Combined Positive Score (CPS) are similar in non-small cell lung cancer (NSCLC). J Clin Pathol. 2021;74(11):735–740. doi:10.1136/jclinpath-2020-206832
14. Carter JM, Polley MC, Leon-Ferre RA, et al. Characteristics and spatially defined immune (micro)landscapes of Early-stage PD-L1-positive triple-negative breast cancer. Clin Cancer Res. 2021;27(20):5628. doi:10.1158/1078-0432.CCR-21-0343
15. Yamashita K, Iwatsuki M, Harada K, et al. Prognostic impacts of the combined positive score and the tumor proportion score for programmed death ligand-1 expression by double immunohistochemical staining in patients with advanced gastric cancer. Gast Cancer. 2020;23(1):95–104. doi:10.1007/s10120-019-00999-9
16. Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
17. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
18. Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer KEYNOTE-164. J Clin Oncol. 2020;38(1):11–19. doi:10.1200/JCO.19.02107
19. Salem ME, Bodor JN, Puccini A, et al. Relationship between MLH1, PMS2, MSH2 and MSH6 gene-specific alterations and tumor mutational burden in 1057 microsatellite instability-high solid tumors. Int J Cancer. 2020;147(10):2948–2956. doi:10.1002/ijc.33115
20. Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-The stable evidence. Nat Rev Clin Oncol. 2010;7(3):153–162. doi:10.1038/nrclinonc.2009.237
21. Guo TA, Wu YC, Tan C, et al. Clinicopathologic features and prognostic value of KRAS, NRAS and BRAF mutations and DNA mismatch repair status: a single-center retrospective study of 1834 Chinese patients with Stage I-IV colorectal cancer. Int J Cancer. 2019;145(6):1625–1634. doi:10.1002/ijc.32489
22. Wen F, Jing R, Yu W, et al. Correlation between the expression of four mismatch repair proteins and clinicopathological features of colorectal cancer in elderly patients. Chin J Geriatr. 2018;1:54–56.
23. Xiao H, Can X, Ye K, et al. Correlation between mismatch-repair protein expression and clinicopathologic features in 658 colorectal cancers. Chin J Pathol. 2018;11:827–833.
24. Yang H, Yu Z, Xiang S, et al. Analysis of mismatch repair protein expression and clinicopathological characteristics in radical resection specimens of colorectal cancer based on random forest algorithm. Chin J Cancer Prev Treat. 2022;29(06):408–413.
25. Ping H, Li L, Qiu W, et al. Detection of mismatch repair protein MLH1 and PMS2 expression in colorectal cancer tissues by optimized automatic immunohistochemical staining scheme. J Mod Oncol. 2021;29(13):2254–2257.
26. Huil YS, Xiang L. Expression of MLH1, MSH2, MSH6 and PMS2 proteins in colorectal cancer and their significance in the screening of Lynch syndrome. Chin J Clin Exp Pathol. 2017;33(04):360–364.
27. Morgan S, Slodkowska E, Parra-Herran C, et al. PD -L1, RB 1 and mismatch repair protein immunohistochemical expression in neuroendocrine carcinoma, small cell type, of the uterine cervix. Histopathology. 2019;74(7):997–1004. doi:10.1111/his.13825
28. Berntsson J, Eberhard J, Nodin B, et al. Expression of programmed cell death protein 1 (PD-1) and its ligand PD-L1 in colorectal cancer: relationship with sidedness and prognosis. Oncoimmunology. 2018;7(8):e1465165. doi:10.1080/2162402X.2018.1465165
29. Kir G, Olgun ZC, Soylemez T, et al. PD-L1 expression in mismatch repair-deficient endometrial carcinoma and tumor-associated immune cells: differences between MLH1 methylated and nonmethylated subgroups. Int J Gynecol Pathol. 2021;40(6):575–586. doi:10.1097/PGP.0000000000000750
30. Gelsomino F, Barbolini M, Spallanzani A, et al. The evolving role of microsatellite instability in colorectal cancer: a review. Cancer Treat Rev. 2016;51:19–26. doi:10.1016/j.ctrv.2016.10.005
31. Xue H, Bin Z, Jun D, et al. Relevance between mismatch repair protein expression status and clinicopathological features of patients with stage II colorectal cancer and its impact on prognosis. Chin Med. 2021;16(05):725–728.
32. Chen L, Chen G, Zheng X, et al. Expression status of four mismatch repair proteins in patients with colorectal cancer: clinical significance in 1238 cases. Int J Clin Exp Pathol. 2019;12(10):3685–3699.
33. Jung KW, Won YJ, Hong S, et al. Prediction of cancer incidence and mortality in Korea, 2021. Cancer Res Treat. 2021;53(2):316–322. doi:10.4143/crt.2021.290
34. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325(7):669–685. doi:10.1001/jama.2021.0106
35. Kahi CJ, Boland CR, Dominitz JA, et al. Colonoscopy surveillance after colorectal cancer resection: recommendations of the US multi-society task force on colorectal cancer. Am J Gastroenterol. 2016;111(3):337–46; quiz 47. doi:10.1038/ajg.2016.22
36. Sharma R, Abbasi-Kangevari M, Abd-Rabu R, et al. Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Gastroenterol Hepatol. 2022;7(7):627–647. doi:10.1016/S2468-1253(22)00044-9
37. Hao C, Jun Q, Yu H. Research progress on the FOLFOXIRI regimen as first-line therapy for advanced colorectal cancer. Chin Clin Oncol. 2021;48(11):587–592.
38. Hong M, Qi Z, Xian Z. Advances in research on maintenance therapy of metastatic colorectal cancer. J Mod Oncol. 2021;29(02):361–364.
39. Akdeniz N, Kaplan MA, Uncu D, et al. The comparison of FOLFOX regimens with different doses of 5-FU for the adjuvant treatment of colorectal cancer: a multicenter study. Int J Colorectal Dis. 2021;36(6):1311–1319. doi:10.1007/s00384-021-03888-9
40. Benson AB, Venook AP, Cederquist L, et al. Colon Cancer, Version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(3):370–398. doi:10.6004/jnccn.2017.0036
41. Liu DX, Li DD, He W, et al. PD-1 blockade in neoadjuvant setting of DNA mismatch repair-deficient/microsatellite instability-high colorectal cancer. Oncoimmunology. 2020;9(1):1711650. doi:10.1080/2162402X.2020.1711650
42. Hu H, Kang L, Zhang J, et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, Phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7(1):38–48. doi:10.1016/S2468-1253(21)00348-4
43. Dienstmann R, Vermeulen L, Guinney J, et al. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;17(2):79–92. doi:10.1038/nrc.2016.126
44. Jiang X, Wang J, Deng X, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18(1):10. doi:10.1186/s12943-018-0928-4
45. Lecocq Q, Keyaerts M, Devoogdt N, et al. The next-generation immune checkpoint LAG-3 and its therapeutic potential in oncology: third time’s a charm. Int J Mol Sci. 2020;22(1):75. doi:10.3390/ijms22010075
46. Gaikwad S, Agrawal MY, Kaushik I, et al. Immune checkpoint proteins: signaling mechanisms and molecular interactions in cancer immunotherapy. Semin Cancer Biol. 2022;86(Pt 3):137–150. doi:10.1016/j.semcancer.2022.03.014
47. Lizardo DY, Kuang C, Hao S, et al. Immunotherapy efficacy on mismatch repair-deficient colorectal cancer: from bench to bedside. Biochim Biophys Acta Rev Cancer. 2020;1874(2):188447. doi:10.1016/j.bbcan.2020.188447
48. Paterson AM, Brown KE, Keir ME, et al. The programmed death-1 ligand 1: B7-1 pathway restrains diabetogenic effector T cells in vivo. J Immunol. 2011;187(3):1097–1105. doi:10.4049/jimmunol.1003496
49. Li Y, Liang L, Dai W, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15(1):55. doi:10.1186/s12943-016-0539-x
50. Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15(3):971–979. doi:10.1158/1078-0432.CCR-08-1608
51. Hu K, Wang ZM, N LJ, et al. CLEC1B expression and PD-L1 expression predict clinical outcome in hepatocellular carcinoma with tumor hemorrhage. Transl Oncol. 2018;11(2):552–558. doi:10.1016/j.tranon.2018.02.010
52. Wang S, Yuan B, Wang Y, et al. Clinicopathological and prognostic significance of PD-L1 expression in colorectal cancer: a meta-analysis. Int J Colorectal Dis. 2021;36(1):117–130. doi:10.1007/s00384-020-03734-4
53. Zhao T, Li Y, Zhang J, et al. PD-L1 expression increased by IFN-γ via JAK2-STAT1 signaling and predicts a poor survival in colorectal cancer. Oncol Lett. 2020;20(2):1127–34.7. doi:10.3892/ol.2020.11647
54. Tang M, Zheng Z, Shang J, et al. Risk analysis of positive PD-L1 expression and clinicopathological features and survival prognosis in patients with colorectal cancer: systematic review and meta-analysis. J Healthc Eng. 2022;2022:8212486. doi:10.1155/2022/8212486
55. Hsieh RC, Krishnan S, Wu RC, et al. ATR-mediated CD47 and PD-L1 up-regulation restricts radiotherapy-induced immune priming and abscopal responses in colorectal cancer. Sci Immunol. 2022;7(72):eabl9330. doi:10.1126/sciimmunol.abl9330
56. Thibaudin M, Limagne E, Hampe L, et al. Targeting PD-L1 and TIGIT could restore intratumoral CD8 T cell function in human colorectal cancer. Cancer Immunol Immunother. 2022;71(10):2549–2563. doi:10.1007/s00262-022-03182-9
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

