Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Exploration of the Risk Factors of Anemia in Patients with Tuberculous Meningitis in South China
Authors Wen A , Leng EL, Cao WF, Xiang ZB, Rao W, Cai W, Zhou YL, Hu F, Wu LF, Zhang P, Liu SM
Received 7 October 2022
Accepted for publication 14 January 2023
Published 16 February 2023 Volume 2023:19 Pages 369—377
DOI https://doi.org/10.2147/NDT.S391751
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
An Wen,1,2 Er-Ling Leng,3 Wen-Feng Cao,1,2 Zheng-bing Xiang,1,2 Wei Rao,1,2 Wen Cai,1,2 Yong-Liang Zhou,1,2 Fan Hu,1,2 Ling-feng Wu,1,2 Ping Zhang,1,2 Shi-Min Liu1,2
1Department of Neurology, Jiangxi Provincial People’s Hospital (The First Affiliated Hospital of Nanchang Medical College), Nanchang, People’s Republic of China; 2Institution of Neurology, Jiangxi Provincial People’s Hospital (The First Affiliated Hospital of Nanchang Medical College), Nanchang, People’s Republic of China; 3Department of Pediatrics, Jiangxi provincial People’s Hospital (The First Affiliated Hospital of Nanchang Medical College), Nanchang, People’s Republic of China
Correspondence: Shi-Min Liu, Department of Neurology, Jiangxi provincial People’s Hospital (The First Affiliated Hospital of Nanchang Medical College), No. 92 Aiguo Road, Nanchang, 330006, People’s Republic of China, Tel +791-8772-1401, Email [email protected]
Objective: Central nervous system (CNS) infection has a high incidence and mortality worldwide. Tuberculous meningitis (TBM) accounts for approximately 5– 6% of all extrapulmonary tuberculosis (TB), and is considered an extremely lethal form of CNS TB, which has become an important threat to human health. Anemia is a common symptom of TB, and its prevalence is generally higher in patients with TBM than in other meningitis patients and healthy individuals. Anemia can increase a person’s susceptibility to common infectious diseases, including TB, by compromising the immune system. Information regarding anemia during the hospitalization of TBM is still scarce in China. This study aimed to describe in detail the prevalence of anemia in patients with TBM in Southern China and its association with the clinical forms of TB, as well as other characteristics of these patients.
Methods: We conducted a retrospective analysis of patients diagnosed with TBM at two tertiary hospitals in southern China. The demographic characteristics, clinical characteristics, and laboratory results of 114 patients with TBM were collected. Multivariate logistic regression analysis was performed to explore the risk factors for anemia in patients with TBM.
Results: Electronic medical record data of adult patients diagnosed with TBM from January 2004 to December 2019 were reviewed. Among 134 patients with TBM, 20 were excluded and 114 were analyzed, of whom 33 had anemic, the prevalence rate of anemia was 28.9%. Among patients with anemia, 51.5% had hypochromic microcytic anemia, 33.3% had normochromic normocytic anemia, and 15.2% had macrocytic anemia. Fever duration, TBM grade III and ESR were found to be independent predictors of anemia.
Conclusion: Anemia was highly prevalent in patients with TBM, mainly hypochromic microcytic anemia. Besides, Fever duration, TBM grade III and ESR are predictors of anemia in patients with TBM.
Keywords: tuberculosis, tuberculous meningitis, anemia, risk factors, prediction
Introduction
In 2019, an estimated 10 million people developed tuberculosis (TB) and 1.4 million died, according to the World Health Organization (WHO) Global Tuberculosis Report 2020. TB is the most common cause of death due to a single infectious agent.1 China is a country with a high TB burden, accounting for 8.4% of new TB cases worldwide.1 Tuberculous meningitis (TBM) is a serious life-threatening presentation of extrapulmonary TB caused by Mycobacterium tuberculosis (MTB), with high rates of mortality and disability, especially among people living with HIV and children.2,3
Anemia is a common clinical complication of TB, besides, its incidence varies from region to region and population to population, ranging from 9.5% to 96%.4 Anemia threatens hundreds of millions of people worldwide, impairing patients’ health and compromising social and economic development. A recent study found that people with TB seem to be more likely to develop anemia than healthy controls and those with TB in close contact.5 Anemia is associated with a higher risk of death and a poor outcomes in TB patients.6–8 It is now thought that hemoglobin (Hb) levels are lower in patients with acid-fast bacilli (AFB) smear-positive tests, delaying TB diagnosis and making anemia more likely.9 TB is a common disease in China. However, to date, no study has yet assessed the incidence of anemia in patients with TBM and its associated factors. Therefore, we attempted to describe the prevalence of anemia and its associated risk factors in patients with TBM in southern China.
Materials and Methods
Study Design and Patient Recruitment
A retrospective study was conducted between January 2004 and December 2019 at Jiangxi Provincial People’s Hospital and Infectious Diseases Hospital of Jiangxi Province; consecutive patients (≥16 years) with a suspected diagnosis of TBM were included. All procedures in this study were performed in accordance with the ethical standards of the 1964 Helsinki declaration and its amendments or comparable ethical standards in Jiangxi provincial People’s Hospital. Patient’s consent was waived due to the retrospective design of the study. Patients’ data confidentialities were maintained in Jiangxi provincial People’s Hospital. This study was approved by the Ethics Committee of Jiangxi Provincial People’s Hospital (NO.2022038). The exclusion criteria were as follows: prior treatment for TB; patients treated with anti-TB drugs for more than one week; patients with HIV, chronic liver disease, chronic renal disease, chronic aplastic anemia, autoimmune disease, and pregnancy/ breast feeding. Patients with missing data and those with other intracranial pathogen infectious were also excluded. Clinical assessment and neurological staging were performed in patients with TBM using the British Medical Research Council (BMRC) criteria.10 Clinical characteristics, laboratory findings (blood indicators, CSF tests), and radiographic findings (chest X-ray [CXR], CT, and/or MRI) results from patients were collected and analyzed.
Definitions
Diagnostic Criteria for TBM
Based on the clinical case definition, including clinical, radiological, cerebrospinal fluid (CSF) standards, and other evidence of TB outside the central nervous system (CNS). Thwaites et al identified a diagnostic scoring system for TBM and used the classifications “definite”, “probable”, and “possible” (Table 1).11 A confirmed case of TBM can be defined if the presence of MTB in the CSF is detected by standard Ziehl–Neelsen staining, CSF culture, or nucleic acid amplification tests (NAATs). According to the latest Lancet consensus scoring system,11 those with a total score ≥12 in the presence of neuroimaging and those with a total score ≥10 in the absence of neuroimaging can be classified as probable TBM. Similarly, when neuroimaging is available, the total score is 6–11; when there is no neuroimaging, the total score is 6–9, which can be classified as possible TBM.
 |
Table 1 Diagnostic Criterias and Severity Grades of Tuberculous Meningitis (TBM) |
TBM Grading Standards
The severity of TBM at admission was assessed using the modified BMRC criteria (Table 1).10 Namely: Stage I was defined as a GCS of 15 points without focal neurological deficits; stage II was defined as a GCS of 15 points with neurological deficits or a GCS of 11–14 points; stage III was defined as a GCS of ≤10 points.12 And these variables also proved to be strong predictors of death.
Definition of Anemia
According to the Guidelines of the WHO, the standard definition of anemia is hemoglobin (Hb) <13 g/dL in men and <12 g/dL in women, and the degree of anemia is categorized as follows: men (mild anemia:11.0–12.9g/dL, moderate anemia:8.0–10.9 g/dL, and severe anemia, <8.0 g/dL), and women (mild anemia: 11.0–11.9 g/dL, moderate anemia:8.0–10.9 g/dL, and severe anemia, <8.0 g/dL).13
The mean corpuscular volume (MCV) was used to classify anemia into macrocytic (>100 fL), normocytic (80–100 fL), and microcytic (<80 fL). Based on the mean corpuscular hemoglobin (MCH) value, anemia can be classified as normochromic (MCH>26 pg) or hypochromic (MHC <26 pg).13 Two blood tests were performed over a period of more than a month; if the Hb concentration was greater than 13 g/dL in men and 12 g/dL in women, anemia was considered eliminated.14
Procedures
All patients enrolled were subjected to a standard history-taking and physical examination. The following information (clinical signs and symptoms) were collected and recorded: body temperature, fever duration, night sweat, nausea and vomiting, seizures, headache, weight loss history, cranial nerve palsies, fundus anomalies, focal neurological deficits, extracranial TB, duration of symptoms before admission, GCS score, TBM score, anorexia and TBM severity grades. Peripheral venous blood was collected for blood WCC, blood % neutrophils, blood % lymphocytes, C-reaction protein (CRP), serum sodium, serum chloride, T cell enzyme-linked immuno-spot assay (T-spot), anti-tuberculous antibody (TB-Ab), HIV status, and erythrocyte sedimentation rate (ESR). All patients underwent lumbar puncture to obtain CSF samples, and the following tests were performed: total cell count, chloride, glucose and total protein levels. CSF stain (Ziehl–Neelsen stain, Gram, and India ink), and CSF culture. To be enrolled, chest X-rays and cranial CT/MRI scans were obtained in all patients at admission when available.
Statistical Analysis
Statistical calculations were performed using SPSS version 17.0 (IBM Corp, Armonk, NY, USA). The 29 clinical and laboratory parameters of patients with and without anemia are compared in Table 2. Data are expressed as mean ± standard deviation (SD) in cases of normal distribution and as median (interquartile range) in cases of non-normal distribution. The t-test or Mann–Whitney U-test was used to compare the two groups of continuous variables. Categorical variables were expressed as frequencies (n) and percentages (%), and analyzed using the χ2-test or Fischer’s exact test. A p-value of < 0.05 was considered statistically significant. The odds ratios and 95% confidence intervals were calculated. For multivariate analysis, factors significantly associated with anemia were included in the logistic regression analysis.
 |
Table 2 Laboratory and Imaging Features in Patients of Tuberculous Meningitis with and without Anemia |
Results
Demographic Data and Clinical Characteristics of the Enrolled Patients
From January 2004 to December 2019, 134 adults with TBM were reviewed, and 20 (15%) patients were excluded as their diagnoses uncertain or their data were incomplete. Among the 114 patients we studied, there were 69 (60.5%) were males and 45 (39.5%) were females. According to the Thwaites score criteria, 12 (10.5%) had a definitive diagnosis, 79 (69.3%) with a probable diagnosis, and 23 (20.2%) had a possible diagnosis. None of the patients received anti-TB drugs before admission. On admission, all patients were given anti-TB treatment. The median treatment duration was 19±23 days. A total of 85 patients (74.6%) had symptoms of TB poisoning before admission, and 59 patients (51.8%) had a history of TB exposure in the past year.
A positive smear for AFB was obtained from CSF of 12 (10.5%) patients. Twenty-two of 33 patients tested positive in the T-cell enzyme-linked immuno-spot assay (T-spot. TB). Forty-five (45.5%) tested positive for anti-TB antibody (TB-Ab). The white blood cell count ranged from 10–500 mm3/mL in 27 (23.6%) and neutrophilic granulocyte percentage was ≥ 70% in 82 (71.9%) patients. Blood tests revealed hyponatremia (<135 mmol/L) in 58/103 (44%) patients, high CRP level in 25 of 53 (47.2%), and high ESR in 38 of 86 (44%) patients. None of the patients were HIV-positive.
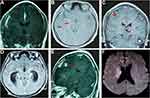
On CSF examination, the median opening pressure of CSF was 17 cmH2O (9–40), and the intracranial pressure increased (≥ 25 cmH2O) in 71 (62.3%) patients. In addition, CSF showed a clear appearance in 84 (73.7%) patients, and CSF WCC ≥ 50 × 106/mL in 88 out of 110 (80%) patients. The CSF protein content ≥ 100 mg/dL in 82 (71.9%) patients, and CSF/serum glucose ratio < 0.5 in 83 out of 107 (77.6%) patients (Table 2). All the adults underwent electroencephalogram (EEG) examination; 53 (75.5%) cases had abnormal results. Similarly, all patients also underwent chest radiographs and cranial CT or MRI scans after admission, wherein signs suggestive of active pulmonary TB were observed in 52 (46%) cases, meningeal reinforcement in 74 (66.1%), hydrocephalus in 36 (31.9%), tuberculoma in 12 (10.6%), and cerebral infarction in 57 (50.4%) (Figure 1).
Prevalence and Characteristics of Anemia
In all, 33 out of 114 (28.9%) TBM patients had anemia, of whom, 19 (57.6%) were men and 14 (42.4%) were women. Among them, there were 21 (64%) cases of mild anemia, 8 (24%) cases of moderate anemia, and 4 (12%) cases of severe anemia. The median age of the patients was 44 years (range 20–78). In the anemia group, 17 (51.5%) patients had pulmonary TB, while in the non-anemia group, 35 (43.2%) patients had pulmonary TB. There were no significant differences in sex, age, and pulmonary TB between the two groups (P > 0.05). Hypochromic microcytic anemia was the most common condition. In the anemia group, 51.5% had hypochromic microcytic anemia, 33.3% had normochromic normocytic anemia, and 15.2% had macrocytic anemia. The most common symptoms were headache in 28 (84.8%), anorexia in 25 (75.8%), and weight loss in 13 (40.6%) patients. The main clinical signs were: focal neurological deficits in 16 (48.5%) patients, and cranial nerve palsies in 15 (45.5%) patients.
Anemia occurred in 22.9% of the 114 patients with TBM enrolled in this study who had a fever lasting 0–30 days. The incidence of anemia was 46.1% in patients with fever lasting 31–60 days, while the incidence of anemia was as high as 100% in patients with fever lasting 61 days to 2 years. According to the modified BMRC criteria, 10 (30.3%) patients had stage I, 16 (48.5%) had stage II, and 7 (21.2%) had stage III TBM (Table 3). As the fever duration increased, the incidence of anemia increased (P=0.001). Additionally, a higher incidence of anemia was observed in patients with stage III than those with stage I or II. Patients with TBM and anemia had a higher ESR.
 |
Table 3 Univariate Analysis, Comparison of the Clinical and Laboratory Characteristics in Tuberculous Meningitis with and without Anemia |
Assessment of Factors Associated with Anemia
On univariate analysis, fever duration, night sweats, cranial nerve palsies, focal neurological deficits, anorexia, TBM severity grades, and ESR were significantly (P < 0.1) associated with anemia (Table 3). A multivariate analysis model using binary logistic regression was then constructed and revealed that three factors (Fever duration, TBM grade III, and ESR) were found to be associated with anemia (Table 4).
 |
Table 4 Multivariate Logistic Regression Analysis |
Evolution of Anemia During Anti-TB Treatment
In this study, patients were treated with a standard four-drug anti-TB therapy (isoniazid, rifampicin, pyrazinamide, and ethambutol) for 12–18 months. Twenty–five (21.9%) patients received adjunctive dexamethasone (0.4 mg/kg/day). Fluoroquinolones were used in combination in 45 patients (levofloxacin in 30 patients and moxifloxacin in 15), depending on their condition. In addition, patients were routinely treated with a combination of dehydration to reduce cranial pressure and improve brain function.
Moreover, 33 patients with anemia were treated with vitamins, compound amino acids and other nutritional support in addition to anti-TB drugs; their median number of days of hospitalization was 18 days. Two patients died due to a complication of foramen magnum hernia, two patients died of infectious shock, and one died because of multiple organ failure; the final mortality rate was 15.1%. At discharge, anemia was improved in 18 (54.5%), resolved in 8 (24.3%), and remained unchanged or aggravated in 7 (21.2%) patients.
Discussion
TBM is an infection that occurs secondary to TB in areas other than the brain, and is associated with the highest rate of mortality and disability.15 Chronic anemia, also known as inflammatory anemia, belongs to a group of clinical syndromes characterized by anemia in patients with infectious diseases such as inflammatory diseases, TB, and neoplastic diseases.16 All common chronic infections, including TB, can lead to anemia.17 Anemia occurred in 28.9% of the enrolled patients with TBM in the present study, and at discharge, anemia was ameliorated or resolved in 26 (78.8%) cases.
The main findings of this study were listed below:1) anemia was a common complication, with an incidence of 28.9% in TBM patients; 2) most anemia cases were mild or moderate, while severe anemia was rare; 3) hypochromic microcytic anemia was the most common, followed by normocytic normochromic anemia, and the least common was macrocytic anemia; 4) anemia was associated with the fever duration, TBM grade III, and ESR.
Our study found that conditions that predict nutritional deficiency (eg, a history of weight loss and low BMI) were more common among anemia patients than non-anemia patients. When we compared the nutritional status of the anemia group with that of the non-anemia group, we found that the former group was more malnourished than the latter. Anorexia was observed in 25 (75.8%) of 33 anemia patients in this study. There were 9 (27.3%) cases of nausea and vomiting, 6 (18%) cases of diarrhea, and 13 (40.6%) cases of weight loss (>10%). The patients’ iron level and absorption were low and impaired owing to an extended period of insufficient iron intake and gastrointestinal dysfunction, respectively.
As the duration of fever is extended, the incidence of anemia increases owing to poor nutritional status, reduced number of bone marrow cells, and reduced folate, riboflavin, and vitamins A and B12 levels.18 Deficiencies in these nutrients can also lead to immune deficiency. Thus, in patients with anemic TB. MTB may require a longer time to proliferate and accumulate, resulting in longer exposure to inflammation. In this study, the incidence of anemia in patients with persistent fever for more than 2 months was 100%, which was considerably higher than that in patients with fever for 0–30 days (22.9%) and 31–60 days (46.1%). Based on this reasoning, fever duration can be considered a risk factor for TBM-associated anemia.
In our study, univariate analysis revealed that TBM with anemia was associated with older age, nausea, vomiting, seizures, a history of weight loss, anorexia, and cranial nerve palsies, indicating that patients with advanced TBM patients were more likely to be anemic.
ESR, a marker of an active inflammation, was significantly elevated in patients with anemia. This observation led us to speculate that an important cause of anemia in patients with TBM may be iron deficiency and/or reduced erythropoiesis due to altered iron metabolism during inflammation. One study evaluating the etiology of anemia estimated that 15.0% to 75.9% and 0.0% to 58.0% patients with anemia had inflammatory anemia and iron deficiency anemia, respectively.4 These differences may be attributed to differences in study designs and reference standards. The pathogenesis of TB-associated anemia varies, while most researches have suggested that inhibition of erythropoiesis by inflammatory mediums is one of the causes of anemia.19,20 It is characterized by mild to moderate normal cytologic hypochromic anemia.21 Some studies have found that undiluted washed red blood cells (RBCs) can hinder the growth of bacteria, but can be damaged when diluted.22–24 Therefore, anemia patients may have immune system disorders and low bactericidal activity owing to low Hb concentration, thus promoting the progression of TB. Previous studies have observed an increase in ESR in anemia, consistent with the results of this study.25 Simultaneously, other studies have also found that a gradual increase in Hb value was related to the significant decrease in ESR and CRP value.26 These researches clearly show that the degree of anemia is directly related to substantial changes in the concentrations of several biochemical parameters associated with significant inflammation in TB patients.
A previous studies showed that anemia may be caused by the tubercle bacillus metabolites as antigens, resulting in bone marrow erythrocyte sensitization.27,28 Anemia can also occur as a defense response, during which iron is abnormally transported and stored in reticuloendothelial cells. This release disorder causes serum iron concentration and cell iron uptake to decrease, thus inhibiting the growth of pathogens.29,30 Long-term fever and excessive release of inflammatory mediators, coupled with inadequate nutrition intake and absorption, easily lead to negative nitrogen balance and increase the susceptibility to co-infection. Simultaneously, bacterial toxins in the blood can accelerate the destruction of RBCs,8,31 affecting the release and utilization of iron, resulting in the suppression of bone marrow hematopoietic function and infectious anemia.
In this study, the incidence of anemia in patients with grade III TBM was higher than that in patients with grade I and grade II TBM. It has been speculated that patients with severe TBM may be more prone to anemia. After admission, all patients were administered anti-TB therapy and corresponding nutritional support therapy. Finally, anemia was resolved in 26 (78.8%) out of 33 patients who did not receive iron supplementation during anti-TB treatment. Further evidence that TB may drive the development of anemia is that after TB treatment, anemia usually disappears.14,32
Conclusions
Anemia is a very common hematologic anomaly of TBM, and fever duration, TBM grade III and ESR are common predictors of anemia. In most patients with TBM, TB-associated anemia is mild and can be ameliorated or resolved with anti-TB therapy and enhanced nutrition.
Acknowledgments
The authors thank the staffs from the Neurology ward of Jiangxi Provincial People’s Hospital for technical assistance as well as Editage (www.editage.cn) for English language editing.
Funding
This work was supported by grant from Health Commission of Jiangxi Province (NO. 202310122).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chakaya J, Khan M, Ntoumi F, et al. Global tuberculosis report 2020 - reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis. 2021;113(Suppl1):S7–S12. doi:10.1016/j.ijid.2021.02.107
2. Brancusi F, Farrar J, Heemskerk D. Tuberculous meningitis in adults: a review of a decade of developments focusing on prognostic factors for outcome. Future Microbiol. 2012;7(9):1101–1116. doi:10.2217/fmb.12.86
3. Ho J, Marais BJ, Gilbert GL, Ralph AP. Diagnosing tuberculous meningitis - have we made any progress? Trop Med Int Health. 2013;18(6):783–793. doi:10.1111/tmi.12099
4. Barzegari S, Afshari M, Movahednia M, Moosazadeh M. Prevalence of anemia among patients with tuberculosis: a systematic review and meta-analysis. Indian J Tuberc. 2019;66(2):299–307. doi:10.1016/j.ijtb.2019.04.002
5. Agrawal Y, Goyal V, Singh A, Lal S. Role of anaemia and magnesium levels at the initiation of tuberculosis therapy with sputum conversion among pulmonary tuberculosis patients. J Clin Diagn Res. 2017;11(6):BC01–BC04. doi:10.7860/jcdr/2017/23734.9975
6. Sarwar M, Saleem MF, Ullah N, et al. Role of mineral nutrition in alleviation of heat stress in cotton plants grown in glasshouse and field conditions. Sci Rep. 2019;9(1):13022. doi:10.1038/s41598-019-49404-6
7. Abay F, Yalew A, Shibabaw A, Enawgaw B. Hematological abnormalities of pulmonary tuberculosis patients with and without HIV at the University of Gondar Hospital, Northwest Ethiopia: a comparative cross-sectional study. Tuberc Res Treat. 2018;2018:5740951. doi:10.1155/2018/5740951
8. Hella J, Cercamondi CI, Mhimbira F, et al. Anemia in tuberculosis cases and household controls from Tanzania: contribution of disease, coinfections, and the role of hepcidin. PLoS One. 2018;13(4):e0195985. doi:10.1371/journal.pone.0195985
9. Saathoff E, Villamor E, Mugusi F, Bosch RJ, Urassa W, Fawzi WW. Anemia in adults with tuberculosis is associated with HIV and anthropometric status in Dar es Salaam, Tanzania. Int J Tubercul Lung Dis. 2011;15(7):925–932. doi:10.5588/ijtld.10.0477
10. Medical Research Council. STREPTOMYCIN treatment of tuberculous meningitis. Lancet. 1948;1(6503):582–596.
11. Marais S, Thwaites G, Schoeman JF, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10(11):803–812. doi:10.1016/s1473-3099(10)70138-9
12. Hosoglu S, Geyik MF, Balik I, et al. Predictors of outcome in patients with tuberculous meningitis. The international journal of tuberculosis and lung disease. Int J Tuberc. 2002;6(1):64–70.
13. Palmer L, Briggs C, McFadden S, et al. ICSH recommendations for the standardization of nomenclature and grading of peripheral blood cell morphological features. Int J Lab Hematol. 2015;37(3):287–303. doi:10.1111/ijlh.12327
14. Lee SW, Kang YA, Yoon YS, et al. The prevalence and evolution of anemia associated with tuberculosis. J Korean Med Sci. 2006;21(6):1028–1032. doi:10.3346/jkms.2006.21.6.1028
15. Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol. 2013;12(10):999–1010. doi:10.1016/s1474-4422(13)70168-6
16. Cançado RD, de Figueiredo PO, Olivato MC, Chiattone CS. Efficacy and safety of intravenous iron sucrose in treating adults with iron deficiency anemia. Rev Bras Hematol Hemoter. 2011;33(6):439–443. doi:10.5581/1516-8484.20110119
17. Weiss G. Pathogenesis and treatment of anaemia of chronic disease. Blood Rev. 2002;16(2):87–96. doi:10.1054/blre.2002.0193
18. Henderson JG, Strachan RW, Beck JS, Dawson AA, Daniel M. The antigastric-antibody test as a screening procedure for vitamin-B12 deficiency in psychiatric practice. Lancet. 1966;2(7468):809–813. doi:10.1016/s0140-6736(66)92250-1
19. Means RT. Recent developments in the anemia of chronic disease. Curr Hematol Rep. 2003;2(2):116–121.
20. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–1023. doi:10.1056/NEJMra041809
21. Antunes SA, Canziani ME. Hepcidin: an important iron metabolism regulator in chronic kidney disease. J Brasileiro de Nefrologia. 2016;38(3):351–355. doi:10.5935/0101-2800.20160053
22. Gelaw Y, Getaneh Z, Melku M. Anemia as a risk factor for tuberculosis: a systematic review and meta-analysis. Environ Health Prev Med. 2021;26(1):13. doi:10.1186/s12199-020-00931-z
23. Cobelens F, Kerkhoff AD. Tuberculosis and anemia-cause or effect? Environ Health Prev Med. 2021;26(1):93. doi:10.1186/s12199-021-01013-4
24. Baluku JB, Mayinja E, Mugabe P, Ntabadde K, Olum R, Bongomin F. Prevalence of anaemia and associated factors among people with pulmonary tuberculosis in Uganda. Epidemiol Infect. 2022;150:e29. doi:10.1017/s0950268822000103
25. Corsonello A, Pedone C, Battaglia S, Paglino G, Bellia V, Incalzi RA. C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) as inflammation markers in elderly patients with stable chronic obstructive pulmonary disease (COPD). Arch Gerontol Geriatr. 2011;53(2):190–195. doi:10.1016/j.archger.2010.10.015
26. Gil-Santana L, Cruz LAB, Arriaga MB, et al. Tuberculosis-associated anemia is linked to a distinct inflammatory profile that persists after initiation of antitubercular therapy. Sci Rep. 2019;9(1):1381. doi:10.1038/s41598-018-37860-5
27. Weinberg ED. Iron depletion: a defense against intracellular infection and neoplasia. Life Sci. 1992;50(18):1289–1297. doi:10.1016/0024-3205(92)90279-x
28. Kontoghiorghes GJ, Kolnagou A, Demetriou T, Neocleous M, Kontoghiorghe CN. New era in the treatment of iron deficiency anaemia using trimaltol iron and other lipophilic iron chelator complexes: historical perspectives of discovery and future applications. Int J Mol Sci. 2021;22(11):5546. doi:10.3390/ijms22115546
29. Rodrigues PN, Gomes SS, Neves JV, et al. Mycobacteria-induced anaemia revisited: a molecular approach reveals the involvement of NRAMP1 and lipocalin-2, but not of hepcidin. Immunobiology. 2011;216(10):1127–1134. doi:10.1016/j.imbio.2011.04.004
30. Sakurai J, Nagahama M, Oda M. Clostridium perfringens alpha-toxin: characterization and mode of action. J Biochem. 2004;136(5):569–574. doi:10.1093/jb/mvh161
31. Kerkhoff AD, Meintjes G, Opie J, et al. Anaemia in patients with HIV-associated TB: relative contributions of anaemia of chronic disease and iron deficiency. Int J Tubercul Lung Dis. 2016;20(2):193–201. doi:10.5588/ijtld.15.0558
32. Minchella PA, Donkor S, Owolabi O, Sutherland JS, McDermid JM. Complex anemia in tuberculosis: the need to consider causes and timing when designing interventions. Clin Infect Dis. 2015;60(5):764–772. doi:10.1093/cid/ciu945
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.