Back to Journals » Journal of Pain Research » Volume 16
Exploring the Effect of Pain Sensitive Questionnaire on Guiding Intravenous Analgesia After Cesarean Section: A Randomised Double Blind Controlled Trial
Authors Liu S, Liu S, Gu D , Zhao X, Zhang H, Deng C, Gu Y
Received 11 March 2023
Accepted for publication 8 September 2023
Published 18 September 2023 Volume 2023:16 Pages 3185—3196
DOI https://doi.org/10.2147/JPR.S412131
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jinlei Li
Shuxin Liu,1,* Siqi Liu,1,* Dengfeng Gu,1 Xiaona Zhao,2 Hong Zhang,1 Chao Deng,1 Yajuan Gu2
1Department of Anesthesiology, First Affiliated Hospital, Shihezi University, Shihezi, People’s Republic of China; 2Department of Obstetrics, First Affiliated Hospital, Shihezi University, Shihezi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chao Deng, Department of Anesthesiology, First Affiliated Hospital, Shihezi University, Shihezi, People’s Republic of China, Email [email protected] Yajuan Gu, Department of Obstetrics, First Affiliated Hospital, Shihezi University, Shihezi, People’s Republic of China, Email [email protected]
Objective: This study investigates the effect of the Pain Sensitivity Questionnaire (PSQ) in guiding patient controlled intravenous analgesia (PCIA) on postoperative analgesia in women undergoing cesarean section.
Methods: A total of 160 women who were to undergo a cesarean section under combined spinal and epidural anaesthesia were included in this study. Women with a preoperative PSQ < 4 were randomly divided into a low pain-sensitive control group (LC group), and a low pain-sensitive observation group (LO group), and women with preoperative PSQ > 6 were randomly divided into a high pain-sensitive control group (HC group) and a high pain-sensitive observation group (HO group). After the surgery, patients received the pump butorphanol concentration was 3.5 μg·kg− 1·h− 1 in the LC and HC groups, 3.0 μg·kg− 1·h− 1 in the LO group and 4.0 μg·kg− 1·h− 1 in the HO group.To compare the analgesic effects of postoperative PCIA and postoperative recovery in women.
Results: Wound pain and uterine contraction pain VAS scores at rest and activity were significantly lower in the LC group than in the LO group at 4 and 8 h postoperatively (P< 0.05). Similarly, wound pain and uterine contraction pain VAS scores at rest and activity were significantly lower in the HO group than in the HC group at 8, 12, and 24 h postoperatively (P< 0.05). The Ramsay scores were significantly higher in the LC than in the LO groups at 4, 8, 12, 24, and 48 h postoperatively (P< 0.05), but there was no statistically significant difference between the Ramsay scores in the HC group and the HO group. There was no statistical difference in any of the post-operative recoveries (P> 0.05).
Conclusion: Compared to the weight-based postoperative PCIA, the PSQ-based postoperative PCIA has better analgesic effects and can improve maternal satisfaction with postoperative analgesia.
Keywords: pain sensitivity, butorphanol, cesarean section, operative analgesia
Introduction
Cesarean section (CS) is one of the most common surgical procedures performed in gynecology and obstetrics.1 With the implementation of the two-child policy, the CS rate in China remained high at 36.7% in 2018.2 Owing to significant trauma of the internal organs caused by the CS, effective analgesia in the postoperative period can be challenging. Furthermore, the uterine contraction agent used after the procedure to promote uterine involution and reduce postoperative hemorrhages can supplement the noxious stimuli and cause cramping pain.3 The birth of the neonate and the resulting decrease in maternal concern, underestimation or neglect of the extent of their postoperative pain all leaves a significant proportion of patients with unsatisfactory analgesia after surgery.4
In this regard, PCIA (patient controlled intravenous analgesia) is currently one of the main modes of analgesia after obstetric surgery due to its convenient management.Satisfactory analgesia is achieved, based on maternal weight, different drug combinations, or mediation methods such as drug concentration. Since pain is a subjective sensation, studies based on maternal pain have been conducted to guide postoperative analgesia based on differences in pain thresholds and serum leptin levels.5 In addition, acupuncture and acupressure have been performed for postoperative pain management, improved maternal satisfaction with postoperative analgesia and reduced the use of postoperative analgesic compared to weight-based pain management.6
Pain sensitivity questionnaire (PSQ)7 Supplementary File 1, a noninvasive assessment for pain sensitivity, has simple and valid characteristics. A significant positive correlation exists between patients with acute postoperative pain, which can predict the degree of acute postoperative pain8–11 Ruscheweyh et al found that the PSQ may be a simple alternative to experimental pain intensity rating procedures in healthy subjects and makes the PSQ an extremely promising tool for clinical and experimental pain research.12 In addition, a study by Tuna et al found an effect of central sensitization syndrome (CSS) on PSQ in patients with chronic pain.13
Because the PSQ score is clinically useful in predicting the level of postoperative pain in patients, it is reasonable to assume that the PSQ can effectively assess the level of postoperative pain in women undergoing cesarean delivery. To our knowledge, no studies have developed postoperative analgesia protocols based on pain sensitivity questionnaire scores. Our preliminary study found that maternal dissatisfaction with postoperative PCIA analgesia was concentrated in those with PSQ scores <4 and PSQ scores >6. We conducted a single-center randomized controlled trial to assess the effectiveness of PSQ-based analgesia for postoperative PCIA and satisfaction with postoperative analgesia in women with PSQ scores less than 4 and PSQ scores greater than 6.
Methods
Study Design
This study was approved by the Research Ethics Committee of the First Affiliated Hospital of Shihezi University School of Medicine, Xinjiang Uygur Autonomous Region, China (approval number: KJX-2021-040-01, registration date: 2022–05-20) and registered with the China Clinical Trials Registry (registration number: ChiCTR2100051387, registration date: 2021–09-22).This randomized, double-blind clinical trial was conducted according to the principles of the Declaration of Helsinki. All participants provided written informed consent, and their anonymized data will be stored for five years and available upon reasonable request to the corresponding author via email.
Study Population
In total, 160 women who were planning to undergo a transverse incisional cesarean delivery (20–40 years old) were recruited from May 2022 to December 2022 at the First Affiliated Hospital of Shihezi University (tertiary A and other hospitals), Uyghur Autonomous Region of Xinjiang, China (Figure 1), of which, 80 had PSQ scores <4 points and 80 had PSQ scores >6 points.The participants with a gestational age between 37 and 40 weeks, singleton pregnancy, body weight between 50 and 80 kg, height between 150 and 170 cm, and BMI between 20 and 35 kg/m2 and voluntarily underwent a PCIA were classified according to the American Society of Anesthesiologists physiological status I–II were eligible for inclusion. Those with contraindications to intralesional anesthesia, a history of chronic pain, analgesic medication, neurological and psychiatric disorders, communication abnormalities, and severe heart, liver, or kidney disease were excluded.
PSQ Assessment Methodology
The PSQ was administered to all patients in the obstetrical ward on the day before surgery (day 1). The scale consisted of 17 items, each describing a daily life situation, and were scored on a numerical rating scale from 0 (no pain) to 10 (most pain) to indicate the pain level. Three items described the absence of pain under normal conditions and provided a reference for pain-free sensation. The other fourteen items covered a variety of different types of pain and intensity levels. The final score was calculated as the mean rating of all items in the questionnaire. Three items describe conditions that was rated by healthy individuals as not normally associated with pain (5,9,13) and do not form part of the final score.8
Experimental Groups and Study Intervention
The participants’ demographic characteristics and preoperative data, such as age, weight, height, gestational week, and the number of previous pregnancies and deliveries, were recorded. The SPSS 26.0 software program (IBM SPSS Statistics, Chicago, Illinois, USA) was used to divide 80 women with a PSQ <4 into two groups in a 1:1 ratio. A computerized random number method was used. Forty pairs of numbers (1 or 2) were randomly generated, where 1 and 2 corresponded to the LC and LO groups, respectively.The intervention methods for the LC or LO groups were kept in opaque envelopes numbered 1 to 80 in the order in which they were generated. The same method was used to divide the 80 women with a PSQ >6 into the HC and HO groups. An anaesthetist, who was unaware of the study, opened the envelopes and determined the PCIA formulation.All patients and investigators involved in the study were unaware of the grouping.
Formulation of the PCIA for each group:
The pump butorphanol (Lot No.:210505BP, Jiangsu Hengrui Pharmaceutical Co.) concentration was 3.5 µg·kg−1·h−1 in the LC and HC groups, 3.0 µg·kg−1·h−1 in the LO group and 4.0 µg·kg−1·h−1 in the HO group. The PCIA parameters were as follows: continuous background dose 2. 0 mL/h, PCA dose 0.5 mL, lock time 15 min. The intraoperative and postoperative data were recorded by the investigator during the follow-up period. All patients and investigators involved in the study were unaware of the grouping.
Anaesthetic Procedures
All patients were monitored by electrocardiogram (ECG), blood pressure (BP), and pulse oxygen saturation (SaO2). Intravenous access was established on the right forearm or back of the hand to inject 10 mL·kg−1·h−1lactic acid Ringer solution. Combined spinal and epidural anaesthesia was performed in the left lateral recumbent position with a puncture gap of L2 to L3. Clear cerebrospinal fluid was seen after the pen-tip lumbar anesthesia needle punctured the dura, 0.5% ropivacaine 12 mg was slowly injected into the subarachnoid space, and the epidural catheter was left in the epidural space for 3–4 cm, oriented toward the cephalad end and properly fixed with adhesive tape. The skin sensory level was tested by the anesthesiologist using a cotton swab, and the anesthetic level was controlled at T6. During anesthesia, 80 μg of phenylephrine was administered when the SBP was less than 90 mmHg, 0.5 mg of atropine was administered when the HR was less than 50 beats/min, 12 mg of ephedrine was administered when the SBP was less than 90 mmHg and HR was less than 60 beats/min. If necessary, the operating bed was tilted, or the waist was pushed to the left to avoid severe hypotension. Butorphanol (1 mg) was pushed intravenously 30 min before the end of the procedure as a loading dose; then, the PCIA analgesic pump was connected to the patient’s peripheral venous access before exiting the room.
After the procedure, the mothers were sent back to the ward by the anesthesiology nurses and were informed of the use of the PCIA analgesia pump. During the analgesic period, if the VAS pain score was >4, the mothers pressed the PCA key for additional medication. If pain was still felt significantly after two compressions of PCA within 1 h, 5 mg diazoxide was given intravenously by the anesthesiologist until the VAS pain score was <4. If sedation was excessive, the analgesic pump was clamped for 1 h, and remedies were administered more than twice; the woman was excluded from this study.
The Data Collected and Calculated
The main outcome indicator of this study was the Visual Analogue Scale/Score (VAS) score (a 10 cm horizontal line drawn on paper with a score of 0 at one end of the line for no pain and a score of 10 at the other end for maximum pain, with the middle part indicating different degrees of pain) on which the mother marks the corresponding pain level.14 The Ramsay score is as follows: 1: anxious, agitated, and irritable; 2: quiet, cooperative, and disoriented; 3: responds only to commands; 4: asleep but sensitive to stimuli; 5: asleep, slow to respond to stimuli; 6: asleep and unable to call.15 Static and dynamic VAS scores of abdominal incisional pain and uterine contraction pain (defined as pain on coughing) and Ramsay scores at the corresponding time points were performed at 4, 8, 12, 24, and 48 h postoperatively.
The secondary outcomes were the amount of PCIA analgesic within 24 h postoperatively; time to first activity; time to first gas; time to first breastfeeding; and the number of analgesic pumps after clamping. Blood pressure, respiratory rate, heart rate, and overall patient satisfaction score (1: very dissatisfied; 2: relatively dissatisfied; 3: fair; 4: relatively satisfied; 5: very satisfied) were recorded on the return to the ward and 24 h postoperatively.16 Postoperative adverse events, including dizziness, drowsiness, pruritus, nausea, vomiting, and respiratory depression (defined as apnea or respiratory rate of 8 breaths/min), were recorded.
Sample Size and Power
Satisfaction with postoperative analgesia based on the conventional weight-based configuration of PCIA for those with PSQ <4 and PSQ >6 was 70%, according to the preliminary study. It was hypothesized that PSQ scores would increase postoperative satisfaction by 25%. Based on a significance level of 0.05 and a power of 0.8, the required minimum sample size was 33 per group according to the sample size calculation software tool PASS version 15.0 (NCSS, Kaysville, UT, USA). Projecting a loss to follow-up of 10%, 160 patients were recruited for this study.
Statistical Analyses
Continuous-type variables conforming to a normal distribution are expressed as mean ± standard deviation, those not conforming to a normal distribution are expressed as median and interquartile spacing, and subtype variables are expressed as number (percentage). Normality tests (Shapiro–Wilk test) were performed for all continuous variables. Independent sample t-tests were used to compare the differences in age and weight. The Mann–Whitney U-test was used to compare differences in height, BMI, gestational week, operative time, bleeding, pain VAS scores, time to the first activity, time to first deflation, time to first breastfeeding, PCIA consumption, preoperative and postoperative systolic blood pressure, diastolic blood pressure, and heart rate. A chi-square test was used to compare differences in place of residence, education, analgesic pump clamping requirements, and complications. All data were analyzed using SPSS version 25 (IBM Corporation, USA).
Results
A total of 225 patients were screened for this study, 180 of whom met the inclusion criteria. As depicted in Figure 1, 20 cases were lost to follow-up, and the remaining 160 patients completed postoperative follow-up. The demographics of the observation and control groups in the high and low sensitivity groups of the PSQ score were not statistically different, as illustrated in Table 1.
 |
Table 1 Demographic, Preoperative, and Intraoperative Data |
As illustrated in Table 2, in the HC group at 8 h (p=0.004), 12 h (p=0.000), and 24 h (p=0.000) postoperatively, the VAS of incisional pain at rest scores were higher in the HC group than in the HO group. At 8 h (p=0.000), 12 h (p=0.000), and 24 h (p=0.000) postoperatively at rest, VAS scores for uterine systolic pain were higher in the HC group than in the HO group. At 8 h (p=0.027), 12 h (p=0.000), and 24 h (p= 0.000), the VAS scores for incisional pain during activity were higher than those in the HO group. The HC group had higher VAS scores at 8 h (p=0.031), 12 h (p=0.000), and 24 h (p=0.000) VAS scores of uterine contraction pain during activity were higher than those of the HO group, indicating that the analgesic effect of the observation group (HO) was better than that of their control group (HC) in the 24 h after surgery (Figure 2).Whereas at 4h and 8h VAS scores were statistically significant in the LO group versus the LC group. (P<0.05) but the VAS scores in each group were less than four, which was not clinically significant.
 |
Table 2 Main Postoperative Outcome Indicators |
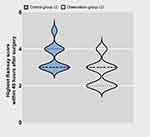
As displayed in Figure 3, the Ramsay scores of the LC group at 4, 8, 12, 24, and 48 h were higher than those of the LO group, indicating that their control group (LC) was more sedated than the observation group (LO) during the 48 h postoperative period.There was no statistically significant difference between the Ramsay scores of the HC and HO groups at the time points recorded (p>0.05).
There were no statistical differences in systolic blood pressure, diastolic blood pressure, heart rate, adverse events, exhaust time after cesarean section, time to first breastfeeding, or early activity for all patients at different time points. The overall postoperative satisfaction scores of patients in the PSQ low-sensitive and high-sensitive groups were statistically higher than those in the control group (Table 3).
 |
Table 3 Secondary Postoperative Outcome Indicators |
Discussion
Our preliminary findings suggested that the population with unsatisfactory outcomes of PCIA for cesarean section had higher or lower PSQ scores. The present study demonstrated that the PSQ-based guided PCIA protocol was superior to the weight-based guided PCIA protocol in preventing maternal over-sedation and inadequate analgesia in this population and improved the overall satisfaction of postoperative analgesia in this group of patients.
This study aimed to improve maternal satisfaction with postoperative PCIA. Most current studies on PCIA protocols focus on selecting analgesic pump drugs and doses, with the optimal dose mostly being explored in a kilogram weight-based manner.17–21 However, pain is a painful experience of sensory, emotional, cognitive, and social dimensions associated with tissue damage or potential tissue damage, and it is a subjective feeling.22 Therefore, the pain perception experienced by patients of the same weight may differ significantly for injurious stimuli of the same magnitude. Recently, many investigators have conducted studies on postoperative analgesia based on patients’ pain thresholds. Luedi et al administered PCIA to patients undergoing anal surgery based on preoperative pressure pain thresholds and found that this approach helped to address the high variability in patient pain sensitivity and reduced the amount of postoperative analgesic medication.23
There are two main methods of pain sensitivity assessment, Quantitative Sensory Testing (QST) in the laboratory and PSQ. The former is a pain sensitivity test performed in the laboratory on subjects with generally applied injurious stimuli, including hot, cold, and pressure stimuli. However, these assessment methods not only require additional human and material resources but also cause additional physical pain to the patients and can also cause more psychological stress.11,12,22,24,25 The PSQ method of pain sensitivity assessment was used in this study, which is less time-consuming and laborious, and easier to perform than the QST method without causing additional harm. The PSQ depicts good reliability with a significant correlation with the QST. The feasibility of PSQ for assessing pain sensitivity has been validated in several countries through clinical trials and QST.7,26–28 Kim et al also found consistency in the extent to which the PSQ could predict the degree of moderate or severe postoperative pain.29 Therefore, the PSQ was deemed appropriate to guide postoperative analgesia.In our study, we combined a weight-based postoperative analgesia protocol with a PSQ score, with a moderate decrease in the amount of analgesic medication for women with PSQ scores less than 4 and a moderate increase in the amount of analgesic medication for women with PSQ scores greater than 6.
Butorphanol was applied in PCIA in the present study, which mainly acts on κ receptors, with insignificant effects on δ receptors, and has a dual agonist-antagonist effect on μ receptors, with an agonist strength of 25:4:1 for κ, δ, and μ receptors. Due to this unique effect on opioid receptors, it has the good analgesic effect of opioids, rarely causes clinically significant respiratory depression, decreased gastrointestinal activity and smooth muscle spasms, skin pruritus or urinary retention, and has very low somatic dependence.30–32 Thus, Butorphanol is widely used in clinical practice, and since it causes no adverse effects in lactating women and newborns, it is increasingly being used for postoperative analgesia in women in labor.33 However, no uniformity has been reached among regions and hospitals. In our previous study, we found that the reasons for unsatisfactory maternal postoperative analgesia were mainly over-sedation and under-sedation, with over-sedation concentrated in the group of women with low pain sensitivity and under-sedation concentrated in the group of women with high pain sensitivity. In the current study, the PSQ-based protocol to guide PICA improved satisfaction with postoperative analgesia in both more pain-sensitive and less pain-sensitive women.
The analgesic pump of women with high Ramsay scores was clamped to reduce excessive sedation, with more women requiring clamping in the pain-insensitive control group than in the pain-insensitive observation group, suggesting that the pain-insensitive control group had overdosed on Butorphanol. Mothers would press the PCA button frequently to increase the drug dose for inadequate analgesia to meet the analgesic demand, so we compared the maternal PCIA drug consumption in 24 h and found that the pain hypersensitive control group consumed 58.5 mL of PCIA drug in 24 h, which was more than 54.4 mL in the pain hypersensitive observation group. This suggests that the maternal Butorphanol dose was too low, and the analgesia was inadequate in the pain-hypersensitive control group.
There was no significant difference in maternal systolic blood pressure, diastolic blood pressure, and heart rate for both analgesic strategies, suggesting that both strategies can maintain maternal hemodynamic stability and ensure life safety. This also suggests that Butorphanol is suitable for postoperative analgesia because of its low impact on the cardiovascular system, in line with the findings of Ding et al.34
Limitations
This study has some limitations. First, only women with a PSQ <4 and PSQ >6 were recruited, so whether the PSQ as the basis for guiding maternal PCIA protocol is also appropriate for women with a PSQ 4–6 needs to be further explored. Second, two women presented with symptoms of inadequate analgesia and excessive sedation drowsiness, which may be due to the single medication used for PCIA in this study and could be resolved by a multimodal combination of medications. Third, this study was a single-center study with a small sample size which may affect the study results, so future large multicenter studies are required to verify the credibility of the clinical results. In addition, our study included only primiparous women and did not study women who had a repeat cesarean delivery, so whether our study protocol is also applicable to women who had repeat cesarean delivery requires further study.
Conclusion
Compared with weight-based postoperative PCIA, PSQ-based postoperative PCIA has better analgesic effects and can improve maternal satisfaction with postoperative analgesia. The PSQ-based postoperative PCIA may be a better analgesic strategy and can be extended for postoperative analgesia after cesarean delivery.
Data Sharing Instructions
Requests to access data should be addressed to [email protected] De-identified individual participant data will be available to medical researchers on request in accordance with local registration and ethical approval when the article is published until 20 April 2028. All proposals requesting data access will need to specify an analysis plan and will need approval of the scientific board before any data can be released.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
We received support from Shihezi University for an independent project (Research and promotion of optimal perioperative treatment strategy in cesarean delivery, project number: ZZZC202070A).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhao Z, Xu K, Zhang Y, Chen G, Zhou Y. Quadratus lumborum block for postoperative analgesia after cesarean section: a meta-analysis of randomized controlled trials with trial sequential analysis. Sci Rep. 2021;11(1):18104. doi:10.1038/s41598-021-96546-7
2. H-T L, Hellerstein S, Zhou Y-B, Liu J-M, Blustein J. Trends in Cesarean Delivery Rates in China, 2008-2018. JAMA. 2020;323(1):89–91. doi:10.1001/jama.2019.17595
3. Sun S, Guo Y, Wang T, Huang S. Analgesic Effect Comparison Between Nalbuphine and Sufentanil for Patient-Controlled Intravenous Analgesia After Cesarean Section. Front Pharmacol. 2020;11:574493. doi:10.3389/fphar.2020.574493
4. Sutton CD, Carvalho B. Optimal Pain Management After Cesarean Delivery. Anesthesiol Clin. 2017;35(1):107–124. doi:10.1016/j.anclin.2016.09.010
5. Akkececi NS, Oksuz G, Urfalioğlu A, et al. Preoperative Serum Leptin Level Is Associated with Preoperative Pain Threshold and Postoperative Analgesic Consumption in Patients Undergoing Cesarean Section. Med Princ Pract. 2019;28(4):333–340. doi:10.1159/000500556
6. Smith CA, Collins CT, Levett KM, et al. Acupuncture or acupressure for pain management during labour. Cochrane Database Systematic Rev. 2020;2(2):CD009232. doi:10.1002/14651858.CD009232.pub2
7. Quan X, Fong DYT, Leung AYM, Liao Q, Ruscheweyh R, Chau PH. Validation of the mandarin Chinese version of the pain sensitivity questionnaire. Pain Pract. 2018;18(2):180–193. doi:10.1111/papr.12587
8. Yaari L, Dolev A, Kittani M, et al. Preoperative pain sensitivity questionnaire helps customize pain management after arthroscopic partial meniscectomy. Knee Surg Sports Traumatol Arthrosc. 2021;29(12):4198–4204. doi:10.1007/s00167-021-06438-6
9. Kaunisto MA, Jokela R, Tallgren M, et al. Pain in 1000 women treated for breast cancer: a prospective study of pain sensitivity and postoperative pain. Anesthesiology. 2013;119(6):1410–1421. doi:10.1097/ALN.0000000000000012
10. Grundström H, Larsson B, Arendt-Nielsen L, Gerdle B, Kjølhede P. Associations between pain thresholds for heat, cold and pressure, and Pain Sensitivity Questionnaire scores in healthy women and in women with persistent pelvic pain. Eur J Pain. 2019;23(9):1631–1639.
11. Ruscheweyh R, Viehoff A, Tio J, Pogatzki-Zahn EM. Psychophysical and psychological predictors of acute pain after breast surgery differ in patients with and without pre-existing chronic pain. Pain. 2017;158(6):1030–1038. doi:10.1097/j.pain.0000000000000873
12. Ruscheweyh R, Marziniak M, Stumpenhorst F, Reinholz J, Knecht S. Pain sensitivity can be assessed by self-rating: development and validation of the pain sensitivity questionnaire. Pain. 2009;146(1–2):65–74. doi:10.1016/j.pain.2009.06.020
13. Tuna T, Obbergh LV, Cutsem NV, Engelman E. Usefulness of the pain sensitivity questionnaire to discriminate the pain behaviour of chronic pain patients. Br J Anaesth. 2018;121(3):616–622. doi:10.1016/j.bja.2018.04.042
14. Johnson C. Measuring Pain. Visual Analog Scale Versus Numeric Pain Scale: what is the Difference? J Chiropr Med. 2005;4(1):43–44. doi:10.1016/S0899-3467(07)60112-8
15. Bråten LCH, Gjefsen E, Gervin K, et al. Cytokine Patterns as Predictors of Antibiotic Treatment Effect in Chronic Low Back Pain with Modic Changes: Subgroup Analyses of a Randomized Trial (AIM Study). J Pain Res. 2023;16:1713–1724. doi:10.2147/JPR.S406079
16. McNicol ED, Ferguson MC, Hudcova J. Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Systematic Rev. 2015;2015(6):CD003348. doi:10.1002/14651858.CD003348.pub3
17. Wang Y, Fang X, Liu C, Ma X, Song Y, Yan M. Impact of Intraoperative Infusion and Postoperative PCIA of Dexmedetomidine on Early Breastfeeding After Elective Cesarean Section: a Randomized Double-Blind Controlled Trial. Drug Des Devel Ther. 2020;14:1083–1093. doi:10.2147/DDDT.S241153
18. Sharpe EE, Molitor RJ, Arendt KW, et al. Intrathecal Morphine versus Intrathecal Hydromorphone for Analgesia after Cesarean Delivery: a Randomized Clinical Trial. Anesthesiology. 2020;132(6):1382–1391. doi:10.1097/ALN.0000000000003283
19. Kaçmaz O, Gülhaş N, Kayhan GE, Durmuş M. Effects of different epidural initiation volumes on postoperative analgesia in cesarean section. Turkish j Med Sci. 2020;50(8):1955–1962. doi:10.3906/sag-1905-44
20. Zimpel SA, Torloni MR, Porfírio GJ, Flumignan RL, Silva E. Complementary and alternative therapies for post-caesarean pain. Cochrane Database Systematic Rev. 2020;9:CD011216. doi:10.1002/14651858.CD011216.pub2
21. Chi X, Li M, Mei W, Liao M. Comparison of patient-controlled intravenous analgesia with sufentanil versus tramadol in post-cesarean section pain management and lactation after general anesthesia - A prospective, randomized, double-blind, controlled study. J Pain Res. 2017;10:1521–1527. doi:10.2147/JPR.S137799
22. Duchow J, Schlöricke E, Hüppe M. Self-rated pain sensitivity and postoperative pain. Schmerz. 2013;27(4):371–379. doi:10.1007/s00482-013-1338-6
23. Luedi MM, Schober P, Hammoud B, Andereggen L, Hoenemann C, Doll D. Preoperative pressure pain threshold is associated with postoperative pain in short-stay anorectal surgery: a prospective observational study. Anesth Analg. 2021;132(3):656–662. doi:10.1213/ANE.0000000000005072
24. Melotti R, Ruscheweyh R, Pramstaller PP, Hicks AA, Pattaro C. Structural Consistency of the Pain Sensitivity Questionnaire in the Cooperative Health Research In South Tyrol (CHRIS) Population-Based Study. J Pain. 2018;19(12):1424–1434. doi:10.1016/j.jpain.2018.06.007
25. Ruscheweyh R, Verneuer B, Dany K, et al. Validation of the pain sensitivity questionnaire in chronic pain patients. Pain. 2012;153(6):1210–1218. doi:10.1016/j.pain.2012.02.025
26. Sellers AB, Ruscheweyh R, Kelley BJ, Ness TJ, Vetter TR. Validation of the English language pain sensitivity questionnaire. Reg Anesth Pain Med. 2013;38(6):508–514. doi:10.1097/AAP.0000000000000007
27. Bell BA, Ruscheweyh R, Kelley BJ, Ness TJ, Vetter TR, Sellers AB. Ethnic Differences Identified by Pain Sensitivity Questionnaire Correlate With Clinical Pain Responses. Reg Anesth Pain Med. 2018;43(2):200–204. doi:10.1097/AAP.0000000000000689
28. Inal FY, Gul K, Camgoz YY, Daskaya H, Kocoglu H. Validation of the Turkish version of the Pain Sensitivity Questionnaire in patients with chronic pain. J Int Med Res. 2021;49(12):3000605211060158. doi:10.1177/03000605211060158
29. Kim H-J, Park J-H, Kim J-W, et al. Prediction of postoperative pain intensity after lumbar spinal surgery using pain sensitivity and preoperative back pain severity. Pain Medicine. 2014;15(12):2037–2045. doi:10.1111/pme.12578
30. Commiskey S, Fan L-W, Ho IK, et al. Butorphanol: effects of a prototypical agonist-antagonist analgesic on kappa-opioid receptors. J Pharmacol Sci. 2005;98(2):109–116. doi:10.1254/jphs.CRJ05001X
31. Du B-X, Song Z-M, Wang K, et al. Butorphanol prevents morphine-induced pruritus without increasing pain and other side effects: a systematic review of randomized controlled trials. Canadian j Anaesthesia. 2013;60(9):907–917. doi:10.1007/s12630-013-9989-4
32. Zhu Z, Zhang W. Efficacy and Safety of Butorphanol Use in Patient-Controlled Analgesia: a Meta-Analysis. Evid Based Complemen Alternative Med. 2021;2021:5530441. doi:10.1155/2021/9854850
33. Yadav J, Regmi MC, Basnet P, Guddy KM, Bhattarai B, Poudel P. Butorphanol in Labour Analgesia. JNMA J Nepal Med Assoc. 2018;56(214):940–944. doi:10.31729/jnma.3905
34. Ding X, Luo Y, Shi L, Liu C, Yan Z. Butorphanol in combination with dexmedetomidine provides efficient pain management in adult burn patients. Burns. 2021;47(7):1594–1601. doi:10.1016/j.burns.2020.12.025
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.