Back to Journals » International Journal of General Medicine » Volume 17
Exploring Symptom Clusters in Chinese Patients with Diabetic Kidney Disease: A Network Analysis
Authors Duan DF , Liu M, Ma DY, Yan LJ, Huang YY, Chen Y, Jiang W, Tang X, Xiong AQ, Shi YY
Received 2 November 2023
Accepted for publication 26 February 2024
Published 7 March 2024 Volume 2024:17 Pages 871—884
DOI https://doi.org/10.2147/IJGM.S447921
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Di-Fei Duan,1,2,* Min Liu,1,2,* Deng-Yan Ma,1,2 Lin-Jia Yan,3 Yue-Yang Huang,1,2 Yi Chen,1,2 Wei Jiang,1,2 Xi Tang,2 An-Qi Xiong,4,5 Yun-Ying Shi2
1West China School of Nursing, West China Hospital, Sichuan University, Chengdu, Sichuan Province, People’s Republic of China; 2Department of Nephrology, Kidney Research Institute, West China Hospital, Sichuan University, Chengdu, Sichuan Province, People’s Republic of China; 3The Nethersole School of Nursing Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, People’s Republic of China; 4Department of Nursing, West China Second University Hospital, Sichuan University, Chengdu, Sichuan Province, People’s Republic of China; 5Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, Chengdu, Sichuan Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Deng-Yan Ma, Email [email protected]
Purpose: The research on symptom management in patients with diabetic kidney disease (DKD) has shifted from separate symptoms to symptom clusters and networks recently. This study aimed to evaluate the unpleasant symptoms of DKD patients, and to investigate how these symptom clusters could affect patients.
Methods: 408 DKD patients were recruited in this cross-sectional study. The symptoms of DKD patients were measured using the modified Dialysis Symptom Index. Network analysis was employed to evaluate the symptom network and the characteristics of individual nodes, while factor analysis was utilized to identify symptom clusters.
Results: Blurred vision was the most prevalent symptom among DKD patients. The symptoms identified as the most distressing, severe, and frequent were light headache or dizziness, arteriovenous fistula/catheterization pain, and diarrhea, respectively. Five symptom clusters were obtained from factor analysis, and the most central symptom cluster in the entire symptom network was sexual dysfunction.
Conclusion: This study identified five symptom clusters in Chinese DKD patients, with sexual dysfunction emerging as the most central cluster. These findings carry significant clinical implications, underscoring the necessity of assessing symptom clusters and their associations to enhance symptom management in DKD patients. Further research is essential to elucidate the underlying mechanisms of symptoms and to clarify the associations among symptoms in DKD patients across different disease trajectories or treatment modalities.
Keywords: diabetic kidney disease, network analysis, symptom cluster, symptom management
Introduction
Diabetic kidney disease (DKD) stands as one of the most frequent and severe complications arising diabetes, becoming the leading cause of end-stage renal disease(ESRD).1,2 Diabetes contributes to approximately 30–50% of ESRD cases in the United States.3 A study in 2020 showed that the total prevalence of DKD in patients with Type 2 diabetes reached 21.8%,4 with an estimated 24.3 million DKD patients in China.5 Consequently, the global prevalence of DKD is witnessing a rapid increase, signifying the growth of a significant public health challenge worldwide.6
Patients with chronic kidney disease (CKD) frequently experience unpleasant symptoms, including psychological, gastrointestinal, cardiopulmonary, neurological, dermatological, painful or sleep-related disorders, as well as sexual dysfunction, and fatigue.7,8 These symptoms often occur in clusters, with one being the primary symptom and the others being secondary.9 A “symptom cluster” is defined as two or more symptoms that occur together in interrelated patterns. These clusters represent cohesive sets of symptoms, maintaining relative independence from other clusters, thereby potentially unveiling distinct underlying symptom dimensions. Within a given cluster, the associations between symptoms are expected to be stronger than those between symptoms belonging to different clusters. It is noteworthy that symptoms within a cluster may not necessarily have a common cause.10 Previous studies have suggested that a symptom cluster has a greater impact on health than that of an individual symptom,11 as a certain cluster has synergistic effects on morbidity, mortality, prognosis, and quality of life. Thus, identifying and addressing symptom clusters is a critical strategy for effective symptom management, offering a more targeted approach to improving patient outcomes.12
The “unpleasant symptoms” theory offers a multidimensional view of symptoms, emphasizing the significance of understanding symptom clusters. This approach provides a comprehensive framework for researchers to explore symptom experiences holistically.13,14 Recognizing the benefits of symptom clustering in enhancing patient survival and prognosis, network analysis emerges as a powerful tool.15 It constructs partial correlation models to delineate the relationships between unpleasant symptoms and their clusters, facilitating the graphical representation of each symptom’s importance and the intricate connections within the network from a holistic standpoint.16 The identification of unpleasant symptoms thus plays a pivotal role in empowering DKD patients in their symptom management journey.17
The 2022 World Kidney Day emphasized quality of life supersedes quantity of life, and recommends the use of symptom clusters for symptom management in CKD patents.18 Many studies focusing on CKD domain have revealed that interventions targeting symptom clusters can greatly improve the quality of life and reduce symptomatic burdens.11,12 As a complication of CKD, DKD presents with numerous CKD-related symptoms,19 thereby increasing the risk of adverse health outcomes and contributing significantly to the global disease burden.20 Hence, prevention and management of DKD symptoms is a key goal in the comprehensive care of kidney and diabetic patients.21
Despite the recognized importance, existing research on symptom clusters has predominantly focused on dialysis treatment, leaving a gap in our understanding of these clusters in DKD patients. This lack of evidence impedes efforts to lessen patient burden and improve prognoses.9,22 Therefore, this study aimed to 1) collect and evaluate the unpleasant symptoms of DKD patients, and 2) explore the symptom clusters affecting these patients by network analysis and factor analysis.
Methods
We used STROBE Checklist for more rigorous study design and improved article quality.
Study Design and Participants
This is a prospective cross-sectional study with data collected from a general tertiary hospital in Chengdu, Sichuan Province, China. Convenience sampling was adopted. Individuals discharged from this hospital and met the following inclusion criteria were eligible to participate: 1) diagnosed according to the Chinese guidelines for the clinical diagnosis and treatment of DKD,23 and 2) willing to participate in this study. Participants with incomplete data were excluded to prevent measurement contamination. Initially, 486 participants were approached, with 408 providing complete data, resulting in a response rate of 83.95%. Details are shown in Supplementary Figure 1. No significant difference was found in sociodemographic data between participants who completed the study and those who did not (Supplementary Table 1).
Sample Size Calculation
As the rule of thumb for exploratory factor analysis,24 the required sample size for this study was 5–10 times the number of variables. Considering the investigation of 40 to 50 symptoms in DKD patients, the minimum sample size was determined to be between 200 to 250 cases. The minimum sample size in network analysis is determined by assuming N symptoms and using the formula N + [N*(N-1)/2]. Increasing the sample size results in more reliable analysis outcomes.16 Considering symptoms with a prevalence of less than 10%, and assuming N ranges from 20 to 30, the estimated minimum sample size for our study was between 210 and 465 cases. As recommended by literature,25 the evaluations of network analysis outcomes should consider supplementary measures such as stability in conjunction with sample size. If these measures indicate favorable results, the findings may be deemed acceptable.
Data Collection and Ethic Consideration
Data were collected from April to July 2023. In addition to sociodemographic data, the primary outcomes of this study focused on the symptoms of DKD, which were evaluated using the CKD-Symptom Burden Index. Data collection was implemented by trained registered nurses via telephone inquiries. Besides, the clinical data of participants could be collected from the hospital information systems by researchers. Prior to the interviews, the purpose of the study, estimated time of completion, participants’ authority, and privacy policy were provided for participants. All respondents were further asked to give their informed consent through a link sent by telephone or WeChat. The questionnaire items were answered independently by the participants and returned to the researcher immediately. During the process, researchers read out the items in a quiet setting and clarified any queries to ensure understanding. Participants had the right to withdraw at any stage without any negative consequences.
Measurements
Sociodemographic Data
A self-designed basic information form was used to record the following information of participants, including marital status, education level, ethnicity, gender, age, height, weight, employment status, medical insurance coverage, CKD stage, and disease duration. The Body Mass Index (BMI) is determined by dividing one’s weight in kilograms by the square of their height in meters.
Symptoms of DKD
The symptoms of DKD were assessed using the CKD-Symptom Burden Index (CKD-SBI),26 aiming to evaluate symptoms at different stages of CKD within the past month. It consists of four symptom dimensions-prevalence, distress, severity, and frequency-across 32 symptoms. Notably, symptoms reported by patients outside the CKD-SBI were also documented and included in the statistical analysis.27 The prevalence scale assesses the presence or absence (Yes/No) of each symptom, and if a symptom is present, then evaluates its distress, severity, and frequency. Each of these four dimensions is rated on a scale from 0 to 10, with the “distress” subscale ranging from “none” to “very much”, “severity” from “none” to “very severe”, and “frequency” from “never” to “constant”. The overall CKD-SBI score is derived by summing the scores from each subscale (prevalence, distress, severity, and frequency) and applying a constant multiplier of 0.1008, where a higher total score reflects a greater symptom burden.
Statistical Analysis
Statistical analyses were performed using SPSS Statistics for Windows, version 26.0. Means, standard deviations, medians, interquartile ranges, proportions, and percentages were utilized to summarize the sociodemographic characteristics of participants and symptom information. To ensure a valid analysis, symptoms with a prevalence of less than 10% were removed from the dataset,28,29 leaving 22 symptoms for further analysis. This adjustment confirmed that a sample size of 408 was sufficient for conducting factor analysis, a widely used technique in symptom cluster research.30 The total score of symptom experience was utilized to create these clusters. The spindle factorization method, in conjunction with the maximum variance rotation method, was applied to extract eigenvalues greater than 1. After 25 iterations, symptoms with a factor loading of 0.45 or higher were categorized into distinct clusters.31
Network Analysis
Network analysis was performed using 22 symptoms to examine and visualize the relationships between the variables. In network analysis, variables are represented as nodes, and the associations between variables are depicted as edges. The strength of these connections, or edges, provides insights into the level of interconnection among the nodes. These connections can occur either directly between variables or indirectly through other variables. The primary focus of the symptom network analysis revolved around determining which symptom activation would more likely trigger the activation of other symptoms within the network. To assess this, three commonly employed centrality measures were utilized, namely strength, closeness, and betweenness.32 The centrality of strength quantified the overall direct connections of a symptom with other symptoms, thereby representing its capacity to influence other symptoms. The centrality of closeness gauged the reciprocal of the distance between a symptom and other symptoms, thereby indicating the symptom’s central position within the network. Centrality between vertices reflected a measure of the number of shortest paths passing through a symptom, thereby highlighting its bridging function in the network. Symptoms exhibiting the highest centrality coefficients were identified as core symptoms. Given previous findings on the reliability of strength centrality, our analysis primarily focused on this measure (rs) to identify key symptoms. The Fruchterman-Reingold algorithm was utilized for visualizing the network, arranging nodes in a manner that minimizes the crossing of edges. The thickness of edges in the network visualization corresponds to the strength of the associations between variables. Stability and accuracy, crucial for assessing the network’s reliability, were evaluated using the correlation stability coefficient (with a recommended threshold of 0.25 and a satisfactory level above 0.50) and the 95% confidence intervals (CIs) of edge weights, respectively.33 To evaluate the accuracy of the estimated network connections, the 95% confidence intervals (CIs) of the edge weight values were calculated.34 Nonparametric bootstrapping (1000 samples) was employed to determine CIs, ensuring the network’s estimations were robust and reliable.
Results
Characteristics of Participants
This study included 408 participants in the final analysis. The sociodemographic and clinical information of the participants is shown in Table 1. The participants had a mean age of 51.56 years and a mean BMI of 24.38. The median scores for symptoms and the duration of kidney disease were 6.25 and 3 years, respectively. A majority of participants were covered by medical insurance (n=398, 97.55%), identified as Han ethnicity (n=382, 93.63%), had attained a high school education or higher (n=208, 50.98%), were married (n=331, 81.13%), unemployed (n=296, 72.55%), and male (n=252, 61.76%). Among them, 65(15.93%) underwent renal replacement therapy, and 183 (44.85%) reported having three or more comorbidities. The majority were in stages 3–5 CKD (n=303, 74.26%).
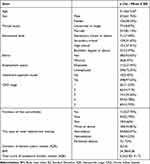 |
Table 1 Demographical and Clinical Characteristics of Participants (n=408) |
Symptom Prevalence, Distress, Severity, and Frequency
Regarding symptom prevalence, blurred vision (n=200, 49.00%) was the most prevalent symptom, followed by foam urine (n=199, 48.80%), and feelings of tired or lack of energy (n=192, 47.10%).
As for symptom distress, light headache or dizziness was the most distressing symptom (average score = 1.69), with arteriovenous fistula/catheterization pain (average score = 1.62) and hiccupping (average score = 1.48) following closely. In terms of symptom severity, the most severe symptom was arteriovenous fistula/catheterization pain (average score = 1.67), followed by coughing (average score = 1.66), and swelling in legs (average score = 1.49). Regarding the frequency of symptoms, the most frequent symptom was diarrhea (average score= 4.71), with changes in stool color (average score = 4.51) and light headache or dizziness (average score = 1.78) also frequently experienced. Detailed symptom characteristics of our sample are given in Table 2.
 |
Table 2 Symptoms of DKD Patients (n=408) |
Summary of Cluster Symptoms
The factor loadings of each symptom and final symptom clusters are presented in Table 3. Through principal component analysis, we identified five distinct symptom clusters. Symptom Cluster A encompasses six symptoms: palpitation, numbness or tingling of hands and feet, bone or joint pain, trouble falling asleep, frequent waking, and feeling anxious. Symptom Cluster B is characterized by constipation, diarrhea, facial oedema, swelling in legs, dry mouth, and dry skin. Sexual dysfunction, defined by decreased interest in sex and difficulty with sexual arousal, constitutes Symptom Cluster C. Symptom Cluster D includes Nausea, muscle cramps, blurred vision, and feelings of being tired or lack of energy. Lastly, Symptom Cluster E comprises urinary symptoms, including 2 symptoms nocturia and foam urine. Itching and decreased appetite were found to have low factor loadings across all clusters, suggesting their classification as individual symptoms rather than part of a cluster.31
 |
Table 3 Factor Loading of Total Symptom Score in DKD Patients (n=408) |
Overall Network
The network models, as depicted in Figures 1, illustrate the relationships among symptoms in the total sample of 408 participants. Notably, decreased interest in sex had a strong connection with difficulty becoming sexually aroused (r = 0.87); foam urine had a strong connection with nocturia (r = 0.50) and a moderate connection with itching coupled with dry skin (r = 0.35); Additionally, facial oedema had a moderate connection with swelling in the legs (r = 0.33), and frequent waking was moderately connected to trouble falling asleep (r = 0.30). There were also weak associations between bone or joint pain and numbness or tingling in the feet (r = 0.29), as well as between facial edema and both diarrhea and constipation (r = 0.23, r = 0.27, r = 0.20), as detailed in Supplementary Table 2.
Centrality analyses, shown in Figure 2 and Supplementary Table 3, revealed that decreased interest in sex (rs = 2.49, rc = 1.77, rb = 0.68) and difficulty becoming sexually aroused (rs = 2.33, rc = 1.77, rb = 1.44) were the most central symptoms based on strength, indicating their significant influence within the symptom network. The result shows that their weights were high in the network, their influence was strong, and they were the most important symptoms in the symptom network.35 Facial oedema (rs = 1.71, rc = 2.09, rb=2.81) had the highest closeness, were located in the center of the symptom network, and had the closest relationship with other symptoms.36 Despite their high prevalence, blurred vision, nocturia, and foam urine had low centrality, suggesting they may act as sentinel symptoms for other conditions.34 The correlation coefficients related to strength consistently exceeded 0.5, affirming the network’s stability (Figure 3). Furthermore, the analysis indicated that the ranking based on strength centrality was more stable compared to the rankings based on closeness and betweenness centrality measures. Bootstrap analysis of edge weights revealed narrow bootstrapped CIs, indicating that the edge weights in the current sample were consistent with those in the bootstrapped sample. This consistency suggests that the network structure is accurate. (Figure 4).
Discussion
This is a first study to demonstrate the symptomatic burden and its associations in Chinese DKD patients. Blurred vision emerged as the most prevalent symptom, while light headache or dizziness was identified as the most distressing, and arteriovenous fistula/catheterization pain as the most severe. The most frequent symptom was diarrhoea. Five symptom clusters were identified. Decreased interest in sex and difficulty becoming sexually aroused were symptoms with high coefficients of the centrality indices. Therefore, sexual dysfunction emerged as the most central symptom cluster within the entire symptom network.
The findings of this study indicate that blurred vision was the most prevalent symptom among DKD patients, aligning with prior research that identifies diabetic retinopathy as a common diabetes complication leading to blurred vision, often associated with diabetes duration, poor glycemic control, and hypertension.37 Additionally, patients undergoing hemodialysis often present with ocular abnormalities, and only a minority have normal vision.38 Light headache or dizziness also emerged as particularly distressing symptoms for DKD patients, potentially linked to depression, anxiety and neuropathy from prolonged DKD.39 Studies have shown that cognitive impairment due to neuropathy could predict diabetic retinopathy progression.40 Furthermore, arteriovenous fistula/catheterization pain was one of the most severe symptoms,41 underscoring its significance in patients with chronic renal failure and diabetes, especially those on hemodialysis.41 Diarrhea was the most frequent symptom, corroborating findings from another study indicating a high prevalence of gastrointestinal symptoms among Chinese diabetes outpatients, with diabetes duration being a key contributing factor.42 Overall, the above-mentioned evidence suggested that healthcare providers need to pay attention to the conscious state and routine vision testing in DKD patients in the future, as well as to the possible important role of pain management in alleviating discomfort and pain.
Our study, employing factor and network analysis, expanded upon previous research by identifying five symptom clusters in DKD patients, significantly enhancing our understanding of their symptomatic experiences. Symptom Cluster A included palpitation, numbness or tingling in feet, bone or joint pain, trouble falling asleep, frequent waking, and feeling anxious, which mainly reflected sleep and emotional problems in DKD patients. Clinical and experimental evidence suggests that sleep apnea, linked to renal injury, may be a covert risk factor for kidney disease progression,43 highlighting the role of sleep disturbances in the worsening of renal function.44 Consistent with previous studies, anxiety and depression are common yet frequently overlooked psychiatric symptom in patients undergoing ESRD,45 and have been shown to be associated with higher morbidity and mortality.46 Our study further stressed the necessity of early guidance to identify sleep and emotional problems in patients with DKD.47 Furthermore, our network analysis revealed moderate correlations among symptoms such as bone or joint pain, numbness or tingling in feet, and sleep disturbances, indicating that the presence of one symptom could help caregivers in identifying related symptoms, thereby improving patient care.
Constipation, diarrhea, facial oedema, swelling in legs, dry mouth, and dry skin were included in Symptom Cluster B, mainly reflecting gastrointestinal and fluid retention symptoms. This is in accordance with previous study displayed that DKD patients may had gastrointestinal symptoms.42 Consistent with research on CKD-related symptom clusters,48 we found that diarrhea and constipation often occur in one symptom cluster, possibly due to a range of factors. For instance, constipation symptoms in patients could potentially be linked to factors such as the presence of uremic toxins and dietary restrictions imposing limitations on fiber-rich foods.49 Moreover, medications like sotagliflozin and Tenapanor, commonly prescribed to Chinese CKD patients, have been noted to contribute to diarrhea.50,51 Additionally, the literature indicates a correlation between edema and DKD,52,53 with fluid retention being associated with adverse renal outcomes,54 potentially due to compensatory ultrafiltration by the remaining nephrons to offset the reduced sodium filtration load.55 Our network analysis showed that facial edema was moderately associated with swelling in legs, and was also related to diarrhea and constipation, suggesting that facial edema may be a predictor of other symptoms, or has more correlation with other symptoms. Thus, it is crucial for healthcare professionals to promptly identify DKD patients with facial edema and implement targeted interventions.
Symptom Cluster C, encompassing decreased interest in sex and difficulty with sexual arousal. This underscores the importance for healthcare professionals to promptly recognize and address sexual dysfunction in DKD patients. As kidney disease management and patient survival rates continue to improve, sexual function should be pursued as the focus of patient-centered care to improve quality of life.56 The impact of sexual dysfunction on quality of life is well recognized, and it has been proved to relate to low self-esteem and confidence, and higher rates of anxiety and depression.57 CKD significantly impacts the body’s hormonal balance, leading to various hormonal disturbances that can affect sexual function.58 In CKD, the kidneys’ diminished ability to filter blood and regulate hormones, such as testosterone in men and estrogen in women, contributes to hormonal imbalances. These imbalances are associated with symptoms like reduced libido, erectile dysfunction in men, and menstrual irregularities in women, directly impacting sexual health and function.59,60 Furthermore, psychological stress and certain antidepressants may exacerbate sexual dysfunction in CKD patients.61,62 Therefore, it is crucial to prioritize the identification and management of diverse factors contributing to sexual dysfunction in these patients.61 Our network analysis identified sexual dysfunction as the most central symptom cluster, indicating its potential association with various symptoms. While the precise mechanisms remain to be fully understood, the significance of addressing sexual health issues, often neglected in clinical practice, cannot be overstated.
Nausea, muscle cramps, blurred vision, and feeling tired or lack of energy were included in Symptom Cluster D. These symptoms may be attributed to retinal neuropathy and neurological changes associated with DKD, as indicated by prior research.40 Muscle cramps, particularly prevalent and painful, not only disrupt sleep and daily activities but also negatively impact the quality of life and dialysis outcomes.63 Literature reports muscle cramp incidence rates of 33–78% in hemodialysis patients and 24–78% in diabetic patients.64,65 In this study, the incidence of muscle cramp in patients with DKD was 12.5%. The connection between DKD and muscle cramps remains incompletely understood. It is hypothesized that one contributing factor is the accumulation of uremic toxins, a result of diminished renal excretion.66 Additionally, complications arising from diabetes mellitus, such as electrolyte imbalances, diabetic neuropathy, and vascular complications, are believed to play a significant role in the development of muscle cramps.67,68 Research suggests that patient-centered symptom management strategies, including incremental dialysis, conservative care, exercise, or dietary modifications (eg, consuming spicy foods to stimulate oropharyngeal reflexes, leading to muscle relaxation), may help alleviate muscle spasms.69 These findings point towards potential management strategies for DKD patients, emphasizing the importance of tailored approaches to symptom management.
Symptom Cluster E encompasses two symptoms: nocturia and foam urine. This finding aligns with a large population-based study indicating that nocturia serves as an independent predictor of albuminuria and is linked to kidney disease.64 Prior research has established a connection between abnormal urination patterns and DKD, potentially due to the increased blood pressure associated with DKD.69 In our study, nocturia and foamy urine were the most prevalent symptoms, yet they exhibited low centrality. This suggests that these symptoms may coexist with or act as predictors for other associated symptoms, highlighting their potential role in the broader symptom network of DKD patients.34
Limitations
This study has several limitations. First, our study only included participants with complete data from a tertiary hospital, potentially limiting the representativeness due to access to specialized care not universally available. Second, the symptoms included in this study were from CKD-SBI, which is not specific for patients with DKD. However, we compensated for this deficiency with the use of a system leveraging the self-reporting of symptoms. Thirdly, our cross-sectional data-based network analysis does not establish causality, requiring theory-supported interpretations for causal inferences. Fourth, since most participants were in stage 3 CKD or higher, we did not differentiate between early and late-stage DKD, highlighting an area for future research to examine symptom associations in patients with distinct disease trajectories or treatment modalities.
Conclusion
This study identified five symptom clusters in Chinese DKD patients, with sexual dysfunction emerging as the most central cluster. These findings carry significant clinical implications, underscoring the necessity of assessing symptom clusters and their interrelations to enhance symptom management in DKD patients. Further research is essential to elucidate the underlying mechanisms of symptoms and to clarify the associations among symptoms in DKD patients across different disease trajectories or treatment modalities.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Biomedical Ethics Review Committee of West China Hospital, Sichuan University (no.2023(1848)). Written informed consent was obtained from all participants.
Funding
The West China Nursing Discipline Development Special Fund Project, Sichuan University, Grant/Award Number: HXHL21013. The Scientific and Technological Department of Sichuan Province, China, Grant/Award Number: 2022YFS0266.
Disclosure
The authors declare they have no competing interests.
References
1. Samsu N. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int. 2021;2021:1497449. doi:10.1155/2021/1497449
2. Guedes M, Pecoits-Filho R. Can we cure diabetic kidney disease? Present and future perspectives from a nephrologist’s point of view. J Internal Med. 2022;291(2):165–180. doi:10.1111/joim.13424
3. Umanath K, Lewis JB. Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis. 2018;71(6):884–895. doi:10.1053/j.ajkd.2017.10.026
4. Zhang XX, Kong J, Yun K. Prevalence of diabetic nephropathy among patients with type 2 diabetes mellitus in China: a meta-analysis of observational studies. J Diabetes Res. 2020;2020:2315607. doi:10.1155/2020/2315607
5. Zhang L, Long J, Jiang W, et al. Trends in Chronic Kidney Disease in China. New Engl J Med. 2016;375(9):905–906. doi:10.1056/NEJMc1602469
6. Thomas B. The global burden of diabetic kidney disease: time trends and gender gaps. Curr Diabetes Rep. 2019;19(4):18. doi:10.1007/s11892-019-1133-6
7. Carney EF. The impact of chronic kidney disease on global health. Nat Rev Nephrol. 2020;16(5):251. doi:10.1038/s41581-020-0268-7
8. Faye M, Legrand K, Le Gall L, et al. Five-year symptom trajectories in nondialysis-dependent CKD patients. Clin J Am Soc Nephrol. 2022;17(11):1588–1597. doi:10.2215/cjn.06140522
9. Ahdoot RS, Kalantar-Zadeh K, Burton JO, Lockwood MB. Novel approach to unpleasant symptom clusters surrounding pruritus in patients with chronic kidney disease and on dialysis therapy. Curr Opin Nephrol Hypertens. 2022;31(1):63–71. doi:10.1097/mnh.0000000000000752
10. Kim H-J, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs. 2005;28:4.
11. Hsu HT, Lin KC, Wu LM, et al. Symptom cluster trajectories during chemotherapy in breast cancer outpatients. J Pain Sympt Manage. 2017;53(6):1017–1025. doi:10.1016/j.jpainsymman.2016.12.354
12. Lockwood MB, Chung S, Puzantian H, et al. Symptom cluster science in chronic kidney disease: a literature review. West J Nurs Res. 2019;41(7):1056–1091. doi:10.1177/0193945918808766
13. Blakeman JR. An integrative review of the theory of unpleasant symptoms. Journal of Advanced Nursing. 2019;75(5):946–961. doi:10.1111/jan.13906
14. Jablonski A. The multidimensional characteristics of symptoms reported by patients on hemodialysis. Nephrol Nurs J. 2007;34(1):29–37; quiz 38.
15. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J clin Oncol. 2016;34(6):557–565. doi:10.1200/jco.2015.63.0830
16. Epskamp S, Fried EI. A tutorial on regularized partial correlation networks. Psychological Methods. 2018;23(4):617–634. doi:10.1037/met0000167
17. Kalantar-Zadeh K, Lockwood MB, Rhee CM, et al. Patient-centred approaches for the management of unpleasant symptoms in kidney disease. Nat Rev Nephrol. 2022;18(3):185–198. doi:10.1038/s41581-021-00518-z
18. Rhee CM, Edwards D, Ahdoot RS, et al. Living well with kidney disease and effective symptom management: consensus conference proceedings. Kidney Int Rep. 2022;7(9):1951–1963. doi:10.1016/j.ekir.2022.06.015
19. Qi C, Mao X, Zhang Z, Wu H. Classification and differential diagnosis of diabetic nephropathy. J Diabetes Res. 2017;2017:8637138. doi:10.1155/2017/8637138
20. Tuttle KR, Agarwal R, Alpers CE, et al. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. 2022;102(2):248–260. doi:10.1016/j.kint.2022.05.012
21. Bonner R, Albajrami O, Hudspeth J, Upadhyay A. Diabetic kidney disease. Primary Care. 2020;47(4):645–659. doi:10.1016/j.pop.2020.08.004
22. You AS, Kalantar SS, Norris KC, et al. Dialysis symptom index burden and symptom clusters in a prospective cohort of dialysis patients. J Nephrol. 2022;35(5):1427–1436. doi:10.1007/s40620-022-01313-0
23. Nephrology EGoCSo. Chinese guidelines for diagnosis and treatment of diabetic kidney disease. Chin J Nephrol. 2021;2:1.
24. Cold S, Cold F, Jensen MB, Cronin-Fenton D, Christiansen P, Ejlertsen B. Systemic or vaginal hormone therapy after early breast cancer: a Danish observational cohort study. J National Cancer Inst. 2022;114(10):1347–1354. doi:10.1093/jnci/djac112
25. Epskamp S, Borsboom D, Fried EI. Estimating psychological networks and their accuracy: a tutorial paper. Behavior Research Methods. 2018;50(1):195–212. doi:10.3758/s13428-017-0862-1
26. Almutary H, Bonner A, Douglas C. Arabic translation, adaptation and modification of the dialysis symptom index for chronic kidney disease stages four and five. BMC Nephrol. 2015;16:36. doi:10.1186/s12882-015-0036-2
27. Aiyegbusi OL, Kyte D, Cockwell P, et al. Measurement properties of patient-reported outcome measures (PROMs) used in adult patients with chronic kidney disease: a systematic review. PLoS One. 2017;12(6):e0179733. doi:10.1371/journal.pone.0179733
28. Ye H, Zalesky A, Lv J, et al. Network analysis of symptom comorbidity in schizophrenia: relationship to illness course and brain white matter microstructure. Schizophrenia Bulletin. 2021;47(4):1156–1167. doi:10.1093/schbul/sbab015
29. Rha SY, Lee J. Stable symptom clusters and evolving symptom networks in relation to chemotherapy cycles. J Pain Sympt Manage. 2021;61(3):544–554. doi:10.1016/j.jpainsymman.2020.08.008
30. Zeng L, Huang H, Qirong C, et al. Multiple myeloma patients undergoing chemotherapy: which symptom clusters impact quality of life? J Clin Nurs. 2023. doi:10.1111/jocn.16791
31. Zhu Z, Hu Y, Xing W, et al. Identifying symptom clusters among people living with HIV on antiretroviral therapy in China: a network analysis. J Pain Sympt Manage. 2019;57(3):617–626. doi:10.1016/j.jpainsymman.2018.11.011
32. Opsahl T, Agneessens F, Skvoretz J. Node centrality in weighted networks: generalizing degree and shortest paths. Soc Network. 2010;32(3):245–251. doi:10.1016/j.socnet.2010.03.006
33. Papachristou N, Barnaghi P, Cooper B, et al. Network analysis of the multidimensional symptom experience of oncology. Sci Rep. 2019;9(1):2258. doi:10.1038/s41598-018-36973-1
34. Zhu Z, Sun Y, Kuang Y, et al. Contemporaneous symptom networks of multidimensional symptom experiences in cancer survivors: a network analysis. Cancer Med. 2023;12(1):663–673. doi:10.1002/cam4.4904
35. Feng ZT, Li X, Zhu H, Yin DQ, Ning YZ, Jia HX. Relationship between Upper-heat and Lower-cold syndrome of generalized anxiety disorder based on hierarchical clustering and complex symptom network. J Cap Med Univ. 2022;1:3.
36. Zeng K, Liang JW, Zhang LL. Identifying core symptoms and symptom clusters in patients during intermittent period of cancer therapy. J Nurs Sci. 2022;2:1.
37. Simó-Servat O, Hernández C, Simó R. Diabetic retinopathy in the context of patients with diabetes. Ophthal Res. 2019;62(4):211–217. doi:10.1159/000499541
38. Herrera Añazco P, Díaz Sánchez MG, Palacios Guillén M, et al. Compromiso ocular en pacientes en hemodialysis X1 - Eye involment in patients undergoin hemodialysis. research-article. Acta Médica Peruana. 2013;30(3):116–119.
39. Roy P, Chandra M, Mishra A, et al. Otological and visual implications of diabetes mellitus in north Indian population. Indian J Otolaryngol Head Neck Surg. 2019;71(Suppl 2):1639–1651. doi:10.1007/s12070-019-01705-y
40. Trento M, Charrier L, Salassa M, et al. Cognitive function may be a predictor of retinopathy progression in patients with type 2 diabetes. Euro J Ophthalmol. 2017;27(3):278–280. doi:10.5301/ejo.5000885
41. Back Y, Lee Y. Optimal time of thermotherapy for reducing pain, anxiety, and side effects in arteriovenous fistula puncture patients: a randomized controlled trial. Int J Environ Res Public Health. 2020;17:19.
42. Ko GT, Chan WB, Chan JC, Tsang LW, Cockram CS. Gastrointestinal symptoms in Chinese patients with Type 2 diabetes mellitus. Diabetic Med. 1999;16(8):670–674. doi:10.1046/j.1464-5491.1999.00135.x
43. Ozkok A, Kanbay A, Odabas AR, Covic A, Kanbay M. Obstructive sleep apnea syndrome and chronic kidney disease: a new cardiorenal risk factor. Ann Clin Exp Hypertens. 2014;36(4):211–216. doi:10.3109/10641963.2013.804546
44. Jhamb M, Ran X, Abdalla H, et al. Association of sleep apnea with mortality in patients with advanced kidney disease. Clin J Am Soc Nephrol. 2020;15(2):182–190. doi:10.2215/cjn.07880719
45. Cohen SD, Cukor D, Kimmel PL. Anxiety in Patients Treated with Hemodialysis. Clin J Am Soc Nephrol. 2016;11(12):2250–2255. doi:10.2215/cjn.02590316
46. Ng HJ, Tan WJ, Mooppil N, Newman S, Griva K. Prevalence and patterns of depression and anxiety in hemodialysis patients: a 12-month prospective study on incident and prevalent populations. Br J Health Psychol. 2015;20(2):374–395. doi:10.1111/bjhp.12106
47. Prabu P, Acree L, Waller JL, et al. Sleep apnea in end-stage renal disease patients: risk factors and mortality. J Invest Med. 2023;71(5):465–470. doi:10.1177/10815589231162541
48. Moore C, Santhakumaran S, Martin GP, et al. Symptom clusters in chronic kidney disease and their association with people’s ability to perform usual activities. PLoS One. 2022;17(3):e0264312. doi:10.1371/journal.pone.0264312
49. Ikee R, Sasaki N, Yasuda T, Fukazawa S. Chronic kidney disease, gut dysbiosis, and constipation: a burdensome triplet. Microorganisms. 2020;8:12.
50. Block GA, Rosenbaum DP, Leonsson-Zachrisson M, et al. Effect of tenapanor on serum phosphate in patients receiving hemodialysis. J Am Soc Nephrol. 2017;28(6):1933–1942. doi:10.1681/ASN.2016080855
51. Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384(2):129–139. doi:10.1056/NEJMoa2030186
52. Goetsch MR, Plott C, Woller JA, et al. Facial swelling and pancytopenia: first features and clues to the etiology of acute kidney injury. Am J Med. 2021;134(10):1238–1241. doi:10.1016/j.amjmed.2021.03.046
53. Wang H, Zhang R, Wu X, et al. The wnt signaling pathway in diabetic nephropathy. Front Cell Develop Biol. 2021;9:701547. doi:10.3389/fcell.2021.701547
54. Hung SC, Kuo KL, Peng CH, Wu CH, Wang YC, Tarng DC. Association of fluid retention with anemia and clinical outcomes among patients with chronic kidney disease. J Am Heart Assoc. 2015;4(1):e001480. doi:10.1161/jaha.114.001480
55. Soi V, Yee J. Sodium homeostasis in chronic kidney disease. Adv Chronic Kidney Dis. 2017;24(5):325–331. doi:10.1053/j.ackd.2017.08.001
56. Chou J, Kiebalo T, Jagiello P, Pawlaczyk K. Multifaceted sexual dysfunction in dialyzing men and women: pathophysiology, diagnostics, and therapeutics. Life. 2021;11:4.
57. Vecchio M, Navaneethan SD, Johnson DW, et al. Interventions for treating sexual dysfunction in patients with chronic kidney disease. Cochrane Database Syst Rev. 2010;12:Cd007747. doi:10.1002/14651858.CD007747.pub2
58. Wang CJ, Cukor D, Johansen KL. Sexual dysfunction among patients with chronic kidney disease. Semin Nephrol. 2021;41(6):534–549. doi:10.1016/j.semnephrol.2021.10.006
59. Ali S, Dave NN. Sexual dysfunction in women with kidney disease. Adv Chronic Kidney Dis. 2020;27(6):506–515. doi:10.1053/j.ackd.2020.07.005
60. Pizzol D, Xiao T, Yang L, et al. Prevalence of erectile dysfunction in patients with chronic kidney disease: a systematic review and meta-analysis. Int J Impot Res. 2021;33(5):508–515. doi:10.1038/s41443-020-0295-8
61. Finkelstein FO, Shirani S, Wuerth D, Finkelstein SH. Therapy Insight: sexual dysfunction in patients with chronic kidney disease. Nat Clin Pract Nephrol. 2007;3(4):200–207. doi:10.1038/ncpneph0438
62. Bostwick JM. A generalist’s guide to treating patients with depression with an emphasis on using side effects to tailor antidepressant therapy. Mayo Clin Proc. 2010;85(6):538–550. doi:10.4065/mcp.2009.0565
63. Kesik G, Altinok Ersoy N. The effect of nonpharmacologic interventions for muscle cramps and restless-leg syndrome in hemodialysis patients: a meta-analysis of randomized controlled trials. Ther Apher Dial. 2023. doi:10.1111/1744-9987.13968
64. Król E, Rutkowski B, Czarniak P, et al. Early detection of chronic kidney disease: results of the PolNef study. Am J Nephrol. 2009;29(3):264–273. doi:10.1159/000158526
65. Afsar B, Elsurer R. Central hemodynamics, vascular stiffness, and nocturia in patients with type 2 diabetes. Renal Failure. 2015;37(10):359–365. doi:10.3109/0886022x.2015.1088335
66. Taguchi K, Fukami K, Elias BC, Brooks CR. Dysbiosis-related advanced glycation endproducts and trimethylamine N-oxide in chronic kidney disease. Toxins. 2021;13(5):361.
67. Ballout RA, Arabi A. Painful and prolonged muscle cramps following insulin injections in a patient with type 2 diabetes mellitus: revisiting the 1992 duke case. Front Endocrinol. 2017;8:1.
68. Roy S. Muscle cramps — a mini review of possible causes and treatment options available with a special emphasis on diabetics — a narrative review. Clin Diabetol. 2020;8:310–317.
69. Kavanagh C. Nephrogenic Diabetes Insipidus. Pediatr Clin N Am. 2019;66(1):227–234. doi:10.1016/j.pcl.2018.09.006
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




