Back to Journals » International Journal of Nanomedicine » Volume 19
Exploiting Nanotechnology for Drug Delivery: Advancing the Anti-Cancer Effects of Autophagy-Modulating Compounds in Traditional Chinese Medicine
Authors Liu Z , Lu T, Qian R, Wang Z, Qi R, Zhang Z
Received 17 December 2023
Accepted for publication 6 March 2024
Published 12 March 2024 Volume 2024:19 Pages 2507—2528
DOI https://doi.org/10.2147/IJN.S455407
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Jie Huang
Zixian Liu, Tianming Lu, Ruoning Qian, Zian Wang, Ruogu Qi, Zhengguang Zhang
School of Medicine, Nanjing University of Chinese Medicine, Jiangsu, Nanjing, People’s Republic of China
Correspondence: Zhengguang Zhang; Ruogu Qi, Email [email protected]; [email protected]
Background: Cancer continues to be a prominent issue in the field of medicine, as demonstrated by recent studies emphasizing the significant role of autophagy in the development of cancer. Traditional Chinese Medicine (TCM) provides a variety of anti-tumor agents capable of regulating autophagy. However, the clinical application of autophagy-modulating compounds derived from TCM is impeded by their restricted water solubility and bioavailability. To overcome this challenge, the utilization of nanotechnology has been suggested as a potential solution. Nonetheless, the current body of literature on nanoparticles delivering TCM-derived autophagy-modulating anti-tumor compounds for cancer treatment is limited, lacking comprehensive summaries and detailed descriptions.
Methods: Up to November 2023, a comprehensive research study was conducted to gather relevant data using a variety of databases, including PubMed, ScienceDirect, Springer Link, Web of Science, and CNKI. The keywords utilized in this investigation included “autophagy”, “nanoparticles”, “traditional Chinese medicine” and “anticancer”.
Results: This review provides a comprehensive analysis of the potential of nanotechnology in overcoming delivery challenges and enhancing the anti-cancer properties of autophagy-modulating compounds in TCM. The evaluation is based on a synthesis of different classes of autophagy-modulating compounds in TCM, their mechanisms of action in cancer treatment, and their potential benefits as reported in various scholarly sources. The findings indicate that nanotechnology shows potential in enhancing the availability of autophagy-modulating agents in TCM, thereby opening up a plethora of potential therapeutic avenues.
Conclusion: Nanotechnology has the potential to enhance the anti-tumor efficacy of autophagy-modulating compounds in traditional TCM, through regulation of autophagy.
Keywords: autophagy, nano-delivery, anti-cancer, traditional Chinese medicine
Introduction
The study of cancer treatment is of paramount importance in the global medical field and faces various challenges.1 The increasing prevalence of cancer, driven by an aging population and changing lifestyles, puts significant pressure on healthcare providers.2–4 Traditional treatments such as chemotherapy and radiotherapy have limitations, including harm to healthy tissues, uncertain effectiveness, and the development of drug resistance.5,6 Consequently, it is crucial for novel technologies and methodologies to be developed to improve the effectiveness of cancer treatment. During recent decades, the advent of emerging technologies, namely nanotechnology, immunotherapy, and gene editing technology, has instilled renewed optimism in the field of cancer treatment.7–9 Nanotechnology enables the precise delivery of drugs to tumor tissues using nanocarriers, reducing the risk of damage to healthy tissues.10 Numerous studies have demonstrated the mechanisms and benefits of various nanoformulations in tumor therapy, showing that nanoformulations are more effective than free drugs.11,12 Furthermore, immunotherapy augments the body’s immune system and enhances its response to tumors.13 The application of gene editing technology has emerged as a prominent area of research, offering promising prospects for cancer treatment.14 In conjunction with the exploration of emerging technologies, there has been a concerted effort to investigate novel pharmaceuticals, particularly Chinese medicines, which are increasingly recognized as valuable assets in the realm of oncological treatment.15 A burgeoning body of research indicates that Chinese medicines offer promising prospects in the field of oncology.16–18 The development of these innovative technologies, methodologies, and pharmaceuticals can improve the precision and effectiveness of cancer therapy, thereby contributing to enhanced patient outcomes and quality of life.
Autophagy, a critical process in eukaryotic cells, plays a pivotal role in organelle regeneration, substance recycling, metabolic homeostasis, and adaptation to external stimuli.19,20 In recent years, there has been a notable increase in research attention towards autophagy in various biological disciplines. Within the field of oncology, autophagy has been acknowledged as a significant mechanism in the regulation of tumor cell survival.21,22 Additionally, ongoing studies focusing on the modernization of TCM have revealed an increasing number of TCM compounds that exhibit potential in modulating autophagy.23–25 These compounds present notable advantages compared to traditional chemotherapeutic agents, including platinum-based drugs and anthracyclines, owing to their reduced toxicity, capacity to target multiple sites, and synergistic effects.26,27 Nevertheless, the widespread presence of indoles and flavonoids in these compounds poses a significant obstacle due to their inadequate water solubility and restricted bioavailability, thereby impeding their potential clinical applicability.26,28 The incorporation of nano-delivery technology holds promise in overcoming this constraint.
The field of cancer therapy requires novel drugs and technologies for advancement, with the active ingredient components of TCM showing promise as anti-tumor small-molecule drugs. However, limitations in their application for tumor therapy exist. The integration of nano-delivery technology addresses these challenges and enhances the benefits of TCM active ingredients. Autophagy is recognized as a crucial target for modulating the viability and apoptosis of cancer cells. Compounds sourced from TCM can either enhance or impede the autophagy process in tumor cells, thereby facilitating autophagy-induced cell death or hindering protective autophagy to inhibit tumor progression. The utilization of nanotechnology for the delivery of active compounds sourced from TCM allows for the development of a personalized targeted delivery approach designed for specific cancer treatments.26 This approach enables controlled release, synergistic effects of multiple drugs, and combination therapy, ultimately enhancing the effectiveness of anti-tumor interventions.
Currently, there is a significant gap in academic literature regarding the application of nanoparticles for targeted delivery of autophagy-modulating compounds in TCM for cancer therapy. This lack of comprehensive synthesis and elucidation has motivated us to undertake a thorough investigation utilizing multiple databases. Our study results can be categorized into three main areas: 1. the application of nanoparticles in cancer therapy; 2. the diverse range of autophagy-modulating compounds in TCM, their mechanisms of action against cancer, and their potential significance; 3. the advantages associated with the nanoparticle-mediated administration of autophagy-modulating compounds in TCM, alongside the progress in research and pertinent case studies.
Application of Nanoparticles in Cancer Therapy
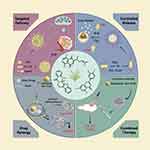
In recent years, the field of nanotechnology has made notable advancements, particularly in the area of drug delivery, which has generated optimism for the treatment of cancer.29–31 Nanoformulations of drugs, characterized by their small dimensions and stable properties, have demonstrated the ability to effectively transport a variety of small molecules including conventional chemotherapeutic drugs, active compounds from TCM, genes, as well as photosensitizers and photothermal agents.32–34 The nanoformulations mentioned above, which include nanoparticles, nanomicelles, metal-on-framework (MOF) nanoparticles, liposomes, and biomimetic nanoparticles, frequently exhibit advantages such as targeted delivery, controlled release, multidrug synergy, and combined therapy.35–37 These distinctive characteristics enable nanoformulations to demonstrate superior efficacy compared to free small-molecule drugs. Numerous studies in the literature have shown that nanoformulations exhibit higher anti-tumor toxicity than conventional therapies at equivalent concentrations, as well as a lower IC50.38–41 The advantages of these nanoformulations are illustrated in Figure 1.
Targeted Delivery
The successful attainment of targeted delivery of nanoformulations can be achieved through a variety of mechanisms, one of which relies on their inherently small size and the enhanced permeability and retention (EPR) effect observed in the vicinity of tumor sites.42 Specifically, the rapid growth of tumor cells leads to irregular neovascularization, causing structural abnormalities in the vessel wall and increased microvascular permeability. As a result, nanoparticles with reduced diameters can traverse the vessel wall more easily and penetrate the tumor tissue.43 This phenomenon facilitates the accumulation and prolonged presence of nanoparticles in the vicinity of the tumor site.
Furthermore, nanoformulations possess the capability to be modified at the surface to enhance targeted delivery. This process entails the integration of antibodies, ligands, or other compounds onto the nanoparticle surface, enabling them to selectively identify and attach to particular receptors on cancer cell surfaces.44,45 Through the implementation of this technique, the efficacy of therapeutic targeting can be enhanced, while reducing potential damage to healthy cells. An exemplar of this concept is the utilization of folic acid (FA) as a surface modification on nanoparticles containing resveratrol.46 This modification allows for an increased drug concentration at the tumor site, thereby enhancing the antitumor toxicity and efficacy.47
Controlled Release
An additional advantage of nanoformulations lies in their ability to facilitate the controlled release of co-delivered drugs. This precise release mechanism guarantees timely and targeted delivery of the drug, thereby enhancing its concentration at the specific site of the lesion.48 As a result, this strategy optimizes the drug’s efficacy while minimizing potential harm to healthy cells. Additionally, the controlled release also serves to prolong drug metabolism and excretion, leading to a decreased frequency of administrations and improved patient compliance with the prescribed treatment regimen.49
The activation of nanoformulation release can be classified into two distinct methodologies. The first method entails the incorporation of polymers or biodegradable materials with specific chemical groups, such as Tk bonds (thioketal) and s-s bonds (disulfide bond), that are designed to be responsive to stimuli such as pH, GSH, ROS, etc., facilitating controlled drug release based on the environmental conditions within the tumor microenvironment.50–53 The second approach entails utilizing external stimuli, such as ultrasound and near-infrared (NIR) signals from external sources, to trigger targeted drug release under specific conditions.54,55 These methods are commonly utilized in combination to develop composite nanomedicine systems, which allow for more precise control over drug release and have gained significant traction in the delivery of active ingredients from TCM.
Multidrug Synergy
Nanoformulations show promise in enhancing treatment effectiveness by enabling the targeted delivery of various therapeutic agents to specific tissues or cells, allowing for the simultaneous exertion of synergistic effects.56 This strategy often proves successful in overcoming the obstacles associated with single-agent therapy, such as the emergence of drug tolerance and resistance, thus presenting hopeful therapeutic opportunities for multidrug-resistant (MDR) cancer cells.57 Notably, the combination of autophagy-modulating compounds derived from TCM with autophagy inhibitors such as chloroquine (CQ) can augment the therapeutic impact, while the integration of autophagy inhibition with conventional chemotherapeutic agents can counteract drug resistance in tumor cells.41,58
Combined Therapy
Nanoformulations can utilize a variety of materials to transport different drugs, transforming them into nanoplatforms with multiple diagnostic and therapeutic functions.59 For example, combining photosensitizers and photothermal agents enables the integration of chemotherapy, photothermal therapy (PTT), and photodynamic therapy (PDT).60 Additionally, the integration of an imaging agent within nanoparticles enables the live observation of a lesion’s physiological condition in an in vivo setting. For instance, the inclusion of nanoparticles loaded with a photosensitizer like indocyanine green in tumor tissue enables the utilization of near-infrared (NIR) molecular imaging for precise visualization of the tumor, thereby enhancing treatment accuracy.61
Although nanotechnology holds potential for drug delivery, it is important to consider the potential side effects associated with nanoformulations. These formulations may be recognized and rejected by the immune system, leading to allergic reactions or inflammation that can be harmful to the body.62,63 Additionally, nanomedicines may be rapidly cleared by the liver or kidneys, reducing their effectiveness.63,64 We are also concerned about the presence of metal nanoparticles with autophagy-regulating properties, particularly those composed of materials such as gold and silver that are challenging to metabolize and eliminate. Localized accumulation of these nanoparticles may contribute to the malignant transformation of healthy cells.65–68 Besides, nanopreparations have been reported to impact platelet function and coagulation in the blood system, potentially leading to thrombus formation.63 Therefore, thorough safety evaluation and vigilant monitoring are imperative throughout the development and application of nanomedicine delivery systems to guarantee the safety and effectiveness of nanopreparations.
The aforementioned multifunctional nanoformulations are currently under investigation and have not yet been integrated into clinical practice. Approved nanomedicines for clinical use include Paclitaxel-loaded albumin nanoparticles (Abraxane) and liposomes loading Doxorubicin (Doxil), among others.69–72 Abraxane has been approved for the treatment of breast cancer, pancreatic cancer, and non-small cell lung cancer, while Doxil is currently approved and utilized in the treatment of multiple cancers, including breast and ovarian cancer.73,74 Although nanoformulations have shown improved drug solubility and bioavailability, their monofunctionality and limited drug loading capacity may impede their ability to substantially enhance clinical efficacy for tumors. Nonetheless, these successful cases provide strong support for the potential of nano-delivery systems in enhancing drug efficacy, reducing toxicity, and broadening treatment options. As a result, they offer important insights and bolster confidence in the future clinical utilization of multifunctional nanoformulations.
TCM Active Ingredients Act as Anti-Tumor Agents by Modulating Autophagy
Autophagy is an evolutionarily conserved cellular process in eukaryotes that aids in the degradation and recycling of intracellular biomolecules and damaged organelles. The initiation of this process is primarily mediated by the ULK1 (UNC-51 Like Kinase 1) complex, which leads to the formation of a phagophore, a flat lipid bilayer structure.75,76 The VPS34-Beclin1 complex, regulated by ULK1, is essential for elongating the phagophore membrane and establishing a link between autophagy and cancer progression.77
The elongation of the phagophore results in the formation of the autophagosome, a double-membrane structure. This crucial process is tightly controlled by two ubiquitin-like pathways, specifically ATG7-ATG3 and ATG7-ATG10.78,79 It entails the transformation of LC3-I, a cytoplasmic protein, into LC3-II, which aids in the elongation of the autophagosome membrane. Furthermore, the p62 protein plays a significant role in the selective sequestration of degradation by autophagosomes, as it interacts with both LC3-II and the ubiquitin-binding domain of the degraded proteins.80
Autophagy plays a crucial role in cancer by exerting varied effects dependent on the specific cancer type, progression, and tumor microenvironment. Recent research has shown that autophagy acts as a protective mechanism, promoting metastasis and drug resistance in cancer cells.81–83 Conversely, other studies have identified autophagy as a process that facilitates programmed cell death.84–86 Additionally, autophagy is closely linked with apoptosis, necroptosis, ferroptosis, and immunogenic cell death (ICD).87–89 A comprehensive examination indicates that Bcl-2 functions as a connection between apoptosis and autophagy, with the potential for mutual activation between autophagy and ferroptosis through the production of reactive oxygen species (ROS) and lipid peroxidation.90,91 Moreover, necroptosis may be associated with autophagy via RIP3-related pathways.92,93 Besides, autophagy has been found to induce ICD either directly or indirectly through ferroptosis.94 Given the capacity of TCM to target multiple components and subsequently stimulate various pathways, a thorough comprehension of the interaction among these pathways becomes essential. The interplay of these cell death pathways is visually represented in Figure 2.
Moreover, autophagy plays a crucial role in the anti-tumor effects of active components found in TCM. Numerous studies have demonstrated that TCM-derived compounds, including alkaloids, flavonoids, coumarins, and lignans, are involved in the regulation of cellular autophagy and show promise as potential cancer therapeutics.95–99 The effectiveness of TCM active ingredients in inducing changes in intracellular autophagy-related proteins, including LC3, p62, Beclin1, ATG5, etc., has been supported by various signaling pathways, such as the PI3K/AKT/mTOR signaling pathway, AMPK-related signaling pathways, and MAPK-related signaling pathways.100,101 Additionally, these TCM active ingredients have demonstrated a significant influence on cancer progression. The targets and mechanisms involved in the regulation of autophagy by these TCM-active ingredients are depicted in Figure 3.
This article aims to provide a comprehensive review of various active ingredients derived from TCM that possess the ability to regulate autophagy and induce cell death in tumors. The significance of autophagy in cancer therapy and the distinct advantages offered by these compounds will be thoroughly examined.
Evodiamine
Evodiamine (EVO), a compound derived from Tetradium Ruticarpum, a traditional Chinese medicinal plant, has been shown to modulate autophagy in tumor cells through multiple signaling pathways.102 Previous research has highlighted the role of EVO in regulating autophagy via the PI3K/AKT/mTOR, Ras/MEK/ERK, STAT3-related, and Ca2+/JNK pathways. Specifically, EVO has been observed to promote protective autophagy in pancreatic and lung cancers,103,104 while inhibiting autophagy in glioblastoma.105 Furthermore, the combination of EVO with 3-methyladenine (3-MA), an autophagy inhibitor has been found to enhance therapeutic efficacy.103,104,106 Additionally, a compound was synthesized by researchers through the combination of EVO and tetravalent platinum, demonstrating potent anti-cancer properties. In contrast to EVO or Pt monomers, this innovative compound notably increased autophagy levels in MCF-7 cells. However, contrary to previous studies, the introduction of 3-MA did not augment cytotoxicity; instead, it led to a decrease.107 This occurrence may be attributed to the inadequate autophagy induction caused by EVO alone, which failed to initiate autophagic cell death. Conversely, the novel compound induced substantial levels of autophagy, excessively activating the autophagy pathway beyond the protective threshold, ultimately culminating in autophagic cell death in the cancer cells. This investigation presents potential avenues for the logical and clinical implementation of EVO-derived compounds.
Icaritin
Icaritin (ICA), an isopentenyl flavonoid derived from Herba Epimedii, has been shown in previous research to modulate autophagy through the PI3K/AKT pathway in tumor cells.108 Its inhibitory effect on autophagy enhances the sensitivity of breast and ovarian tumor cells to tamoxifen and cisplatin.108,109 However, a separate study found that when combined with Curcumol, ICA promotes autophagy in prostate cancer cells by inhibiting mTOR downstream.110 These findings suggest that the regulation of autophagy is a complex process, as ICA can have contrasting effects on autophagy induction or inhibition, potentially varying across different cell lines.
Luteolin
Luteolin (LUT), a naturally occurring flavonoid compound derived from medicinal plants, has shown considerable potential as a therapeutic agent against various types of cancer.111 The intricate and diverse mechanisms by which LUT modulates tumor cells have been extensively studied.112 In liver cancer and nasopharyngeal carcinoma cells, LUT has been found to induce autophagic cell death,113 whereas, in breast and colon cancers, it promotes protective autophagy.114 Interestingly, in ovarian cancer, LUT inhibits autophagy while simultaneously enhancing cisplatin-induced apoptosis.115 To identify potential targets for LUT therapy, a recent study utilized advanced techniques such as RNA sequencing and molecular docking programs.116
Epigallocatechin Gallate
Extensive research has been conducted on Epigallocatechin gallate (EGCG), a catechin compound, to explore its therapeutic properties in cancer treatment. It has been observed that EGCG can induce cell autophagy and exert toxic effects by inhibiting the PTEN pathway and suppressing the PI3K/AKT/mTOR signaling pathway.117 Additionally, EGCG has been found to interfere with the JAK/STAT3 signaling pathway at low concentrations.118 For instance, a research investigation centered on oral cancer revealed that EGCG not only triggers autophagy via the AKT/STAT pathway but also substantially augments the functionality of apoptosis-related caspases while downregulating the expression of multi-drug resistance gene. This underscores its potential in the treatment of oral cancer from various perspectives.119 In a separate study concentrating on liver cancer cells, EGCG was observed to stimulate autophagy and diminish AFP levels.120 Interestingly, in non-small cell lung cancer, EGCG hampers autophagy by suppressing the Ras/MEK/ERK signaling pathway and overcomes Gefitinib resistance in A549 cells.121
α-Mangostin
α-mangostin (MGT), derived from Garcinia sp., exhibits a diverse array of pharmacological activities. Extensive research has substantiated the ability of MGT and its derivatives to elicit cellular apoptosis and stimulate defensive autophagy in the context of cancer investigations.122 Intriguingly, the co-administration of autophagy inhibitors notably augments the anti-cancer efficacy of MGT.123,124 A specific investigation has elucidated that MGT induces autophagy via the PI3K/AKT/mTOR pathway in skin cancer.125 Furthermore, MGT has been observed to induce autophagic cell death in glioblastoma studies. In contrast to previous findings, this research has unveiled that MGT suppresses mTOR by inhibiting the liver kinase B1/AMPK pathway and directly phosphorylating Raptor, thereby leading to autophagic cell death.126 Additionally, MGT has been discovered to enhance the susceptibility of gastric cancer cells to cisplatin by inducing autophagy through the inhibition of the STAT3 pathway.127
Shikonin or Alkannin
Shikonin (SKN), an active naphthoquinone derivative obtained from Radix Lithospermi, a traditional Chinese medicine plant, has been extensively investigated for its anti-cancer properties. Previous research has demonstrated that SKN is capable of inducing reactive oxygen species (ROS), regulating autophagy, and initiating apoptosis. Recent studies have further elucidated the mechanisms by which SKN induces autophagy and apoptosis in gastric cancer cells, specifically through the inhibition of the PI3K/AKT/mTOR pathway, thereby reversing their resistance to oxaliplatin.128,129 In a separate investigation, it was discovered that SKN effectively triggers autophagy in SK-OV-3 ovarian cancer cells by activating the Keap1/Nrf2 signaling pathway, thereby inhibiting both cellular autophagy and invasive properties.130 Additionally, in the context of liver cancer research, the suppression of the PYCR1 gene further augmented SKN-induced autophagy and apoptosis. These findings imply that a combined therapeutic approach involving drug-gene therapy holds promise as a viable strategy for combating cancer.131
Recent investigations have revealed that SKN instigates autophagy and apoptosis by inducing an accumulation of ROS, which is facilitated via MAPK-related pathways.132–134 Furthermore, SKN has been observed to induce cell necroptosis. In both bladder and lung cancers, SKN regulates autophagy and triggers cellular necrosis through the RIP3/p62/Keap1 pathway. Additionally, the introduction of CQ augments the incidence of necroptosis, implying an antagonistic association between necrosis and autophagy.93,135,136
Honokiol
Honokiol (HNK), a biphenolic compound obtained from Magnolia officinalis, a medicinal plant belonging to the Magnoliaceae family, has been demonstrated in previous research to possess the ability to induce autophagy in diverse tumor types. LUO et al conducted a study that identified HNK as an autophagy inducer in A549 cells by inhibiting the mTOR pathway.137 Additionally, HUANG et al discovered that HNK activates autophagy via the ROS/ERK pathway.138 In a study conducted on glioblastoma, the involvement of HNK in cell autophagy was observed through the activation of both the PI3K/Akt/mTOR and ERS/ROS/ERK signaling pathways, leading to the inhibition of cell migration and the promotion of apoptosis.139 Furthermore, XU et al synthesized HNK derivatives (1,3,4-thiadiazole/oxadiazole-linked honokiol) through covalent chemical modifications.140 These derivatives demonstrated a significant tenfold increase in cytotoxicity against various cancer cell types compared to free HNK, while still inducing autophagy via the PI3K/AKT/mTOR pathway.
Other Active Ingredients of TCM
Several other active ingredients of Traditional Chinese Medicine (TCM) have been identified for their potential to regulate autophagy in cancer. It has been confirmed that Echinatin and Tanshinone I possess the ability to induce cell autophagy and promote apoptosis by inhibiting the PI3K/AKT/mTOR pathway.141,142 Additionally, Triptolide is a potent autophagy inducer and can trigger autophagic cell death in drug-resistant ovarian cancer through the JAK2/STAT3 pathway.143 Furthermore, Curcumin and Resveratrol, both well-known autophagy-inducers, are capable of mediating autophagy via the PI3K/AKT/mTOR and AMPK pathways.144,145 These compounds hold promise as anti-cancer agents due to their potential to induce autophagic cell death, inhibit tumor migration, and reverse resistance to Gefitinib. Additionally, numerous other active ingredients derived from TCM possess autophagy-modulating properties; however, the mechanisms underlying their effects remain unknown, necessitating further investigation.
Interestingly, our collective research indicates that the active ingredients of TCM that modulate autophagy appear to utilize common signaling pathways (Figure 3). Specifically, MGT, SKN, and HNK have been shown to influence the AMPK pathways, while EVO, ICA, LUT, and EGCG are involved in regulating autophagy through the PI3K/AKT/mTOR pathway. Additionally, EVO, EGCG, SKN, and HNK have been linked to MAPK-related signaling pathways. This phenomenon could potentially be attributed to the analogous chemical structures of these compounds, necessitating additional research to elucidate the precise underlying mechanisms. Moreover, the potential for certain drugs to interact with multiple targets and pathways, thereby modulating autophagy and other cell death pathways concurrently, raises concerns. Further investigation into these mechanisms may enhance the efficacy of anticancer drugs in inhibiting cancer growth.
The Integration of TCM-Derived Autophagy Compounds with Nanotechnology Offers Great Promise and Advantages
Significant progress has been achieved in the study of nanoparticles containing autophagy-regulating TCM components for cancer treatment. Particularly in the realm of breast cancer, nanoparticles have shown promise in delivering both conventional chemotherapeutic drugs and autophagy-modulating TCM compounds.146 This strategy enhances drug concentration in tumor sites and induces autophagic cell death, ultimately aiding in the breakdown of solid tumors. In the case of hepatocellular carcinoma (HCC), nanoparticles demonstrate the capacity to increase drug concentration, with specific nanoformulations allowing for targeted therapy through selective binding to surface molecules on HCC cells, thus enabling more precise therapeutic interventions.46,147,148 Additionally, nanoformulations are instrumental in aiding the permeation of the blood-brain barrier and improving drug bioavailability in the treatment of gliomas.149,150 Similarly, in the realm of colon cancer,151,152 nanoformulations containing autophagy-regulating herbal compounds have attracted considerable interest. Moreover, multifunctional nanoformulations that offer targeted delivery, controlled release, combination therapies, and the integration of diagnostic and therapeutic functionalities have shown promising results in combating tumors.
Furthermore, in addition to the aforementioned benefits, there is a notable focus on the safety of the drug. Numerous studies have provided evidence that nanoformulations incorporating active ingredients from TCM demonstrate minimal harm to surrounding tissues, maintain stability in the bloodstream, and do not typically trigger hemolytic reactions.153,154 Additionally, researchers have evaluated liver and kidney function, as well as blood biochemical markers, in mice following drug administration, with results indicating a favorable safety profile for these nanopreparations.155
The efficiency of drug delivery, which is intricately linked to drug effectiveness, is impacted by various factors such as the intrinsic characteristics of the nanopreparation and the physiological environment.156 The size of the nanoparticle is a key determinant in its biodistribution, with smaller particles displaying improved tissue penetration but also increased susceptibility to clearance in circulation.157 Furthermore, the zeta potential of the nanoformulation is a critical parameter for assessing drug stability.158 Unstable nanoformulations are susceptible to premature depolymerization in the circulatory system, hindering their ability to reach the desired target site. Additionally, the body’s natural clearance mechanisms, such as the immune system, liver, and kidneys, present obstacles to successful drug delivery.159 To overcome immunological clearance, researchers have sought to coat the external surface of nanoformulations with biologically-derived membranes, such as those sourced from red blood cells, white blood cells, platelets, and other sources.160–163 This strategy aims to evade recognition and phagocytosis by immune cells present in the circulatory system.
The synergistic benefits of integrating autophagy-regulating active components from TCM with nanoformulations have been demonstrated to effectively leverage complementary advantages. As previously elucidated, TCM’s active components possess advantages over conventional chemotherapeutic agents, including multi-targeting capabilities, reduced toxicity, and potent synergistic effects. Nevertheless, these active components are hampered by limitations such as poor solubility, low bioavailability, and a short blood circulation cycle, which impede their clinical utility in cancer therapy. Fortunately, the utilization of nano-delivery platforms offers a promising solution to overcome these challenges. The active components of TCM demonstrate multitarget effects by concurrently activating the autophagy-related pathway and inducing apoptosis, ferroptosis, and ICD. Autophagy, in this context, plays a “double-edged sword” role.164 Numerous studies have shown that autophagy induction impedes apoptosis, diminishing the protective effects of autophagy when autophagy inhibitors are utilized.165,166 On the contrary, additional research has demonstrated that autophagy facilitates ferroptosis and ICD, resulting in heightened anti-tumor efficacy when autophagy-inducing agents are utilized in combination.167 Nanoformulations provide a mechanism for implementing multi-drug regimens, enabling the concurrent administration of multiple drugs at the tumor site, thereby facilitating synergistic therapeutic outcomes.
The following Table 1 summarizes the autophagy-modulating ingredients of TCM and their nanoformulations.
 |
Table 1 Ingredients of TCM Regulating Autophagy and Its Nano-Delivery Strategy in Cancer Therapy |
Evodiamine Loaded NPs
As previously stated, EVO can modulate autophagy through its interactions with the PI3K/AKT and MAPK/ERK signaling pathways. Furthermore, it has been documented that the combination of EVO with autophagy inhibitors enhances the induction of apoptosis. In a particular investigation, the combination of EVO and CQ resulted in a substantial accumulation of autophagosomes, leading to a notable decrease in cellular activity.197 Expanding upon these findings, researchers have developed EVO-CQ-Lips, which exhibit enhanced effectiveness against cancer and reduced toxicity toward normal renal embryonic cells when compared to unencapsulated drugs.169 Subsequent studies have provided evidence that the aforementioned multifunctional delivery platforms exhibit superior biocompatibility, targeting capabilities, and controlled release in comparison to standard liposomes. For instance, the utilization of a folic acid (FA)-modified EVO-loaded micelle resulted in enhanced antitumor activity by specifically targeting cell membrane folate-binding proteins through FA.170 Another study171 involved the development of a nanoparticle co-loaded with EVO and DOX, triphenylphosphine (TPP) was utilized as an agent, while Dextran and star-polycaprolactone were connected through disulfide bonds (-s-s-) to allow micelle depolymerization in response to GSH signals within the tumor microenvironment. Furthermore, the development of EVO-loaded nanoplatforms that enhance imaging capabilities has been achieved. This has been accomplished through the utilization of heterostructures consisting of lanthanide-doped upconversion nanoparticles (DUCNPs) to transport EVO derivatives, thereby enabling in vivo fluorescence imaging using near-infrared (NIR) light.172 Simultaneously, this approach optimizes solubility and augments the inhibition of tumor growth. In a similar vein, LI et al have undertaken surface modifications with GE11, a fluorescent label, and EGFR-targeting agent, to enhance targeting efficacy and facilitate in vivo tumor imaging.173
Icaritin Loaded NPs
As previously mentioned, the induction of autophagy through the mTOR-related pathway by ICA has been observed. To treat HCC, a nano-delivery platform loaded with DOX and ICA, which combines autophagy with ICD, was developed.147 It has been demonstrated that the activation of autophagy leads to the production of ICD markers, which have been found to have positive effects on ICD. To specifically target HCC, the researchers incorporated AEAA, a functional group that binds to the σ-1 receptor, which is highly expressed on the surface of HCC cells, onto the surface of the nano-delivery platform. Additionally, other researchers have successfully created self-assembled nanoparticles consisting of ICA-pyropheophorbide-a self-assembled nanoparticles (IP NPs),174 which have shown remarkable stability and uptake capacity. In a separate investigation, the combination of ICA and Coix seed oil was enclosed within heat-sensitive liposomes, resulting in a favorable ability to infiltrate tumor spheroids.148 Certain scholars employed a cyclic tumor-penetrating peptide (iRGD), which aids in the traversal of nanocarriers through tumor vasculature and their subsequent penetration into tumor parenchyma. This peptide was covalently attached to DSPE-PEG, serving as a carrier for ICA. Additionally, the outer layer of the liposomes was modified with an erythrocyte membrane, thus creating functionalized biomimetic nanoplatforms that enhance the capability of drug delivery.175
Luteolin Loaded NPs
Prior research has demonstrated that LUT is involved in the regulation of autophagy and exerts antitumor effects by modulating the PI3K/AKT pathway. Nevertheless, the efficacy of LUT against glioblastoma is hindered by its limited ability to traverse the blood-brain barrier. To tackle this challenge, WU et al have developed FA-modified NPs (FA-PEG-PCL) that enhance biocompatibility and facilitate the transportation of LUT across the blood-brain barrier, leading to its accumulation at the tumor site.149 In the context of malignant cancers characterized by elevated levels of ROS, the utilization of ROS-responsive materials for drug delivery presents a promising approach for achieving controlled drug release. FU et al developed ROS-responsive nanoparticles (NPs) utilizing Poly (propylene sulfide)-poly (ethylene glycol) (PPS-PEG) polymers specifically for the treatment of melanoma.177 The LUT-PPS-PEG NPs exhibited a significant in vivo anti-cancer effect, as expected. Expanding on these findings, WANG et al further modified LUT-loaded NPs with tumor-targeting FA and ROS-responsive Oxi-αCD, resulting in a substantial improvement in the antitumor efficacy of LUTs.178 However, there is currently a lack of direct evidence demonstrating the ability of LUT-loaded NPs to modulate autophagy in cancer cells. In light of this, further research is needed to examine the potential role of LUT-loaded anti-cancer NPs in regulating autophagy, which provides an intriguing area for future investigation.
Epigallocatechin Gallate-Loaded NPs
Despite extensive studies on the upregulation of intracellular ATG5, Beclin1, and LC3-II induced by EGCG through various pathways, additional research is required to ascertain the comparative effectiveness of nano-delivered EGCG in inducing autophagy. To facilitate more comprehensive investigations, we present a summary of several potential strategies for delivering EGCG. Notably, FAN et al have recently developed nanoparticles by conjugating EGCG with hyaluronic acid, which specifically targets CD44, a protein that exhibits high expression in GBM cell lines. These nanoparticles facilitate the accumulation of EGCG at the tumor site and stimulate the generation of ROS, thereby inducing ferroptosis.181 In a separate investigation, YANG et al employed manganese carbonyl and dendritic mesoporous silicon as carriers for delivering EGCG. The study provided evidence that EGCG functions as an inhibitor of HSP90, thereby enhancing PTT.182 Considering the established correlation between HSP90 and autophagy, it would be intriguing to investigate whether autophagy plays a more direct role in tumor treatment when utilizing these nanodrugs.179 Furthermore, extensive research has been conducted on a nano-delivery system employing inorganic zinc as a carrier for loading EGCG.183 Similarly, the development of EGCG-loaded liposomes incorporating FA has been pursued for the targeted treatment of colon cancer.184
α-Mangostin Loaded NPs
As previously stated, MGT exhibits significant antitumor efficacy, prompting the development of diverse PEG-based nano-delivery systems for its administration. Notably, MGT-loaded MPEG-PCL nanoparticles have been employed for melanoma therapy,186 while MGT-loaded PEG-PLA nanoparticles have been explored for pancreatic cancer treatment.187 In a breast cancer study, liposomes were utilized as carriers for MGT, leading to the dissolution of breast cancer tumors at lower concentrations compared to free MGT, thereby underscoring the enhanced effectiveness of the nanoparticle formulation.188 In addition, both cyclodextrins and PEI have been employed as carriers for the delivery of MGT. The encapsulation of MGT in nanoparticles has demonstrated enhancements in drug biocompatibility and penetration, reductions in hematotoxicity, and improvements in its anti-cancer proliferative effects.189,190 It is important to highlight that autophagy induction by MGT has been extensively established through various pathways, including PI3K/AKT/mTOR, AMPK, and STAT3. Therefore, the utilization of MGT-containing nanoparticles presents a promising strategy for inducing autophagy in cancer cells.
Shikonin/Alkannin Loaded NPs
In a study conducted by LI et al, it was found that SKN, a potent inducer of autophagy, possesses various anti-cancer activities.58 To enhance the anti-cancer effect, liposomes were developed to deliver SKN and CQ. SKN not only induces ICD and autophagy but also initiates the autophagy-mediated degradation of tumor antigens, potentially attenuating the ICD response. Fortunately, the autophagy inhibitor CQ can rescue this effect. In a recent study by SHI et al, cytosine guanine dinucleotide (CpG) oligodeoxynucleotide (ODN) was designed and constructed. These nanoparticles were loaded with CpG ODN and capped by a Fe-Shikonin metal-phenol network (Alum-CpG@Fe-Shikonin NPs, MPNs).191 Upon internalization by tumor cells, these nanoparticles undergo decomposition, resulting in the formation of Fe2+ and SKN. This process subsequently triggers ICD through the induction of ferroptosis and necroptosis. Concurrently, CpG ODN initiates a cascade of anti-cancer immune responses. The combined action of both components exhibits significant anti-cancer efficacy, leading to the elimination of tumor cells and suppression of distant tumors. In a separate investigation involving the use of SKN-NPs to induce ICD, lactoferrin was employed as a carrier to encapsulate JQ1, a BET bromodomain inhibitor, along with SKN. This formulation facilitated the promotion of ICD in cancer cells.192
Honokiol Loaded NPs
HNK has been verified to induce autophagy, and this review aims to analyze current nano-delivery approaches as a basis for further comprehensive inquiries. In a study conducted by WANG et al, HNK was enclosed within hyaluronic acid (HA)-modified liposomes to achieve active targeting.194 Furthermore, in addition to liposomes, natural small molecules have been employed to generate nanocellular micelles for drug delivery. Rebaudioside A (RA), a small molecule derived from stevia, exhibits the ability to self-assemble into micelles that serve as carriers for HNK. Notably, EGCG, an autophagy inducer previously mentioned, has also been recognized as a suitable material for the creation of nanocarriers.195 In a separate investigation, a polymer composed of Chitin and EGCG was developed to load HNK.196
NPs Loaded with Other Active Ingredients of TCM
The encapsulation of paclitaxel NPs (Nab-PTX) with albumin enhanced the intracellular uptake of lung and colorectal cancer cells and has already been used in clinical practice.198 In recent research, it was observed that Nab-PTX induces autophagosome formation and upregulates LC3-II levels via the selective autophagy receptor SQSTM1/p62 (Figure 4A).199 Furthermore, there exist diverse strategies presently accessible for the delivery of curcumin via nanoparticles.200,201 Notably, in a study of chitosan liposomes co-loaded with nicotinic and curcumin, nicotinic-induced autophagy and curcumin increased the number of autophagosomes, achieving synergy at the level of autophagy induction (Figure 4B).202 In a separate investigation, scientists devised a versatile nanoplatform employing ROS-responsive Tk bonds (thioketal) for the transportation of triptolide and photosensitizers.203
 |
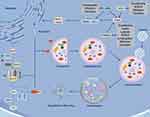
Figure 4 Autophagic activity depicted by compounds from TCM-loaded NPs in cancer. (A) Enhanced expression of autophagic proteins LC3-II and SQSTM1/p62 was observed in lung and colorectal cancer cells after treatment with Nab-PTX in Western blots and immunofluorescence. Reprinted from Lin YW, Lin TT, Chen CH, et al. Enhancing Efficacy of Albumin-Bound Paclitaxel for Human Lung and Colorectal Cancers through Autophagy Receptor Sequestosome 1 (SQSTM1)/p62-Mediated Nanodrug Delivery and Cancer therapy. ACS Nano. Oct 10 2023;17 (19):19,033–1905. Copyright 2023 American Chemical Society.199 (B) Nicotinic, curcumin, and their nanoparticles regulate expressions of autophagy-related genes (ATG7, Beclin1, mTOR, and p62) and promote autophagosomes formation. The data are shown as the means ± SD (n = 3). * p ≤ 0.05, **p ≤ 0.01, *** p ≤ 0.001 and **** p ≤ 0.0001. Reprinted from Int J Biol Macromol. Volume: 245. Hanafy NAN, Sheashaa RF, Moussa EA, Mahfouz ME. Potential of curcumin and niacin-loaded targeted chitosan-coated liposomes to activate autophagy in hepatocellular carcinoma cells: An in vitro evaluation in HePG2 cell line. 125,572. Copyright 2023, with permission from Elsevier.202 |
Discussions and Prospects
The field of oncology therapeutics has witnessed substantial advancement over the last decade in the realm of nano-delivery platforms. Approved nanomedicines for clinical application encompass Paclitaxel-loaded albumin nanoparticles (Abraxane), liposomes loading Doxorubicin (Doxil), and other formulations.70,71 Nevertheless, these nanocarriers demonstrate limited functionality, low drug delivery efficacy, and susceptibility to the emergence of drug resistance. Consequently, the adoption of multifunctional nano-delivery platforms presents a promising strategy to overcome these obstacles. The inclusion of targeting agents, such as FA and TPP, in various compounds has been demonstrated to improve the accumulation of drugs specifically at the tumor site.204–206 Controlled release mechanisms can be attained by utilizing materials that are sensitive to GSH and ROS. Additionally, the incorporation of photosensitizers and photothermal agents allows for the integration of PDT and PTT with traditional chemotherapy.60 As highlighted in this review, these versatile nanoplatforms have exhibited significant anti-cancer efficacy in both in vitro and in vivo experiments. Although multifunctional nanoplatforms show great promise, their high cost of preparation, complex production methods, and differences between human and animal environments pose significant obstacles to their clinical application.207–209 Additionally, recent research has shown that the enhanced EPR effect, a key targeting mechanism for nanomedicines, may not be effective for all types of tumors, highlighting the need for new targeting approaches.210
The incorporation of autophagy-modulating herbal compounds into nanoparticles represents a promising pursuit. Nanodelivery technology facilitates the circumvention of delivery obstacles to the tumor site, allowing for controlled release to reduce toxicity. Furthermore, this method complements other chemotherapeutic agents or PDT and PTT for a more holistic treatment approach. Research indicates that the modulation of cellular autophagy is more pronounced following drug delivery through nano-formulations as opposed to free drugs, possibly due to the concentration of drug accumulation at the tumor site. It is important to highlight that, despite the considerable research efforts directed toward integrating autophagy-regulating TCM compounds into nanopreparations, there is a lack of literature specifically examining the mechanisms by which these nanopreparations modulate autophagy in tumor cells. Therefore, a deeper comprehension of these mechanisms would significantly enhance the progress of precision tumor therapy.
Interestingly, autophagy, a cellular process of self-degradation, plays a crucial role in tumor development, progression, and treatment. Its complex involvement results in a dualistic nature, with some aspects promoting tumor cell death and others providing protection. While TCM has been found to contain active components that modulate autophagy and exhibit strong anticancer effects, the exact mechanisms remain poorly understood, necessitating further comprehensive research. Furthermore, autophagy possesses the capacity to augment the susceptibility of cancer cells to radiotherapy and chemotherapy, as well as reverse drug resistance.
In prospective studies, it is theorized that the integration of artificial intelligence (AI) may improve the effectiveness of the nanoformulation preparation process and tackle existing obstacles.211 Specifically, machine learning (ML) can be applied throughout various stages of creating versatile nano-delivery systems that transport active ingredients from TCM. ML can assist in identifying and refining new small molecule drugs, as well as forecasting potential targets for investigating novel autophagy signaling pathways.212,213 Moreover, ML is instrumental in predicting and prioritizing characterization parameters and optimizing delivery strategies in the design of nanomedicines.214 When designing nanomedicines, ML plays a crucial role in predicting and prioritizing characterization parameters, as well as optimizing delivery strategies.215 Additionally, ML can be utilized to assess and predict treatment efficacy.216 This review emphasizes the importance of autophagic flux levels and different autophagic pathways in the treatment of tumors, as well as the interplay between autophagy and other mechanisms of cell death. Therefore, ML can be employed to gather past data, develop models, and examine the relationship between autophagy and patient outcomes, as well as the crosstalk between autophagy and other forms of cell death.
Conclusions
This comprehensive review provides a summary of autophagy-regulating compounds in TCM, detailing their anticancer mechanisms and potential therapeutic value. Additionally, we synthesize multiple sources of literature to assess the potential of nanotechnology in addressing delivery challenges and augmenting the anticancer properties of these compounds. However, it is imperative to acknowledge limitations in our study, particularly the lack of in-depth exploration into the mechanisms of autophagy regulation by active compounds in TCM. Nevertheless, it is imperative to recognize specific constraints within our research. Firstly, our examination of the autophagy regulation mechanism by active compounds of TCM lacks thoroughness. Secondly, our comprehension of the interplay between autophagic death induced by autophagy-regulating active compounds of TCM and other cell death pathways is inadequate, warranting additional comprehensive investigation.
In summary, our research indicates that the incorporation of nanotechnology for the precise delivery of active TCM compounds, in conjunction with autophagy modulation as a therapeutic strategy for cancer, holds significant potential. The integration of nanotechnology, TCM components, and autophagy in cancer therapy offers a synergistic approach that overcomes the limitations of individual strategies and provides avenues for personalized and precise treatment. It is imperative to prioritize the development of affordable and safe nano-delivery systems for delivering autophagy-modulating compounds derived from TCM to advance both fundamental and clinical research in this field.
Abbreviation
AKT, Protein kinase B; AMPK, AMP-activated protein kinase; ATG, Autophagy-associated protein; Beclin1, Recombinant Beclin 1; EPR, Enhanced permeability and retention; ERK, Extracellular regulated protein kinases; ICD, Immunogenic cell death; JNK, c-Jun N-terminal kinase; Keap1, Kelch-like ECH- associated protein l; LC3, Microtubule-Associated Protein 1 Light Chain 3; MAPK, Mitogen-activated protein kinase; MEK, Mitogen activation inhibitor; ML, Machine learning; mTOR, Mammalian target of rapamycin; Nrf2, Nuclear Factor erythroid 2-Related Factor 2; PI3K, Phosphatidylinositol 3 kinase; PTEN, Phosphatase and tensin homolog deleted on chromosome ten; RIP, Receptor interacting protein; SQSTM1, Recombinant Sequestosome 1; TCM, Traditional Chinese Medicine; TPP, Triphenylphosphine; ULK1, UNC-51 Like Kinase 1; VPS34, Vacuolar Protein Sorting 3.
Data Sharing Statement
The current study does not have any datasets generated or analyzed for data sharing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting and writing, substantially revised or critically reviewed the article; have agreed on the journal to which the article will be submitted; reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication; and agree to be accountable for all aspects of the work.
Funding
This study was not supported by grants from the institutional funds.
Disclosure
The authors declare no competing interests in this work.
References
1. Wild CP. The global cancer burden: necessity is the mother of prevention. Nat Rev Cancer. 2019;19(3):123–124. doi:10.1038/s41568-019-0110-3
2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. Ca a Cancer J Clinicians. 2023;73(1):17–48. doi:10.3322/caac.21763
3. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi:10.3322/caac.20107
4. Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72(5):409–436. doi:10.3322/caac.21731
5. Zhang QY, Wang FX, Jia KK, Kong LD. Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects. Front Pharmacol. 2018;9:1253. doi:10.3389/fphar.2018.01253
6. Moding EJ, Kastan MB, Kirsch DG. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat Rev Drug Discov. 2013;12(7):526–542. doi:10.1038/nrd4003
7. Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol. 2020;15(4):313–320. doi:10.1038/s41565-020-0669-6
8. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70(2):86–104. doi:10.3322/caac.21596
9. Zhang Y, Li M, Gao X, Chen Y, Liu T. Nanotechnology in cancer diagnosis: progress, challenges and opportunities. J Hematol Oncol. 2019;12(1):137. doi:10.1186/s13045-019-0833-3
10. Dessale M, Mengistu G, Mengist HM. Nanotechnology: a Promising Approach for Cancer Diagnosis, Therapeutics and Theragnosis. Int J Nanomed. 2022;17:3735–3749. doi:10.2147/ijn.S378074
11. Ghanbari-Movahed M, Mondal A, Farzaei MH, Bishayee A. Quercetin- and rutin-based nano-formulations for cancer treatment: a systematic review of improved efficacy and molecular mechanisms. Phytomedicine. 2022;97:153909. doi:10.1016/j.phymed.2021.153909
12. Kim B, Park JE, Im E, et al. Recent Advances in Nanotechnology with Nano-Phytochemicals: molecular Mechanisms and Clinical Implications in Cancer Progression. Int J Mol Sci. 2021;22(7):3571. doi:10.3390/ijms22073571
13. Joshi S, Durden DL. Combinatorial Approach to Improve Cancer Immunotherapy: rational Drug Design Strategy to Simultaneously Hit Multiple Targets to Kill Tumor Cells and to Activate the Immune System. J Oncol. 2019;2019:5245034. doi:10.1155/2019/5245034
14. Zhang H, Qin C, An C, et al. Application of the CRISPR/Cas9-based gene editing technique in basic research, diagnosis, and therapy of cancer. Mol Cancer. 2021;20(1):126. doi:10.1186/s12943-021-01431-6
15. Wei Z, Chen J, Zuo F, et al. Traditional Chinese Medicine has great potential as candidate drugs for lung cancer: a review. J Ethnopharmacol. 2023;300:115748. doi:10.1016/j.jep.2022.115748
16. Wang X, Li J, Chen R, Li T, Chen M. Active Ingredients from Chinese Medicine for Combination Cancer Therapy. Int J Biol Sci. 2023;19(11):3499–3525. doi:10.7150/ijbs.77720
17. Wang S, Long S, Deng Z, Wu W. Positive Role of Chinese Herbal Medicine in Cancer Immune Regulation. Am J Chin Med. 2020;48(7):1577–1592. doi:10.1142/s0192415x20500780
18. Peng F, Xie X, Peng C. Chinese Herbal Medicine-Based Cancer Therapy: novel Anticancer Agents Targeting MicroRNAs to Regulate Tumor Growth and Metastasis. Am J Chin Med. 2019;47(8):1711–1735. doi:10.1142/s0192415x19500873
19. Yan Q, Zhang Y, Wang Q, Yuan L. Autophagy: a Double-Edged Sword in Male Reproduction. Int J Mol Sci. 2022;23(23):15273. doi:10.3390/ijms232315273
20. Klionsky DJ, Petroni G, Amaravadi RK, et al. Autophagy in major human diseases. EMBO j. 2021;40(19):e108863. doi:10.15252/embj.2021108863
21. White E. The role for autophagy in cancer. J Clin Invest. 2015;125(1):42–46. doi:10.1172/jci73941
22. Choi KS. Autophagy and cancer. Exp Mol Med. 2012;44(2):109–120. doi:10.3858/emm.2012.44.2.033
23. Wang ZY, Liu J, Zhu Z, et al. Traditional Chinese medicine compounds regulate autophagy for treating neurodegenerative disease: a mechanism review. Biomed Pharmacother. 2021;133:110968. doi:10.1016/j.biopha.2020.110968
24. Shi X, Chang M, Zhao M, Shi Y, Zhang Y. Traditional Chinese medicine compounds ameliorating glomerular diseases via autophagy: a mechanism review. Biomed Pharmacother. 2022;156:113916. doi:10.1016/j.biopha.2022.113916
25. Cui B, Yu JM. Autophagy: a new pathway for traditional Chinese medicine. J Asian Nat Prod Res. 2018;20(1):14–26. doi:10.1080/10286020.2017.1374948
26. Zhao W, Zheng XD, Tang PY, et al. Advances of antitumor drug discovery in traditional Chinese medicine and natural active products by using multi-active components combination. Med Res Rev. 2023;43(5):1778–1808. doi:10.1002/med.21963
27. Dehelean CA, Marcovici I, Soica C, et al. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules. 2021;26(4):1109. doi:10.3390/molecules26041109
28. Yousefi M, Narmani A, Jafari SM. Dendrimers as efficient nanocarriers for the protection and delivery of bioactive phytochemicals. Adv Colloid Interface Sci. 2020;278:102125. doi:10.1016/j.cis.2020.102125
29. Avula LR, Grodzinski P. Nanotechnology-aided advancement in the combating of cancer metastasis. Cancer Metastasis Rev. 2022;41(2):383–404. doi:10.1007/s10555-022-10025-7
30. Singh A, Amiji MM. Application of nanotechnology in medical diagnosis and imaging. Curr Opin Biotechnol. 2022;74:241–246. doi:10.1016/j.copbio.2021.12.011
31. Nasir A, Khan A, Li J, et al. Nanotechnology, A Tool for Diagnostics and Treatment of Cancer. Curr Top Med Chem. 2021;21(15):1360–1376. doi:10.2174/1568026621666210701144124
32. Liu XL, Dong X, Yang SC, et al. Biomimetic Liposomal Nanoplatinum for Targeted Cancer Chemophototherapy. Adv Sci (Weinh). 2021;8(8):2003679. doi:10.1002/advs.202003679
33. Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71. doi:10.1186/s12951-018-0392-8
34. Fu S, Li G, Zang W, Zhou X, Shi K, Zhai Y. Pure drug nano-assemblies: a facile carrier-free nanoplatform for efficient cancer therapy. Acta Pharm Sin B. 2022;12(1):92–106. doi:10.1016/j.apsb.2021.08.012
35. Hu T, Qin Z, Shen C, Gong HL, He ZY. Multifunctional Mitochondria-Targeting Nanosystems for Enhanced Anticancer Efficacy. Front Bioeng Biotechnol. 2021;9:786621. doi:10.3389/fbioe.2021.786621
36. Wathoni N, Rusdin A, Motoyama K, Joni IM, Lesmana R, Muchtaridi M. Nanoparticle Drug Delivery Systems for α-Mangostin. Nanotechnol Sci Appl. 2020;13:23–36. doi:10.2147/nsa.S243017
37. Wu K, Yu B, Li D, Tian Y, Liu Y, Jiang J. Recent Advances in Nanoplatforms for the Treatment of Osteosarcoma. Front Oncol. 2022;12:805978. doi:10.3389/fonc.2022.805978
38. Jampilek J, Kralova K. Anticancer Applications of Essential Oils Formulated into Lipid-Based Delivery Nanosystems. Pharmaceutics. 2022;14(12):2681. doi:10.3390/pharmaceutics14122681
39. Wahi A, Bishnoi M, Raina N, et al. Recent updates on nano-phyto-formulations based therapeutic intervention for cancer treatment. Oncol Res. 2023;32(1):19–47. doi:10.32604/or.2023.042228
40. Kumari S, Goyal A, Sönmez Gürer E, et al. Bioactive Loaded Novel Nano-Formulations for Targeted Drug Delivery and Their Therapeutic Potential. Pharmaceutics. 2022;14(5):1091. doi:10.3390/pharmaceutics14051091
41. Yang X, Zhao M, Wu Z, et al. Nano-ultrasonic Contrast Agent for Chemoimmunotherapy of Breast Cancer by Immune Metabolism Reprogramming and Tumor Autophagy. ACS Nano. 2022;16(2):3417–3431. doi:10.1021/acsnano.2c00462
42. Björnmalm M, Thurecht KJ, Michael M, Scott AM, Caruso F. Bridging Bio-Nano Science and Cancer Nanomedicine. ACS Nano. 2017;11(10):9594–9613. doi:10.1021/acsnano.7b04855
43. Fang J, Islam W, Maeda H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv Drug Deliv Rev. 2020;157:142–160. doi:10.1016/j.addr.2020.06.005
44. Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14(1):85. doi:10.1186/s13045-021-01096-0
45. Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed Engl. 2014;53(46):12320–12364. doi:10.1002/anie.201403036
46. Lian B, Wu M, Feng Z, Deng Y, Zhong C, Zhao X. Folate-conjugated human serum albumin-encapsulated resveratrol nanoparticles: preparation, characterization, bioavailability and targeting of liver tumors. Artif Cells Nanomed Biotechnol. 2019;47(1):154–165. doi:10.1080/21691401.2018.1548468
47. Fu X, Shi Y, Qi T, et al. Precise design strategies of nanomedicine for improving cancer therapeutic efficacy using subcellular targeting. Signal Transduct Target Ther. 2020;5(1):262. doi:10.1038/s41392-020-00342-0
48. Xiao Y, Zhang T, Ma X, et al. Microenvironment-Responsive Prodrug-Induced Pyroptosis Boosts Cancer Immunotherapy. Adv Sci (Weinh). 2021;8(24):e2101840. doi:10.1002/advs.202101840
49. Lu CH, Willner I. Stimuli-responsive DNA-functionalized nano-/microcontainers for switchable and controlled release. Angew Chem Int Ed Engl. 2015;54(42):12212–12235. doi:10.1002/anie.201503054
50. Price R, Poursaid A, Ghandehari H. Controlled release from recombinant polymers. J Control Release. 2014;190:304–313. doi:10.1016/j.jconrel.2014.06.016
51. Cheng X, Xu HD, Ran HH, Liang G, Wu FG. Glutathione-Depleting Nanomedicines for Synergistic Cancer Therapy. ACS Nano. 2021;15(5):8039–8068. doi:10.1021/acsnano.1c00498
52. Liu J, Huang Y, Kumar A, et al. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol Adv. 2014;32(4):693–710. doi:10.1016/j.biotechadv.2013.11.009
53. Duan J, Li Y, Gao J, Cao R, Shang E, Zhang W. ROS-mediated photoaging pathways of nano- and micro-plastic particles under UV irradiation. Water Res. 2022;216:118320. doi:10.1016/j.watres.2022.118320
54. Hameed S, Zhang M, Bhattarai P, Mustafa G, Dai Z. Enhancing cancer therapeutic efficacy through ultrasound-mediated micro-to-nano conversion. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(3):e1604. doi:10.1002/wnan.1604
55. Zhu R, He Q, Li Z, et al. ROS-cleavable diselenide nanomedicine for NIR-controlled drug release and on-demand synergistic chemo-photodynamic therapy. Acta Biomater. 2022;153:442–452. doi:10.1016/j.actbio.2022.09.061
56. Staffa SJ, Kohane DS, Zurakowski D. Synergy in Nanomedicine: what It Is Not, and What It Might Be. Nano Lett. 2021;21(13):5457–5460. doi:10.1021/acs.nanolett.1c01894
57. Bar-Zeev M, Livney YD, Assaraf YG. Targeted nanomedicine for cancer therapeutics: towards precision medicine overcoming drug resistance. Drug Resist Updat. 2017;31:15–30. doi:10.1016/j.drup.2017.05.002
58. Li J, Cai W, Yu J, et al. Autophagy inhibition recovers deficient ICD-based cancer immunotherapy. Biomaterials. 2022;287:121651. doi:10.1016/j.biomaterials.2022.121651
59. Menon JU, Jadeja P, Tambe P, Vu K, Yuan B, Nguyen KT. Nanomaterials for photo-based diagnostic and therapeutic applications. Theranostics. 2013;3(3):152–166. doi:10.7150/thno.5327
60. Pan WL, Tan Y, Meng W, et al. Microenvironment-driven sequential ferroptosis, photodynamic therapy, and chemotherapy for targeted breast cancer therapy by a cancer-cell-membrane-coated nanoscale metal-organic framework. Biomaterials. 2022;283:121449. doi:10.1016/j.biomaterials.2022.121449
61. Chen Z, Zhao P, Luo Z, et al. Cancer Cell Membrane-Biomimetic Nanoparticles for Homologous-Targeting Dual-Modal Imaging and Photothermal Therapy. ACS Nano. 2016;10(11):10049–10057. doi:10.1021/acsnano.6b04695
62. Kozma GT, Shimizu T, Ishida T, Szebeni J. Anti-PEG antibodies: properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv Drug Deliv Rev. 2020;154-155:163–175. doi:10.1016/j.addr.2020.07.024
63. Guo S, Shi Y, Liang Y, Liu L, Sun K, Li Y. Relationship and improvement strategies between drug nanocarrier characteristics and hemocompatibility: what can we learn from the literature. Asian J Pharm Sci. 2021;16(5):551–576. doi:10.1016/j.ajps.2020.12.002
64. Wang B, He X, Zhang Z, Zhao Y, Feng W. Metabolism of nanomaterials in vivo: blood circulation and organ clearance. Acc Chem Res. 2013;46(3):761–769. doi:10.1021/ar2003336
65. Villeret B, Dieu A, Straube M, et al. Silver Nanoparticles Impair Retinoic Acid-Inducible Gene I-Mediated Mitochondrial Antiviral Immunity by Blocking the Autophagic Flux in Lung Epithelial Cells. ACS Nano. 2018;12(2):1188–1202. doi:10.1021/acsnano.7b06934
66. Wan HY, Chen JL, Zhu X, Liu L, Wang J, Zhu XM. Titania-Coated Gold Nano-Bipyramids for Blocking Autophagy Flux and Sensitizing Cancer Cells to Proteasome Inhibitor-Induced Death. Adv Sci (Weinh). 2018;5(3):1700585. doi:10.1002/advs.201700585
67. Xu Q, Zhang H, Liu H, Han Y, Qiu W, Li Z. Inhibiting autophagy flux and DNA repair of tumor cells to boost radiotherapy of orthotopic glioblastoma. Biomaterials. 2022;280:121287. doi:10.1016/j.biomaterials.2021.121287
68. Ruan S, Xie R, Qin L, et al. Aggregable Nanoparticles-Enabled Chemotherapy and Autophagy Inhibition Combined with Anti-PD-L1 Antibody for Improved Glioma Treatment. Nano Lett. 2019;19(11):8318–8332. doi:10.1021/acs.nanolett.9b03968
69. De Dosso S, Siebenhüner AR, Winder T, et al. Treatment landscape of metastatic pancreatic cancer. Cancer Treat Rev. 2021;96:102180. doi:10.1016/j.ctrv.2021.102180
70. Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Control Release. 2013;170(3):365–372. doi:10.1016/j.jconrel.2013.05.041
71. Barenholz Y. Doxil®--The first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–134. doi:10.1016/j.jconrel.2012.03.020
72. Hulst MB, Grocholski T, Neefjes JJC, van Wezel GP, Metsä-Ketelä M. Anthracyclines: biosynthesis, engineering and clinical applications. Nat Prod Rep. 2022;39(4):814–841. doi:10.1039/d1np00059d
73. Pujade-Lauraine E, Fujiwara K, Ledermann JA, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, Phase 3 study. Lancet Oncol. 2021;22(7):1034–1046. doi:10.1016/s1470-2045(21)00216-3
74. Emens LA, Adams S, Barrios CH, et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: iMpassion130 final overall survival analysis. Ann Oncol. 2021;32(8):983–993. doi:10.1016/j.annonc.2021.05.355
75. Zou L, Liao M, Zhen Y, et al. Autophagy and beyond: unraveling the complexity of UNC-51-like kinase 1 (ULK1) from biological functions to therapeutic implications. Acta Pharm Sin B. 2022;12(10):3743–3782. doi:10.1016/j.apsb.2022.06.004
76. Grunwald DS, Otto NM, Park JM, Song D, Kim DH. GABARAPs and LC3s have opposite roles in regulating ULK1 for autophagy induction. Autophagy. 2020;16(4):600–614. doi:10.1080/15548627.2019.1632620
77. Tanida I. Autophagy basics. Microbiol Immunol. 2011;55(1):1–11. doi:10.1111/j.1348-0421.2010.00271.x
78. Frudd K, Burgoyne T, Burgoyne JR. Oxidation of Atg3 and Atg7 mediates inhibition of autophagy. Nat Commun. 2018;9(1):95. doi:10.1038/s41467-017-02352-z
79. Kaiser SE, Mao K, Taherbhoy AM, et al. Noncanonical E2 recruitment by the autophagy E1 revealed by Atg7-Atg3 and Atg7-Atg10 structures. Nat Struct Mol Biol. 2012;19(12):1242–1249. doi:10.1038/nsmb.2415
80. Ichimura Y, Komatsu M. Selective degradation of p62 by autophagy. Semin Immunopathol. 2010;32(4):431–436. doi:10.1007/s00281-010-0220-1
81. Sui X, Chen R, Wang Z, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4(10):e838. doi:10.1038/cddis.2013.350
82. Zhang Y, Zhang L, Gao J, Wen L. Pro-Death or Pro-Survival: contrasting Paradigms on Nanomaterial-Induced Autophagy and Exploitations for Cancer Therapy. Acc Chem Res. 2019;52(11):3164–3176. doi:10.1021/acs.accounts.9b00397
83. Usman RM, Razzaq F, Akbar A, et al. Role and mechanism of autophagy-regulating factors in tumorigenesis and drug resistance. Asia Pac J Clin Oncol. 2021;17(3):193–208. doi:10.1111/ajco.13449
84. Liu S, Yao S, Yang H, Liu S, Wang Y. Autophagy: regulator of cell death. Cell Death Dis. 2023;14(10):648. doi:10.1038/s41419-023-06154-8
85. Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer. 2020;19(1):12. doi:10.1186/s12943-020-1138-4
86. Towers CG, Wodetzki D, Thorburn A. Autophagy and cancer: modulation of cell death pathways and cancer cell adaptations. J Cell Biol. 2020;219(1):33. doi:10.1083/jcb.201909033
87. Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013;1833(12):3448–3459. doi:10.1016/j.bbamcr.2013.06.001
88. Saleem S. Apoptosis, Autophagy, Necrosis and Their Multi Galore Crosstalk in Neurodegeneration. Neuroscience. 2021;469:162–174. doi:10.1016/j.neuroscience.2021.06.023
89. Wu J, Ye J, Xie Q, Liu B, Liu M. Targeting Regulated Cell Death with Pharmacological Small Molecules: an Update on Autophagy-Dependent Cell Death, Ferroptosis, and Necroptosis in Cancer. J Med Chem. 2022;65(4):2989–3001. doi:10.1021/acs.jmedchem.1c01572
90. Gao W, Wang X, Zhou Y, Wang X, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. 2022;7(1):196. doi:10.1038/s41392-022-01046-3
91. He C, Bassik MC, Moresi V, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481(7382):511–515. doi:10.1038/nature10758
92. Wu W, Wang X, Sun Y, et al. TNF-induced necroptosis initiates early autophagy events via RIPK3-dependent AMPK activation, but inhibits late autophagy. Autophagy. 2021;17(12):3992–4009. doi:10.1080/15548627.2021.1899667
93. Liu X, Liu L, Wang X, et al. Necroptosis inhibits autophagy by regulating the formation of RIP3/p62/Keap1 complex in shikonin-induced ROS dependent cell death of human bladder cancer. Phytomedicine. 2023;118:154943. doi:10.1016/j.phymed.2023.154943
94. Lee S, Hwang N, Seok BG, Lee S, Lee SJ, Chung SW. Autophagy mediates an amplification loop during ferroptosis. Cell Death Dis. 2023;14(7):464. doi:10.1038/s41419-023-05978-8
95. Barman R, Bora PK, Saikia J, et al. Nutmegs and wild nutmegs: an update on ethnomedicines, phytochemicals, pharmacology, and toxicity of the Myristicaceae species. Phytother Res. 2021;35(9):4632–4659. doi:10.1002/ptr.7098
96. Qin R, You FM, Zhao Q, et al. Naturally derived indole alkaloids targeting regulated cell death (RCD) for cancer therapy: from molecular mechanisms to potential therapeutic targets. J Hematol Oncol. 2022;15(1):133. doi:10.1186/s13045-022-01350-z
97. Khan H, Ullah H, Martorell M, et al. Flavonoids nanoparticles in cancer: treatment, prevention and clinical prospects. Semin Cancer Biol. 2021;69:200–211. doi:10.1016/j.semcancer.2019.07.023
98. Küpeli Akkol E, Genç Y, Karpuz B, Sobarzo-Sánchez E, Capasso R. Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers (Basel). 2020;12(7):1959doi. doi:10.3390/cancers12071959
99. Jang WY, Kim MY, Cho JY. Antioxidant, Anti-Inflammatory, Anti-Menopausal, and Anti-Cancer Effects of Lignans and Their Metabolites. Int J Mol Sci. 2022;23(24):15482. doi:10.3390/ijms232415482
100. Debnath J, Gammoh N, Ryan KM. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol. 2023;24(8):560–575. doi:10.1038/s41580-023-00585-z
101. Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528–542. doi:10.1038/nrc.2017.53
102. Fan L, Wu L, Yu XH, Chen YB, Lin L, Li SG. The ethnopharmacology, phytochemistry, pharmacology and toxicology of the genus Erycibe (Convolvulaceae). J Ethnopharmacol. 2021;278:114312. doi:10.1016/j.jep.2021.114312
103. Tu YJ, Fan X, Yang X, Zhang C, Liang HP. Evodiamine activates autophagy as a cytoprotective response in murine Lewis lung carcinoma cells. Oncol Rep. 2013;29(2):481–490. doi:10.3892/or.2012.2125
104. Hong Z, Wang Z, Zhou B, et al. Effects of evodiamine on PI3K/Akt and MAPK/ERK signaling pathways in pancreatic cancer cells. Int J Oncol. 2020;56(3):783–793. doi:10.3892/ijo.2020.4956
105. Liu AJ, Wang SH, Chen KC, et al. Evodiamine, a plant alkaloid, induces calcium/JNK-mediated autophagy and calcium/mitochondria-mediated apoptosis in human glioblastoma cells. Chem Biol Interact. 2013;205(1):20–28. doi:10.1016/j.cbi.2013.06.004
106. Rasul A, Yu B, Zhong L, Khan M, Yang H, Ma T. Cytotoxic effect of evodiamine in SGC-7901 human gastric adenocarcinoma cells via simultaneous induction of apoptosis and autophagy. Oncol Rep. 2012;27(5):1481–1487. doi:10.3892/or.2012.1694
107. Liu XM, Li Z, Xie XR, et al. Combination of DNA Damage, Autophagy, and ERK Inhibition: novel Evodiamine-Inspired Multi-Action Pt(IV) Prodrugs with High-Efficiency and Low-Toxicity Antitumor Activity. J Med Chem. 2023;66(3):1852–1872. doi:10.1021/acs.jmedchem.2c01660
108. Jiang S, Chang H, Deng S, Fan D. Icariin enhances the chemosensitivity of cisplatin resistant ovarian cancer cells by suppressing autophagy via activation of the AKT/mTOR/ATG5 pathway. Int J Oncol. 2019;54(6):1933–1942. doi:10.3892/ijo.2019.4785
109. Cheng X, Tan S, Duan F, Yuan Q, Li Q, Deng G. Icariin induces apoptosis by suppressing autophagy in tamoxifen-resistant breast cancer cell line MCF-7/TAM. Breast Cancer. 2019;26(6):766–775. doi:10.1007/s12282-019-00980-5
110. Xu W, Ding J, Li B, et al. Effects of icariin and curcumol on autophagy, ferroptosis, and lipid metabolism based on miR-7/m-TOR/SREBP1 pathway on prostate cancer. Biofactors. 2023;49(2):438–456. doi:10.1002/biof.1927
111. Çetinkaya M, Baran Y. Therapeutic Potential of Luteolin on Cancer. Vaccines. 2023;11(3):554. doi:10.3390/vaccines11030554
112. Imran M, Rauf A, Abu-Izneid T, et al. Luteolin, a flavonoid, as an anticancer agent: a review. Biomed Pharmacother. 2019;112:108612. doi:10.1016/j.biopha.2019.108612
113. Cao Z, Zhang H, Cai X, et al. Luteolin Promotes Cell Apoptosis by Inducing Autophagy in Hepatocellular Carcinoma. Cell Physiol Biochem. 2017;43(5):1803–1812. doi:10.1159/000484066
114. Monti E, Marras E, Prini P, Gariboldi MB. Luteolin impairs hypoxia adaptation and progression in human breast and colon cancer cells. Eur J Pharmacol. 2020;881:173210. doi:10.1016/j.ejphar.2020.173210
115. Liu Q, Zhu D, Hao B, Zhang Z, Tian Y. Luteolin promotes the sensitivity of cisplatin in ovarian cancer by decreasing PRPA1-medicated autophagy. Cell Mol Biol (Noisy-le-Grand). 2018;64(6):17–22.
116. Wu L, Lin Y, Gao S, et al. Luteolin inhibits triple-negative breast cancer by inducing apoptosis and autophagy through SGK1-FOXO3a-BNIP3 signaling. Front Pharmacol. 2023;14:1200843. doi:10.3389/fphar.2023.1200843
117. Ferrari E, Bettuzzi S, Naponelli V. The Potential of Epigallocatechin Gallate (EGCG) in Targeting Autophagy for Cancer Treatment: a Narrative Review. Int J Mol Sci. 2022;23(11):6075. doi:10.3390/ijms23116075
118. Senggunprai L, Kukongviriyapan V, Prawan A, Kukongviriyapan U. Quercetin and EGCG exhibit chemopreventive effects in cholangiocarcinoma cells via suppression of JAK/STAT signaling pathway. Phytother Res. 2014;28(6):841–848. doi:10.1002/ptr.5061
119. Yuan CH, Horng CT, Lee CF, et al. Epigallocatechin gallate sensitizes cisplatin-resistant oral cancer CAR cell apoptosis and autophagy through stimulating AKT/STAT3 pathway and suppressing multidrug resistance 1 signaling. Environ Toxicol: Int J. 2017;32(3):845–855. doi:10.1002/tox.22284
120. Zhao L, Liu S, Xu J, et al. A new molecular mechanism underlying the EGCG-mediated autophagic modulation of AFP in HepG2 cells. Cell Death Dis. 2017;8(11):e3160. doi:10.1038/cddis.2017.563
121. Meng J, Chang C, Chen Y, Bi F, Ji C, Liu W. EGCG overcomes gefitinib resistance by inhibiting autophagy and augmenting cell death through targeting ERK phosphorylation in NSCLC. Onco Targets Ther. 2019;12:6033–6043. doi:10.2147/ott.S209441
122. Alam M, Rashid S, Fatima K, et al. Biochemical features and therapeutic potential of α-Mangostin: mechanism of action, medicinal values, and health benefits. Biomed Pharmacother. 2023;163:114710. doi:10.1016/j.biopha.2023.114710
123. Kim SM, Han JM, Le TT, Sohng JK, Jung HJ. Anticancer and Antiangiogenic Activities of Novel α-Mangostin Glycosides in Human Hepatocellular Carcinoma Cells via Downregulation of c-Met and HIF-1α. Int J Mol Sci. 2020;21(11):4043. doi:10.3390/ijms21114043
124. Huang W, Liang Y, Ma X. Alpha-mangostin induces endoplasmic reticulum stress and autophagy which count against fatty acid synthase inhibition mediated apoptosis in human breast cancer cells. Cancer Cell Int. 2019;19:151. doi:10.1186/s12935-019-0869-z
125. Wang F, Ma H, Liu Z, Huang W, Xu X, Zhang X. α-Mangostin inhibits DMBA/TPA-induced skin cancer through inhibiting inflammation and promoting autophagy and apoptosis by regulating PI3K/Akt/mTOR signaling pathway in mice. Biomed Pharmacother. 2017;92:672–680. doi:10.1016/j.biopha.2017.05.129
126. Chao AC, Hsu YL, Liu CK, Kuo PL. α-Mangostin, a dietary xanthone, induces autophagic cell death by activating the AMP-activated protein kinase pathway in glioblastoma cells. J Agric Food Chem. 2011;59(5):2086–2096. doi:10.1021/jf1042757
127. Li RR, Zeng DY. The effects and mechanism of α-mangostin on chemosensitivity of gastric cancer cells. Kaohsiung J Med Sci. 2021;37(8):709–717. doi:10.1002/kjm2.12388
128. Xin Z, Haoran H, ZHenyi Z, Liyang Y, Pingping F, Zengjiang Y. Effect of Shikonin on autophagy and apoptosis of human gastric cancer MGC803 cells by regulating PI3K/Akt/mTOR. China CurrAdv Gen Surg. 2023;26(07):511–515.
129. Xin Z, Haoran H, Ruifeng Q. Shikonin Reverses Oxaliplatin Resistance of Human Gastric Cancer Cells by lnhibiting Pl3K/Akt/mTOR Pathway. J RES MED SCI. 2023;52(06):127–131+35.
130. Kun F, Fang Q, Yani D. Effects of Shikonin on Ovarian Cancer SK-OV-3 Cells Through Autophagy Mediated by Keap1/Nrf2 Signaling Pathway. Tradit Chin Drug Res Pharmacol. 2023;34(08):1053–1060. doi:10.19378/j.issn.1003-9783.2023.08.005
131. Zhang J, Shang L, Jiang W, Wu W. Shikonin induces apoptosis and autophagy via downregulation of pyrroline-5-carboxylate reductase1 in hepatocellular carcinoma cells. Bioengineered. 2022;13(3):7904–7918. doi:10.1080/21655979.2022.2052673
132. Zhang N, Peng F, Wang Y, et al. Shikonin induces colorectal carcinoma cells apoptosis and autophagy by targeting galectin-1/JNK signaling axis. Int J Biol Sci. 2020;16(1):147–161. doi:10.7150/ijbs.36955
133. Liu Y, Kang X, Niu G, et al. Shikonin induces apoptosis and prosurvival autophagy in human melanoma A375 cells via ROS-mediated ER stress and p38 pathways. Artif Cells Nanomed Biotechnol. 2019;47(1):626–635. doi:10.1080/21691401.2019.1575229
134. Zheng Q, Li Q, Zhao G, et al. Alkannin induces cytotoxic autophagy and apoptosis by promoting ROS-mediated mitochondrial dysfunction and activation of JNK pathway. Biochem Pharmacol. 2020;180:114167. doi:10.1016/j.bcp.2020.114167
135. Ju X, Zhang H, Wang J, Sun Z, Guo L, Wang Q. Shikonin triggers GSDME-mediated pyroptosis in tumours by regulating autophagy via the ROS-MAPK14/p38α axis. Phytomedicine. 2023;109:154596. doi:10.1016/j.phymed.2022.154596
136. Kim HJ, Hwang KE, Park DS, et al. Shikonin-induced necroptosis is enhanced by the inhibition of autophagy in non-small cell lung cancer cells. J Transl Med. 2017;15(1):123. doi:10.1186/s12967-017-1223-7
137. Min LUO, Ping MIU, Ai-xiang WU, Zhong-yi SHAO, Hua YANG. Honokiol induces autophagy by inhibiting mTOR signaling pathway in lung cancer A549 cells. Chin J Pathophysiol. 2019;35(07):1195–1198.
138. Huang K, Chen Y, Zhang R, et al. Honokiol induces apoptosis and autophagy via the ROS/ERK1/2 signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2018;9(2):157. doi:10.1038/s41419-017-0166-5
139. Yeh PS, Wang W, Chang YA, Lin CJ, Wang JJ, Chen RM. Honokiol induces autophagy of neuroblastoma cells through activating the PI3K/Akt/mTOR and endoplasmic reticular stress/ERK1/2 signaling pathways and suppressing cell migration. Cancer Lett. 2016;370(1):66–77. doi:10.1016/j.canlet.2015.08.030
140. Xu T, Tian W, Zhang Q, et al. Novel 1,3,4-thiadiazole/oxadiazole-linked honokiol derivatives suppress cancer via inducing PI3K/Akt/mTOR-dependent autophagy. Bioorg Chem. 2021;115:105257. doi:10.1016/j.bioorg.2021.105257
141. Hong P, Liu QW, Xie Y, et al. Echinatin suppresses esophageal cancer tumor growth and invasion through inducing AKT/mTOR-dependent autophagy and apoptosis. Cell Death Dis. 2020;11(7):524. doi:10.1038/s41419-020-2730-7
142. Zhou J, Jiang YY, Chen H, Wu YC, Zhang L. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 2020;53(2):e12739. doi:10.1111/cpr.12739
143. El-Baba C, Baassiri A, Kiriako G, et al. Terpenoids’ anti-cancer effects: focus on autophagy. Apoptosis. 2021;26(9–10):491–511. doi:10.1007/s10495-021-01684-y
144. Li J, Fan Y, Zhang Y, Liu Y, Yu Y, Ma M. Resveratrol Induces Autophagy and Apoptosis in Non-Small-Cell Lung Cancer Cells by Activating the NGFR-AMPK-mTOR Pathway. Nutrients. 2022;14(12):22413. doi:10.3390/nu14122413
145. Cao S, Wang C, Yan J, Li X, Wen J, Hu C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway. Free Radic Biol Med. 2020;147:8–22. doi:10.1016/j.freeradbiomed.2019.12.004
146. Shrivastava N, Parikh A, Dewangan RP, et al. Solid Self-Nano Emulsifying Nanoplatform Loaded with Tamoxifen and Resveratrol for Treatment of Breast Cancer. Pharmaceutics. 2022;14(7):1486. doi:10.3390/pharmaceutics14071486
147. Yu Z, Guo J, Hu M, Gao Y, Huang L. Icaritin Exacerbates Mitophagy and Synergizes with Doxorubicin to Induce Immunogenic Cell Death in Hepatocellular Carcinoma. ACS Nano. 2020;14(4):4816–4828. doi:10.1021/acsnano.0c00708
148. Guo J, Zeng H, Liu Y, et al. Multicomponent thermosensitive lipid complexes enhance desmoplastic tumor therapy through boosting anti-angiogenesis and synergistic strategy. Int J Pharm. 2021;601:120533. doi:10.1016/j.ijpharm.2021.120533
149. Wu C, Xu Q, Chen X, Liu J. Delivery luteolin with folacin-modified nanoparticle for glioma therapy. Int J Nanomed. 2019;14:7515–7531. doi:10.2147/ijn.S214585
150. Jhaveri A, Deshpande P, Pattni B, Torchilin V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J Control Release. 2018;277:89–101. doi:10.1016/j.jconrel.2018.03.006
151. Chandra Boinpelly V, Verma RK, Srivastav S, Srivastava RK, Shankar S. α-Mangostin-encapsulated PLGA nanoparticles inhibit colorectal cancer growth by inhibiting Notch pathway. J Cell Mol Med. 2020;24(19):11343–11354. doi:10.1111/jcmm.15731
152. Zhang Z, Ji Y, Hu N, et al. Ferroptosis-induced anticancer effect of resveratrol with a biomimetic nano-delivery system in colorectal cancer treatment. Asian J Pharm Sci. 2022;17(5):751–766. doi:10.1016/j.ajps.2022.07.006
153. Ma BL, Ma YM. Pharmacokinetic herb-drug interactions with traditional Chinese medicine: progress, causes of conflicting results and suggestions for future research. Drug Metab Rev. 2016;48(1):1–26. doi:10.3109/03602532.2015.1124888
154. Wei D, Yang H, Zhang Y, et al. Nano-traditional Chinese medicine: a promising strategy and its recent advances. J Mater Chem B. 2022;10(16):2973–2994. doi:10.1039/d2tb00225f
155. Wang M, Chen L, Liu D, Chen H, Tang DD, Zhao YY. Metabolomics highlights pharmacological bioactivity and biochemical mechanism of traditional Chinese medicine. Chem Biol Interact. 2017;273:133–141. doi:10.1016/j.cbi.2017.06.011
156. Sithole MN, Marais S, Maree SM, Du Plessis LH, Du Plessis J, Gerber M. Development and characterization of nano-emulsions and nano-emulgels for transdermal delivery of statins. Expert Opin Drug Deliv. 2021;18(6):789–801. doi:10.1080/17425247.2021.1867533
157. Wang Y, Li S, Wang X, et al. Smart transformable nanomedicines for cancer therapy. Biomaterials. 2021;271:120737. doi:10.1016/j.biomaterials.2021.120737
158. Solanki R, Rostamabadi H, Patel S, Jafari SM. Anticancer nano-delivery systems based on bovine serum albumin nanoparticles: a critical review. Int J Biol Macromol. 2021;193(Pt A):528–540. doi:10.1016/j.ijbiomac.2021.10.040
159. Jin Y, Wu Z, Wu C, et al. Size-adaptable and ligand (biotin)-sheddable nanocarriers equipped with avidin scavenging technology for deep tumor penetration and reduced toxicity. J Control Release. 2020;320:142–158. doi:10.1016/j.jconrel.2020.01.040
160. Tan H, Song Y, Chen J, et al. Platelet-Like Fusogenic Liposome-Mediated Targeting Delivery of miR-21 Improves Myocardial Remodeling by Reprogramming Macrophages Post Myocardial Ischemia-Reperfusion Injury. Adv Sci (Weinh). 2021;8(15):e2100787. doi:10.1002/advs.202100787
161. Xia Q, Zhang Y, Li Z, Hou X, Feng N. Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm Sin B. 2019;9(4):675–689. doi:10.1016/j.apsb.2019.01.011
162. Yang R, Xu J, Xu L, et al. Cancer Cell Membrane-Coated Adjuvant Nanoparticles with Mannose Modification for Effective Anticancer Vaccination. ACS Nano. 2018;12(6):5121–5129. doi:10.1021/acsnano.7b09041
163. Kang T, Zhu Q, Wei D, et al. Nanoparticles Coated with Neutrophil Membranes Can Effectively Treat Cancer Metastasis. ACS Nano. 2017;11(2):1397–1411. doi:10.1021/acsnano.6b06477
164. Choi Y, Bowman JW, Jung JU. Autophagy during viral infection - a double-edged sword. Nat Rev Microbiol. 2018;16(6):341–354. doi:10.1038/s41579-018-0003-6
165. Russo M, Russo GL. Autophagy inducers in cancer. Biochem Pharmacol. 2018;153:51–61. doi:10.1016/j.bcp.2018.02.007
166. Xi G, Hu X, Wu B, et al. Autophagy inhibition promotes paclitaxel-induced apoptosis in cancer cells. Cancer Lett. 2011;307(2):141–148. doi:10.1016/j.canlet.2011.03.026
167. Long X, Wang H, Yan J, et al. Tailor-Made Autophagy Cascade Amplification Polymeric Nanoparticles for Enhanced Tumor Immunotherapy. Small. 2023;19(24):e2207898. doi:10.1002/smll.202207898
168. Kinsey CG, Camolotto SA, Boespflug AM, et al. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat Med. 2019;25(4):620–627. doi:10.1038/s41591-019-0367-9
169. Chengli Y, Zhipneg W, Ming L, Zhichang Z. Pilot Study on the Preparation of EVO-CQ-Lips and Its Anti-cancer Effect on Breast Cancer. J Cancer Control Treatment. 2022;35(02):93–101.
170. Solanki R, Jangid AK, Jadav M, Kulhari H, Patel S. Folate Functionalized and Evodiamine-Loaded Pluronic Nanomicelles for Augmented Cervical Cancer Cell Killing. Macromol Biosci. 2023;23(9):e2300077. doi:10.1002/mabi.202300077
171. Tan X, Zhou Y, Shen L, Jia H, Tan X. A mitochondria-targeted delivery system of doxorubicin and evodiamine for the treatment of metastatic breast cancer. RSC Adv. 2019;9(63):37067–37078. doi:10.1039/c9ra07096f
172. Zhao X, He S, Li B, et al. DUCNP@Mn-MOF/FOE as a Highly Selective and Bioavailable Drug Delivery System for Synergistic Combination Cancer Therapy. Nano Lett. 2023;23(3):863–871. doi:10.1021/acs.nanolett.2c04042
173. Li C, Cai G, Song D, et al. Development of EGFR-targeted evodiamine nanoparticles for the treatment of colorectal cancer. Biomater Sci. 2019;7(9):3627–3639. doi:10.1039/c9bm00613c
174. Run-tian G, Rong-rong Z, Ni Y, Xiao-na R, Shi-ying L. Icartin and pyropheophorbide-a self-assembled nanomedicine for enchanced the efficacy of photodynamic tumour therapy by increase the cell autophagy. Acta Pharm Sin. 2023;58(08):2483–2493. doi:10.16438/j.0513-4870.2022-0997
175. Ji Y, Zhang Z, Hou W, et al. Enhanced antitumor effect of icariin nanoparticles coated with iRGD functionalized erythrocyte membrane. Eur J Pharmacol. 2022;931:175225. doi:10.1016/j.ejphar.2022.175225
176. Wang S, Wuniqiemu T, Tang W, et al. Luteolin inhibits autophagy in allergic asthma by activating PI3K/Akt/mTOR signaling and inhibiting Beclin-1-PI3KC3 complex. Int Immunopharmacol. 2021;94:107460. doi:10.1016/j.intimp.2021.107460
177. Fu QT, Zhong XQ, Chen MY, et al. Luteolin-Loaded Nanoparticles for the Treatment of Melanoma. Int J Nanomed. 2023;18:2053–2068. doi:10.2147/ijn.S400329
178. Wang Y, Wang Q, Feng W, et al. Folic acid-modified ROS-responsive nanoparticles encapsulating luteolin for targeted breast cancer treatment. Drug Deliv. 2021;28(1):1695–1708. doi:10.1080/10717544.2021.1963351
179. Backe SJ, Sager RA, Heritz JA, et al. Activation of autophagy depends on Atg1/Ulk1-mediated phosphorylation and inhibition of the Hsp90 chaperone machinery. Cell Rep. 2023;42(7):112807. doi:10.1016/j.celrep.2023.112807
180. Humbert M, Seiler K, Mosimann S, et al. Reducing FASN expression sensitizes acute myeloid leukemia cells to differentiation therapy. Cell Death Differ. 2021;28(8):2465–2481. doi:10.1038/s41418-021-00768-1
181. Fan R, Chen C, Mu M, et al. Engineering MMP-2 Activated Nanoparticles Carrying B7-H3 Bispecific Antibodies for Ferroptosis-Enhanced Glioblastoma Immunotherapy. ACS Nano. 2023;17(10):9126–9139. doi:10.1021/acsnano.2c12217
182. Yang G, Song T, Zhang H, et al. Stimulus-Detonated Biomimetic “Nanobomb” with Controlled Release of HSP90 Inhibitor to Disrupt Mitochondrial Function for Synergistic Gas and Photothermal Therapy. Adv Healthc Mater. 2023;12(26):e2300945. doi:10.1002/adhm.202300945
183. Wu P, Zhang H, Yin Y, et al. Engineered EGCG-Containing Biomimetic Nanoassemblies as Effective Delivery Platform for Enhanced Cancer Therapy. Adv Sci (Weinh). 2022;9(15):e2105894. doi:10.1002/advs.202105894
184. Granja A, Neves AR, Sousa CT, Pinheiro M, Reis S. EGCG intestinal absorption and oral bioavailability enhancement using folic acid-functionalized nanostructured lipid carriers. Heliyon. 2019;5(7):e02020. doi:10.1016/j.heliyon.2019.e02020
185. Sudha T, Salaheldin TA, Darwish NH, Mousa SA. Antitumor/anti-angiogenesis efficacy of epigallocatechin gallate nanoformulated with antioxidant in melanoma. Nanomedicine. 2022;17(15):1039–1053. doi:10.2217/nnm-2021-0362
186. Yang S, Gao X, He Y, et al. Applying an innovative biodegradable self-assembly nanomicelles to deliver α-mangostin for improving anti-melanoma activity. Cell Death Dis. 2019;10(3):146. doi:10.1038/s41419-019-1323-9
187. Feng J, Xu M, Wang J, et al. Sequential delivery of nanoformulated α-mangostin and triptolide overcomes permeation obstacles and improves therapeutic effects in pancreatic cancer. Biomaterials. 2020;241:119907. doi:10.1016/j.biomaterials.2020.119907
188. Bonafè F, Pazzini C, Marchionni S, Guarnieri C, Muscari C. Complete Disaggregation of MCF-7-derived Breast Tumour Spheroids with Very Low Concentrations of α-Mangostin Loaded in CD44 Thioaptamer-tagged Nanoparticles. Int J Med Sci. 2019;16(1):33–42. doi:10.7150/ijms.28135
189. Doan VTH, Takano S, Doan NAT, et al. Anticancer efficacy of cyclodextrin-based hyperbranched polymer nanoparticles containing alpha-mangostin. Polym J. 2021;53:481–492. doi:10.1038/s41428-020-00441-3
190. Pham DT, Saelim N, Tiyaboonchai W. Alpha mangostin loaded crosslinked silk fibroin-based nanoparticles for cancer chemotherapy. Colloids Surf B Biointerfaces. 2019;181:705–713. doi:10.1016/j.colsurfb.2019.06.011
191. Shi W, Feng W, Li S, et al. Ferroptosis and Necroptosis Produced Autologous Tumor Cell Lysates Co-Delivering with Combined Immnoadjuvants as Personalized In Situ Nanovaccines for Antitumor Immunity. ACS Nano. 2023;17(15):14475–14493. doi:10.1021/acsnano.3c00901
192. Wang H, Tang Y, Fang Y, et al. Reprogramming Tumor Immune Microenvironment (TIME) and Metabolism via Biomimetic Targeting Codelivery of Shikonin/JQ1. Nano Lett. 2019;19(5):2935–2944. doi:10.1021/acs.nanolett.9b00021
193. Muniraj N, Siddharth S, Shriver M, et al. Induction of STK11-dependent cytoprotective autophagy in breast cancer cells upon honokiol treatment. Cell Death Discov. 2020;6:81. doi:10.1038/s41420-020-00315-w
194. Wang J, Liu D, Guan S, et al. Hyaluronic acid-modified liposomal honokiol nanocarrier: enhance anti-metastasis and antitumor efficacy against breast cancer. Carbohydr Polym. 2020;235:115981. doi:10.1016/j.carbpol.2020.115981
195. Wang J, Yang H, Li Q, et al. Novel nanomicelles based on rebaudioside A: a potential nanoplatform for oral delivery of honokiol with enhanced oral bioavailability and antitumor activity. Int J Pharm. 2020;590:119899. doi:10.1016/j.ijpharm.2020.119899
196. Tang P, Sun Q, Yang H, Tang B, Pu H, Li H. Honokiol nanoparticles based on epigallocatechin gallate functionalized chitin to enhance therapeutic effects against liver cancer. Int J Pharm. 2018;545(1–2):74–83. doi:10.1016/j.ijpharm.2018.04.060
197. Xing-xing S, Le S, Liang-yu Y, Yu-zhen N, Tao L. Effect of Evodiamine Combined with Chloroquine on Hepatocarcinoma. Acta Univ Med Nanjing. 2021;37(03):400–403. doi:10.14148/j.issn.1672-0482.2021.0400
198. Yamaguchi J, Yokoyama Y, Fujii T, et al. Results of a Phase II Study on the Use of Neoadjuvant Chemotherapy (FOLFIRINOX or GEM/nab-PTX) for Borderline-resectable Pancreatic Cancer (NUPAT-01). Ann Surg. 2022;275(6):1043–1049. doi:10.1097/sla.0000000000005430
199. Lin YW, Lin TT, Chen CH, et al. Enhancing Efficacy of Albumin-Bound Paclitaxel for Human Lung and Colorectal Cancers through Autophagy Receptor Sequestosome 1 (SQSTM1)/p62-Mediated Nanodrug Delivery and Cancer therapy. ACS Nano. 2023;17(19):19033–19051. doi:10.1021/acsnano.3c04739
200. Batra H, Pawar S, Bahl D. Curcumin in combination with anti-cancer drugs: a nanomedicine review. Pharmacol Res. 2019;139:91–105. doi:10.1016/j.phrs.2018.11.005
201. Ratan C, Arian AM, Rajendran R, Jayakumar R, Masson M, Mangalathillam S. Nano-based formulations of curcumin: elucidating the potential benefits and future prospects in skin cancer. Biomed Mater. 2023;18(5):548. doi:10.1088/1748-605X/acf0af
202. Hanafy NAN, Sheashaa RF, Moussa EA, Mahfouz ME. Potential of curcumin and niacin-loaded targeted chitosan coated liposomes to activate autophagy in hepatocellular carcinoma cells: an in vitro evaluation in HePG2 cell line. Int J Biol Macromol. 2023;245:125572. doi:10.1016/j.ijbiomac.2023.125572
203. Zhang X, Gao H, Wei D, et al. ROS Responsive Nanoparticles Encapsulated with Natural Medicine Remodel Autophagy Homeostasis in Breast Cancer. ACS Appl Mater Interfaces. 2023;15(25):29827–29840. doi:10.1021/acsami.3c03068
204. Bamburowicz-Klimkowska M, Poplawska M, Grudzinski IP. Nanocomposites as biomolecules delivery agents in nanomedicine. J Nanobiotechnology. 2019;17(1):48. doi:10.1186/s12951-019-0479-x
205. Mei Y, Qin X, Yang Z, et al. Engineered a dual-targeting HA-TPP/A nanoparticle for combination therapy against KRAS-TP53 co-mutation in gastrointestinal cancers. Bioact Mater. 2024;32:277–291. doi:10.1016/j.bioactmat.2023.10.003
206. Sun X, Chen H, Gao R, et al. Intravenous Transplantation of an Ischemic-specific Peptide-TPP-mitochondrial Compound Alleviates Myocardial Ischemic Reperfusion Injury. ACS Nano. 2023;17(2):896–909. doi:10.1021/acsnano.2c05286
207. Feng Y, Liao Z, Li M, et al. Mesoporous Silica Nanoparticles-Based Nanoplatforms: basic Construction, Current State, and Emerging Applications in Anticancer Therapeutics. Adv Healthc Mater. 2023;12(16):e2201884. doi:10.1002/adhm.202201884
208. Mabrouk M, Das DB, Salem ZA, Beherei HH. Nanomaterials for Biomedical Applications: production, Characterisations, Recent Trends and Difficulties. Molecules. 2021;26(4):1077. doi:10.3390/molecules26041077
209. Liu J, Shi J, Nie W, Wang S, Liu G, Cai K. Recent Progress in the Development of Multifunctional Nanoplatform for Precise Tumor Phototherapy. Adv Healthc Mater. 2021;10(1):e2001207. doi:10.1002/adhm.202001207
210. Zhu M, Zhuang J, Li Z, et al. Machine-learning-assisted single-vessel analysis of nanoparticle permeability in tumour vasculatures. Nat Nanotechnol. 2023;18(6):657–666. doi:10.1038/s41565-023-01323-4
211. Zaslavsky J, Bannigan P, Allen C. Re-envisioning the design of nanomedicines: harnessing automation and artificial intelligence. Expert Opin Drug Deliv. 2023;20(2):241–257. doi:10.1080/17425247.2023.2167978
212. Bannigan P, Aldeghi M, Bao Z, Häse F, Aspuru-Guzik A, Allen C. Machine learning directed drug formulation development. Adv Drug Deliv Rev. 2021;175:113806. doi:10.1016/j.addr.2021.05.016
213. Issa NT, Stathias V, Schürer S, Dakshanamurthy S. Machine and deep learning approaches for cancer drug repurposing. Semin Cancer Biol. 2021;68:132–142. doi:10.1016/j.semcancer.2019.12.011
214. Vora LK, Gholap AD, Jetha K, Thakur RRS, Solanki HK, Chavda VP. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics. 2023;15(7):1916. doi:10.3390/pharmaceutics15071916
215. Ding J, Wang C, Sun Y, Guo J, Liu S, Cheng Z. Identification of an Autophagy-Related Signature for Prognosis and Immunotherapy Response Prediction in Ovarian Cancer. Biomolecules. 2023;13(2):339. doi:10.3390/biom13020339
216. Badwan BA, Liaropoulos G, Kyrodimos E, Skaltsas D, Tsirigos A, Gorgoulis VG. Machine learning approaches to predict drug efficacy and toxicity in oncology. Cell Rep Methods. 2023;3(2):100413. doi:10.1016/j.crmeth.2023.100413
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.