Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Exploring Patterns of COPD Exacerbations and Comorbid Flare-Ups
Authors van Dijk SHB , Brusse-Keizer MGJ , Effing T, van der Valk PDLPM, Ploumen EH, van der Palen J , Doggen CJM , Lenferink A
Received 4 July 2023
Accepted for publication 9 November 2023
Published 16 November 2023 Volume 2023:18 Pages 2633—2644
DOI https://doi.org/10.2147/COPD.S428960
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Sanne HB van Dijk,1,2 Marjolein GJ Brusse-Keizer,1,3 Tanja Effing,4,5 Paul DLPM van der Valk,2 Eline H Ploumen,1,6 Job van der Palen,3,7 Carine JM Doggen,1,8 Anke Lenferink1,2,8
1Health Technology & Services Research, TechMed Centre, University of Twente, Enschede, the Netherlands; 2Department of Pulmonary Medicine, Medisch Spectrum Twente, Enschede, the Netherlands; 3Medical School Twente, Medisch Spectrum Twente, Enschede, the Netherlands; 4College of Medicine and Public Health, School of Medicine, Flinders University, Adelaide, Australia; 5School of Psychology, Faculty of Health and Medical Sciences, University of Adelaide, Adelaide, Australia; 6Department of Cardiology, Medisch Spectrum Twente, Enschede, the Netherlands; 7Cognition, Data & Education, BMS Faculty, University of Twente, Enschede, the Netherlands; 8Clinical Research Centre, Rijnstate Hospital, Arnhem, the Netherlands
Correspondence: Anke Lenferink, Tel +31 534896311, Email [email protected]
Background: Comorbidities are known to complicate disease management in patients with Chronic Obstructive Pulmonary Disease (COPD). This is partly due to lack of insight into the interplay of acute exacerbations of COPD (AECOPD) and comorbid flare-ups. This study aimed to explore patterns of AECOPDs and comorbid flare-ups.
Methods: Data of increased symptoms were extracted from a 12-month daily symptom follow-up database including patients with COPD and comorbidities (chronic heart failure (CHF), anxiety, depression) and transformed to visualizations of AECOPDs and comorbid flare-up patterns over time. Patterns were subsequently categorized using an inductive approach, based on both predominance (ie, which occurs most often) of AECOPDs or comorbid flare-ups, and their simultaneous (ie, simultaneous start in ≥ 50%) occurrence.
Results: We included 48 COPD patients (68 ± 9 years; comorbid CHF: 52%, anxiety: 40%, depression: 38%). In 25 patients with AECOPDs and CHF flare-ups, the following patterns were identified: AECOPDs predominant (n = 14), CHF flare-ups predominant (n = 5), AECOPDs nor CHF flare-ups predominant (n = 6). Of the 24 patients with AECOPDs and anxiety and/or depression flare-ups, anxiety and depression flare-ups occurred simultaneously in 15 patients. In 9 of these 24 patients, anxiety or depression flare-ups were observed independently from each other. In 31 of the included 48 patients, AECOPDs and comorbid flare-ups occurred mostly simultaneously.
Conclusion: Patients with COPD and common comorbidities show a variety of patterns of AECOPDs and comorbid flare-ups. Some patients, however, show repetitive patterns that could potentially be used to improve personalized disease management, if recognized.
Keywords: chronic obstructive pulmonary disease, heart failure, anxiety, depression, personalized medicine, disease monitoring
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a progressive lung disease, characterized by acute exacerbations (AECOPD): periods of suddenly worsened respiratory symptoms requiring additional medication or hospitalization.1 COPD rarely is the only chronic disease a patient is suffering from. To illustrate, up to 68% of patients with COPD suffer from cardiovascular disease, up to 45% from anxiety and/or depression, and up to 20% from diabetes mellitus.2 These comorbidities can severely complicate disease management in patients with COPD. Acute dyspnea, for example, can be a symptom of both an AECOPD and comorbid flare-ups, such as decompensation of chronic heart failure (CHF), or a panic attack.3 Because symptoms are often not COPD-specific and may also indicate worsening of a comorbid disease, it is challenging to offer adequate acute treatment in these patients.4,5
Patients suffering from both COPD and CHF are at increased risk of AECOPDs compared to those with COPD without CHF because up to 26% of the AECOPDs are triggered by cardiovascular disease.6 Both COPD and CHF may require hospitalization and are potentially life-threatening. To illustrate, one in five patients who decease shortly after AECOPD hospitalization die from a cardiovascular rather than respiratory cause.7,8 Moreover, about 8 to 45% of COPD patients suffer from mental health disease,2,9 further increasing the disease burden.1 Also the presence of anxiety and depression increases the risk of AECOPDs, for example by impairing the immune system, reducing medication adherence, and worsening (the perception of) acute symptoms leading to seeking early medical attention.10–12 Not only can mental health diseases impact the course of COPD, but vice versa, COPD may also contribute to anxiety and depression severity.11,13 Pathophysiological pathways for anxiety and depression in COPD are not yet fully understood, but include factors such as smoking, systemic inflammation, and hypoxia.11,13
Although plausible pathophysiological pathways of interaction between COPD and comorbidities have been explored, a lack of knowledge persists into the interplay of AECOPDs and comorbid flare-ups.5,6,11,14,15 In this paper, we aimed to explore multimorbid disease patterns in COPD patients with comorbidities (ie, CHF, anxiety, depression) through visualization of AECOPDs and comorbid flare-ups by means of a secondary analysis using daily symptom data. Visualizing multimorbid patient patterns from daily symptom data is novel and could contribute to personalized disease management by improving the recognition of AECOPD and flare-up patterns. It could thereby facilitate faster and optimal treatment actions in patients with multimorbidity.
Materials and Methods
Study Design & Population
A randomized controlled trial (COPE-III study) was conducted from 2012 to 2016 in two hospitals in the Netherlands and three in South Australia.16,17 The COPE-III study was designed in accordance with the Declaration of Helsinki and was approved by the Medical Ethical Committee Twente (NL39516.044.12) and the Southern Adelaide Clinical Human Research Ethics Committee (37-12). Participants’ written informed consent was obtained prior to data collection. For inclusion in the COPE-III study, patients needed to have a clinical diagnosis of COPD GOLD stage II–IV1 and at least one comorbidity (ie, CHF, ischemic heart disease (IHD), symptomatic anxiety/depression, diabetes). Detailed in- and exclusion criteria are described in the COPE-III protocol.16 The COPE-III study investigated the effectiveness of a self-management exacerbation action plan. For this purpose, patients kept a multimorbid symptom diary during a 12-month follow-up.5 Each day, patients were asked whether their symptoms in the last 24 hours (eg, sputum color for COPD, sudden weight gain for CHF) were not, slightly, or significantly increased compared to usual (ie, symptoms they experience during stable phase). An overview of the diary including all symptoms can be retrieved from the online supplement of the COPE-III study.17 All patients received training on how to fill in their daily symptom diaries.
For the current analysis, we only used the symptom data of COPE-III patients who had: 1) comorbid CHF (ie, symptoms and signs typical of heart failure and objective evidence of a structural or functional abnormality of the heart at rest),18 and/or comorbid anxiety (ie, ≥ 11 Hospital Anxiety and Depression Scale (HADS), anxiety domain19 and/or having anxiety symptoms that are being treated) and/or comorbid depression (ie, ≥ 11 HADS, depression domain19 and/or having depression symptoms that are being treated); and 2) at least one AECOPD and at least one flare-up of CHF, anxiety, and/or depression during the one-year follow-up period. COPE-III patients with only comorbid IHD and/or diabetes were, thus, excluded.
Definitions
Based on the change in daily symptoms, AECOPDs and comorbid flare-ups were identified and extracted from the daily symptom diaries. Definitions detailing the start and end of AECOPDs and comorbid flare-ups are described in Table 1.
 |
Table 1 Definitions of COPD Exacerbations and Comorbid Flare-Upsa |
Data Processing and Analysis
The data processing (including visualizations) and statistical analyses were conducted with the statistical software R version 4.2.2. Patient baseline data and follow-up data (eg, number of (respiratory-related) hospitalizations, deaths) were analyzed by computing the mean with standard deviation for parametric continuous data, the median with interquartile range in case of non-parametric continuous data, or numbers and percentages in case of discrete data. Exacerbation and flare-up rates were calculated as per person-year (ie, total number of days patients participated in the COPE-III study divided by 365 days). Daily symptom data were used to identify the start and end of AECOPDs and flare-ups of CHF, anxiety, and depression. The per-patient visualizations of AECOPDs and comorbid flare-ups during one year of follow-up were subsequently computed on a timeline following the definitions described in Table 1. Based on the change in daily symptoms, AECOPDs and comorbid flare-ups were identified, starting from the day of inclusion (day 1).
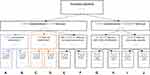
Given the explorative aim of our study, a so-called “inductive” categorization approach was taken, as this approach is considered most appropriate in exploratory, qualitative research to describe the results at a group level.22 After visualizing all patient patterns over time, these were studied by three authors (SvD, MBK, AL). Based on all patterns observed, the three authors independently drafted a flowchart to categorize all patterns based on: 1a) predominance of either AECOPDs or comorbid CHF flare-ups (ie, which is most frequently (≥ 50%) observed), or 1b) simultaneous occurrence of flare-ups of anxiety and depression (ie, whether they most frequently (≥ 50%) start simultaneously), and 2) simultaneous occurrence of AECOPD and comorbid flare-ups of CHF, anxiety or depression. The three flowchart drafts were then used to decide on a final flowchart (Figure 1) by means of a discussion between the three authors. In the next step, the authors independently assigned all observed patterns to categories using the final flowchart. If there were discrepancies between the authors, the final decision regarding the category assignment was made through discussion.
Results
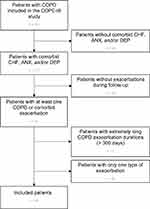
The patient inclusion for the current analyses is presented in Figure 2. Out of the 201 COPE-III patients,17 92 patients had at least one AECOPD or one comorbid flare-up. Two of these patients had extreme long AECOPDs (>300 days) and were excluded from further analyses. A further 42 patients were excluded, because they had only AECOPDs (n = 40) or experienced only one flare-up of CHF (n = 1) or depression (n = 1) during the one-year follow-up. Baseline characteristics of the 48 included patients are detailed in Table 2. The mean age was 68.2 years and 60.4% were male. There were 25 (52.1%) patients with COPD and comorbid CHF, 19 (39.6%) with anxiety, and 18 (37.5%) with depression. The comorbidities and their co-existence at baseline are shown in Figure 3.
 |
Table 2 Baseline Characteristics of 48 Included Patients with COPD |
From the 48 included patients, 37 completed follow-up, 5 patients died, and 6 patients were lost to follow-up (too heavily deteriorated health (n = 2), lung cancer (n = 1), palliative care for terminal CHF (n = 1), other reason (n = 2)). An average of 5.3 AECOPDs, 4.3 CHF flare-ups, 2.0 anxiety flare-ups, and 1.7 depression flare-ups were observed per person-year. A summary of the follow-up information of all included patients is provided in Table 3.
 |
Table 3 Exacerbations of COPD, Comorbid Flare-Ups, Hospitalizations and Deaths and Their Rates per Person-Yeara During Follow-Up |
Figure 1 displays the flowchart with which patient follow-up visualizations were categorized. Eventually, patients were divided over ten sub-categories (Figure 1). Twenty-five of the 48 patients (52%) experienced both AECOPD and flare-ups of CHF during follow-up. A combination of AECOPDs and flare-ups of anxiety and/or depression was observed in 24 patients (50%). Figure 4 presents patient pattern examples for each of the ten defined sub-categories. All 48 visualized patient patterns are presented in the e-Appendix. One patient was assigned to two categories as this patient had two AECOPDs that both started simultaneously with flare-ups of CHF, anxiety, and depression (Figure 4K).
 |
Figure 4 Examples of 11 patterns of acute exacerbations of COPD and comorbid flare-ups. (A) COPD exacerbations predominant – AECOPDs & CHF flare-ups start mostly simultaneous; (B) COPD exacerbations predominant – AECOPDs & CHF flare-ups start mostly separate; (C) CHF flare-ups predominant – AECOPDs & CHF flare-ups start mostly simultaneous; (D) CHF flare-ups predominant – AECOPDs & CHF flare-ups start mostly separate; (E) AECOPDs/CHF flare-ups not clearly predominant – AECOPDs & CHF flare-ups start mostly simultaneous; (F) AECOPDs/CHF flare-ups not clearly predominant – AECOPDs & CHF flare-ups start mostly separate; (G) ANX & DEP flare-ups start mostly simultaneous – AECOPDs & ANX&DEP flare-ups start mostly simultaneous; (H) ANX & DEP flare-ups start mostly simultaneous – AECOPDs & ANX&DEP flare-ups start mostly separate; (I) ANX/DEP flare-ups start mostly separate – AECOPDs & ANX&DEP flare-ups start mostly simultaneous; (J) ANX/DEP flare-ups start mostly separate – AECOPDs & ANX&DEP flare-ups start mostly separate; (K) Due to the presence of AECOPD and flare-ups of CHF, ANX and DEP, patient 11 (K) was assigned to two groups: 1) “COPD exacerbations + CHF flare-ups – AECOPDs/CHF flare-ups not clearly predominant – AECOPDs & CHF flare-ups start mostly simultaneous” (Figure 1C), 2) “COPD exacerbations + ANX/DEP flare-ups – ANX & DEP flare-ups start mostly simultaneous – AECOPDs & ANX&DEP flare-ups start mostly simultaneous” (Figure 1G). Abbreviations: COPD, Chronic Obstructive Pulmonary Disease; CHF, Chronic Heart Failure; ANX, anxiety; DEP, depression; AECOPD, Acute Exacerbation of COPD. |
AECOPDs and Flare-Ups of CHF
In the 25 patients who experienced both AECOPDs and CHF flare-ups (Figure 1, bottom left), AECOPDs were more frequently present than CHF flare-ups (ie, AECOPDs were predominant over CHF flare-ups) in 14 patients (56%). In this subgroup of 14 patients, CHF flare-ups occurred simultaneously with AECOPDs in eight patients (Figure 4A), and separately from AECOPDs in six (Figure 4B). In patients in whom CHF flare-ups were predominant (n = 5; 20%), CHF flare-ups occurred simultaneously with AECOPDs in four patients (Figure 4C), and separately from AECOPDs in one (Figure 4D). In patients with no AECOPD or CHF flare-up predominance (n = 6; 24%), AECOPDs and CHF flare-ups occurred simultaneously in three patients (Figure 4E) and separately in three patients (Figure 4F).
AECOPDs and Flare-Ups of Anxiety and/or Depression
In the 24 patients with both AECOPDs and flare-ups of anxiety and/or depression (Figure 1), the majority of the flare-ups of anxiety and depression occurred simultaneously (15 patients; 63%). In 13 of these 15 patients, anxiety and depression flare-ups started simultaneously with AECOPDs (Figure 4G), and separately from AECOPDs in two patients (Figure 4H). From those in whom flare-ups of anxiety and depression started separately from each other (n = 9; 38%), one of these flare-ups started simultaneously with AECOPDs in three patients (Figure 4I) and at different points in time from AECOPDs in six patients (Figure 4J).
Discussion
In this study, patients’ AECOPD and comorbid flare-up patterns were explored by means of visualizations using patient-reported daily symptom data. A large variety in multimorbid patterns was observed across patients. In patients with COPD and comorbidities, AECOPDs and comorbid flare-ups regularly occur simultaneously, but certainly not always. These insights will probably be recognized by many caregivers from daily practice, where an AECOPD will or will not be accompanied by decompensated CHF23 or episodes of worsened anxiety and/or depression.24–26
In patients with both AECOPDs and CHF flare-ups, we identified patients in whom either AECOPDs or flare-ups of CHF were predominantly present during follow-up, as well as patients in whom neither of the two were predominantly present. Differences in severity between the two diseases may explain these differences in predominance, given that more severe COPD or CHF increase the risk of AECOPDs27 or decompensated CHF,28,29 respectively. In the majority of patients who showed patterns containing both AECOPDs and CHF flare-ups, these occurred repeatedly simultaneously. Several pathophysiological mechanisms underlying this interplay are known.6 For example, due to CHF, oxygen transport becomes inefficient as congestion builds up in the lungs, causing and/or intensifying acute COPD symptoms.6 Vice versa, an AECOPD may trigger decompensated CHF: respiratory infections, often underlying an AECOPD, are also associated with decompensated CHF in 10 to 16% of the hospital admissions.30 Furthermore, AECOPD increases the airflow obstruction, causing hypercapnia potentially leading to oxygen insufficiency for the heart.14,31 Several researchers, therefore, speak of a cardiopulmonary continuum rather than of COPD and CHF as two separate diseases.31,32 Our observation that many patients having simultaneous AECOPDs and CHF flare-ups, seems to be in line with this statement. In some patients, however, AECOPDs seem not to relate to CHF flare-ups, indicating that distinct mechanisms may be driving deterioration of COPD or CHF in some patients.
Notably, flare-ups of anxiety and depression most frequently occurred together in our study. This is in line with research showing that symptoms of anxiety and depression do not emerge isolated very often.33,34 Flare-ups of anxiety and depression were mainly observed simultaneously with AECOPDs. Although pathways of increased symptoms of mental diseases and COPD are not yet fully understood, three mechanisms could explain this simultaneous occurrence. First, AECOPDs reduce the patients’ sense of control of their health and, consequently, increase symptoms of anxiety and/or depression.12 Second, patients with anxiety and/or depression may have a worsened perception of acute symptoms, given that their physiological parameters are less severe when hospitalized compared with patients without comorbid anxiety or depression.11 They may, therefore, be more prone to being hospitalized for less severe or misdiagnosed AECOPDs.11 Regardless of perception, anxiety and depression can also contribute to actual increased dyspnea.12 Third, symptomatic depression may directly impair the immune system, which increases the risk of respiratory infections and, consequently, AECOPDs.10,11 Since patients with comorbid mental diseases recover relatively slowly after an AECOPD,26 addressing mental health issues when offering acute support is important in addition to treatment of AECOPD.25,35
The wide variety of patterns observed in this study argue for personalized, but more importantly multidisciplinary, disease management in COPD patients with multimorbidity. For instance, if within-patient patterns were recognized in usual clinical practice, these could be communicated to patients. Providing feedback about the disease trajectory would empower patients in timely recognizing their need for care by increased symptoms and taking appropriate action (eg, contact caregiver, self-treatment, relaxation exercises).36 Furthermore, by sharing multimorbid disease patterns, patients could gain more insight into their multimorbid disease, which may improve medication adherence, prevent hospital admissions, and ultimately benefit the patient’s health.36
A “one size fits all” care plan is certainly inappropriate, as our results have confirmed once again that “no single person-centered care plan could or should be the same”.37 It is about time that multidisciplinary approaches will actually be used for these patients, something that is already advised,2,3 but rarely implemented in clinical practice.38 For instance, the fact that departments of pulmonology and cardiology often function as two separate disciplines is not in line with the fact that the lungs and heart are inseparable. Whereas it will require additional effort from clinicians to work beyond the boundaries of one’s own discipline, ignoring the cardiopulmonary interplay is costly, for the patient’s health as well as for societal economic burden.39
Our study has several strengths. First and foremost, our study is unique in visualizing patterns of AECOPDs and comorbid flare-ups based on daily patient-reported symptom data. These can be generalized to the large group of patients with COPD with comorbidities and are typically treated in practice. Second, the categorization of individual patient patterns into ten categories has been conducted by three independent researchers in a systematic and robust way. An inductive approach was chosen (ie, the categories were derived from the patterns observed), which is considered most suitable for exploratory purposes.22 Third, the symptom diaries were designed to minimize recall bias: symptoms were prospectively and daily collected, extra information was only asked if symptoms had worsened, and patients were extensively trained in keeping the diary.40 In addition, the COPD exacerbations and comorbid flare-ups were defined based on a combination of symptoms,17 which gives detailed day-to-day information compared to a definition based on pharmacologic treatment only. Fourth, 37 of 48 included patients completed the one-year follow-up, resulting in a mean follow-up of 302 days, reflecting a high compliance rate (82.7%) comparable to the original COPE-III study.17 Finally, using diaries instead of face-to-face symptom reporting, may have given a feeling of safety to report mental health symptoms, whereas the possibility that anxiety is underlying to increased dyspnea is likely frequently ignored in the clinic because respiratory symptoms are often attributed to an AECOPD only.25
Our study also has several limitations. First, next to CHF, anxiety, and depression, we could have taken other comorbidities into account, such as IHD, of which symptoms were collected during the COPE-III study. However, an increase in at least one of the IHD symptoms was defined as an event, lasting only one day, rather than as a longer period of time with an on- and offset. Combined with the fact that these events were defined based on unspecific symptoms, justifiably interpreting IHD events in our study would have been difficult. Second, the fact that comorbid flare-ups are difficult to differentiate from AECOPDs based on increased symptoms,4,30 potentially meant that patients had difficulty reporting disease-specific symptoms, which may have influenced our data. Nonetheless, in patients who show AECOPDs and comorbid flare-ups, these certainly do not always occur simultaneously, as shown by our results. Thus, to a considerable extent, distinct AECOPDs and comorbid flare-ups could be identified based on symptoms reported by patients when properly trained in completing daily symptoms diaries (ie, at least two sessions including coaching on how to recognize and differentiate increased symptoms compared to stable state16). Third, whereas we advocate for personalized and multidisciplinary care, our categorization was partly based on distinct disease domains. However, the derived categories should not be seen as the main results of the study, but rather as a tool to summarize this study’s results in order to draw conclusions on a group-level.
Our study provides novel insights into the interplay of AECOPDs and comorbid flare-ups, but future research should validate our findings. A considerable addition would be to look at consistent repetition of patterns within patients over a longer period of follow-up, after which treatment approaches could be developed taking these patterns into account. What such a new treatment approach should include and how AECOPD and comorbid flare-up pattern visualizations can be of additional value would be an important research question. Another essential next step would be to investigate the interplay of COPD with comorbidities in terms of timing: what comes first, and potentially triggers the other? Also, are daily symptom diaries sensitive enough to measure this, or should alternative measures be considered? It has become clear from our results that AECOPDs frequently occur simultaneously with comorbid flare-ups, so pathophysiological mechanisms underlying this interplay also require further research.
Conclusion
This study revealed that patients with COPD and common comorbidities show a variety of AECOPD and comorbid flare-up patterns. If repetitive within-patient patterns of AECOPD and comorbid flare-ups would be recognized in clinical practice, personalized and multidisciplinary disease management could be optimized, which could ultimately lead to improved health outcomes. Future research should focus on validating these patterns in patients with multimorbidity over a longer period of follow-up.
Abbreviations
AECOPD, Acute Exacerbation of Chronic Obstructive Pulmonary Disease; CHF, Chronic Heart Failure; COPD, Chronic Obstructive Pulmonary Disease; HADS, Hospital Anxiety and Depression Scale; IHD, Ischemic Heart Disease.
Data Sharing Statement
Individual multimorbid patterns are shared in this paper’s Supplementary Table 1. Detailed deidentified patient data are stored in archived datasets for a total of 15 years and can be requested from the corresponding author Anke Lenferink. The R coding used to visualize the patterns can also be requested from the corresponding author.
Ethics Approval
This study was approved by the Medical Ethical Committee Twente and the Flinders Southern Adelaide Clinical Human Research Ethics Committee, and was registered in the public Australian New Zealand Clinical Trials Registry (ACTRN12612000514808).
Acknowledgments
We would like to thank Martijn Oude Wolcherink (University of Twente) for his assistance in the data wrangling, Karel Kroeze (University of Twente) for his assistance in the data visualizations, and Meike Seuntiëns (University of Twente) for her related work during her internship, which provided us some initial ideas. In addition, we would like to thank all patients and people who have made data collection of the COPE-III study possible, that is, the disease-experts in respiratory, cardiac, and mental health diseases, nurses, data managers in the participating hospitals (The Netherlands: Medisch Spectrum Twente in Enschede, Canisius Wilhelmina Ziekenhuis in Nijmegen; Australia (Adelaide): Repatriation General Hospital, Royal Adelaide Hospital, Flinders Medical Centre), and the members of the Data and Safety Monitoring Board.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; drafted, revised or critically reviewed the article; gave approval of the final version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The COPE-III study was supported by Stichting Astma Bestrijding, the Lung Foundation Netherlands (grant number 3.4.11.061), Lung Foundation Australia (Australian Lung Foundation Boehringer Ingelheim COPD Research Fellowship 2010), Repat Foundation, and GlaxoSmithKline (unrestricted grant). This work was supported by the European Union’s Horizon 2020 research and innovation program (grant number 965315).
Disclosure
The authors report no conflicts of interest in this work.
References
1. GOLD comittee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 report); 2022.
2. Hillas G, Perlikos F, Tsiligianni I, Tzanakis N. Managing comorbidities in COPD. Int J COPD. 2015;10:95–109.
3. Vanfleteren LEGW, Fabbri LM. Self-management interventions in COPD patients with multimorbidity. Eur Respir J. 2019;54(5):1901850. doi:10.1183/13993003.01850-2019
4. Yohannes AM, Willgoss TG, Baldwin RC, Connolly MJ. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: prevalence, relevance, clinical implications and management principles. Int J Geriatr Psychiatry. 2010;25(12):1209–1221. doi:10.1002/gps.2463
5. Effing T, Lenferink A. Self-management: personalized action plans for COPD exacerbations. In: Enhancing Patient Engagement in Pulmonary Healthcare. The Art and Science; 2020:205–230.
6. Axson EL, Bottle A, Cowie MR, Quint JK. Relationship between heart failure and the risk of acute exacerbation of COPD. Thorax. 2021;76(8):807–814. doi:10.1136/thoraxjnl-2020-216390
7. Gudmundsson G, Ulrik CS, Gislason T, et al. Long-term survival in patients hospitalized for chronic obstructive pulmonary disease: a prospective observational study in the Nordic countries. Int J COPD. 2012;7:571–576.
8. Tinè M, Bazzan E, Semenzato U, et al. Heart failure is highly prevalent and difficult to diagnose in severe exacerbations of COPD presenting to the emergency department. J Clin Med. 2020;9(8):1–12. doi:10.3390/jcm9082644
9. Dos Santos NC, Miravitlles M, Camelier AA, De Almeida VD, Maciel RR, Camelier FWR. Prevalence and impact of comorbidities in individuals with COPD: a systematic review. Tuberc Respir Dis. 2022;85(3):205–220. doi:10.4046/trd.2021.0179
10. Laurin C, Moullec G, Bacon SL, Lavoie KL. Impact of anxiety and depression on chronic obstructive pulmonary disease exacerbation risk. Am J Respir Crit Care Med. 2012;185(9):918–923. doi:10.1164/rccm.201105-0939PP
11. Pumar MI, Gray CR, Walsh JR, Yang IA, Rolls TA, Ward DL. Anxiety and depression-important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615–1631. doi:10.3978/j.issn.2072-1439.2014.09.28
12. Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J COPD. 2014;9:315–330. doi:10.2147/COPD.S53255
13. Hill K, Geist R, Goldstein RS, Lacasse Y. Anxiety and depression in end-stage COPD. Eur Respir J. 2008;31(3):667–677. doi:10.1183/09031936.00125707
14. MacDonald MI, Shafuddin E, King PT, Chang CL, Bardin PG, Hancox RJ. Cardiac dysfunction during exacerbations of chronic obstructive pulmonary disease. Lancet Respir Med. 2016;4(2):138–148. doi:10.1016/S2213-2600(15)00509-3
15. Sapey E, Bafadhel M, Bolton CE, Wilkinson T, Hurst JR, Quint JK. Building toolkits for COPD exacerbations: lessons from the past and present. Thorax. 2019;74(9):898–905. doi:10.1136/thoraxjnl-2018-213035
16. Lenferink A, Frith P, van der Valk P, et al. A self-management approach using self-initiated action plans for symptoms with ongoing nurse support in patients with Chronic Obstructive Pulmonary Disease (COPD) and comorbidities: the COPE-III study protocol. Contemp Clin Trials. 2013;36(1):81–89. doi:10.1016/j.cct.2013.06.003
17. Lenferink A, van der Palen J, van der Valk PDLPM, et al. Exacerbation action plans for patients with COPD and comorbidities: a randomised controlled trial. Eur Respir J. 2019;54(5):1802134. doi:10.1183/13993003.02134-2018
18. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi:10.1093/eurheartj/ehab368
19. Zigmond AS, Snalth RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
20. Anthonisen NR, Manfreda J, Warren CPW, Hershfield E, Harding GKM, Nelson NA. Antibiotic therapy in acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196–204. doi:10.7326/0003-4819-106-2-196
21. Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest. 2000;117(5 Suppl 2):398S–401S. doi:10.1378/chest.117.5_suppl_2.398S
22. Thomas DR. A general inductive approach for analyzing qualitative evaluation data. Am J Eval. 2006;27(2):237–246. doi:10.1177/1098214005283748
23. Leong P, MacDonald MI, King PT, et al. Treatable cardiac disease in hospitalised COPD exacerbations. ERJ Open Res. 2021;7(1):00756–2020. doi:10.1183/23120541.00756-2020
24. Hill K, Goldstein RS, Guyatt GH, et al. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ. 2010;182(7):673–678. doi:10.1503/cmaj.091784
25. Marsh S, Guck TP. Anxiety and depression: easing the burden in COPD patients. J Fam Pract. 2016;65(4):246–256.
26. Wen-tao D, Xue-xiu C, Zun-jiang C, Wei C, Cheng-feng P, Xing-ken F. The relationship between hospitalization frequency of acute exacerbation of chronic obstructive pulmonary disease and anxiety and depression. Front Genet. 2022;13:817727. doi:10.3389/fgene.2022.817727
27. Hogea SP, Tudorache E, Fildan AP, Fira-Mladinescu O, Marc M, Oancea C. Risk factors of chronic obstructive pulmonary disease exacerbations. Clin Respir J. 2020;14(3):183–197. doi:10.1111/crj.13129
28. Angaran P, Dorian P, Ha AC, et al. Association of left ventricular ejection fraction with mortality and hospitalizations. J Am Soc Echocardiogr. 2020;33(7):802–811. doi:10.1016/j.echo.2019.12.016
29. Ahmed A, Aronow WS, Fleg JL. Higher New York Heart association classes and increased mortality and hospitalization in heart failure patients with preserved left ventricular function. Bone. 2006;151(2):444–450.
30. Hawkins NM, Petrie MC, Jhund PS, Chalmers GW, Dunn FG, McMurray JJV. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail. 2009;11(2):130–139. doi:10.1093/eurjhf/hfn013
31. Čelutkienė J, Balčiūnas M, Kablučko D, Vaitkevičiūte L, Vaitkevičiūte JB, Danila E. Challenges of treating acute heart failure in patients with Chronic Obstructive Pulmonary Disease. Card Fail Rev. 2017;3(1):56. doi:10.15420/cfr.2016:23:2
32. Ukena C, Mahfoud F, Kindermann M, et al. The cardiopulmonary continuum systemic inflammation as “common soil” of heart and lung disease. Int J Cardiol. 2010;145(2):172–176. doi:10.1016/j.ijcard.2010.04.082
33. Kalin NH. The critical relationship between anxiety and depression. Am J Psychiatry. 2020;177(5):365–367. doi:10.1176/appi.ajp.2020.20030305
34. McElroy E, Fearon P, Belsky J, Fonagy P, Patalay P. Networks of depression and anxiety symptoms across development. J Am Acad Child Adolesc Psychiatry. 2018;57(12):964–973. doi:10.1016/j.jaac.2018.05.027
35. Cafarella PA, Effing TW, Usmani ZA, Frith PA. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17(4):627–638. doi:10.1111/j.1440-1843.2012.02148.x
36. Powers TL, Bendall D. Improving health outcomes through patient empowerment. J Hosp Mark Public Relat. 2003;15(1):45–59. doi:10.1300/J375v15n01_05
37. Kaehne A. Care integration – from “one size fits all” to person centred care: comment on “achieving integrated care for older people: shuffling the deckchairs or making the system watertight for the future? Int J Health Policy Manag. 2018;7(10):955–957. doi:10.15171/ijhpm.2018.51
38. Rabe KF, Hurst JR, Suissa S. Cardiovascular disease and COPD: dangerous liaisons? Eur Respir Rev. 2018;27(149):180057. doi:10.1183/16000617.0057-2018
39. Hawkins NM, Virani S, Ceconi C. Heart failure and chronic obstructive pulmonary disease: the challenges facing physicians and health services. Eur Heart J. 2013;34(36):2795–2803. doi:10.1093/eurheartj/eht192
40. Leidy NK, Sexton CC, Jones PW, et al. Measuring respiratory symptoms in clinical trials of COPD: reliability and validity of a daily diary. Thorax. 2014;69(5):443–449. doi:10.1136/thoraxjnl-2013-204428
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.