Back to Journals » Integrated Pharmacy Research and Practice » Volume 10
Expert Consensus on a List of Inappropriate Prescribing after Prescription Review in Pediatric Units in Abidjan, Côte d’Ivoire
Authors Doffou E , Avi C, Yao KC, Abrogoua DP
Received 4 June 2021
Accepted for publication 5 August 2021
Published 27 August 2021 Volume 2021:10 Pages 79—91
DOI https://doi.org/10.2147/IPRP.S322141
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Walter Jäger
Elisée Doffou,1,2 Christelle Avi,3 Kouassi Christian Yao,3 Danho Pascal Abrogoua1,4
1Therapeutic and Clinical Pharmacy Laboratory, Faculty of Pharmaceutical and Biological Sciences, Félix Houphouët-Boigny University, Abidjan, Côte d’Ivoire; 2Department of Pharmacy, Teaching Hospital of Cocody, Abidjan, Côte d’Ivoire; 3Department of Pediatrics, Teaching Hospital of Bouaké, Bouaké, Côte d’Ivoire; 4Department of Clinical Pharmacology, Teaching Hospital of Cocody, Abidjan, Côte d’Ivoire
Correspondence: Elisée Doffou Email [email protected]
Introduction: Inappropriate prescribing (IP) includes inappropriate prescription and omission of prescription. IP can adversely affect the quality of health care in pediatric units. A list of IP taking into account frequently encountered drug-related problems (DRPs) can be useful to optimize prescriptions in pediatrics. The aim of this study was to validate by expert consensus a list of IP after a prescription review in pediatric units in Abidjan.
Materials and Methods: A list of IPs was developed from a prescription review of inpatients and outpatients aged 1 month to 15 years and followed in pediatric units at teaching hospitals of Abidjan during 16 months. A two-round Delphi method was used to validate a qualitative list of IPs by experts according to their level of agreement on a six-point Likert scale of 0– 5 (0, no opinion; 5, strongly agree). Only propositions obtaining the agreement (rating 4 or 5) of > 70% of experts who gave a non-zero rating for the first round and 80% for the second round were retained.
Results: A qualitative list of 54 IPs was drawn up from 267 DRPs detected after prescription review of 4,992 prescription lines for 881 patients. Our panel comprised 22 pediatricians (96%) and one clinical pharmacist (4%). Mean agreement ratings were 4.43/5 (95% CI 4.39– 4.48) and 4.6/5 (95% CI 4.56– 4.64), respectively, during the first Delphi round and the second (p< 0.001). At the end of the first round, all items submitted (54) were retained, including 13 items that had been reworded. In the second round, 20 experts participated and two IPs (4%) were not retained for the final list. This list comprised 52 IPs (44 inappropriate prescriptions and eight omissions of prescription).
Conclusion: The list of IP validated in this study should help in the detection of DRPs and optimize prescriptions in pediatric units in Côte d’Ivoire.
Keywords: inappropriate prescribing, consensus, experts, pediatrics, Côte d’Ivoire
Introduction
Improving the safety of therapy and optimizing prescription are core objectives of health systems. Inappropriate drug prescription can adversely affect the quality of health care. Inappropriate prescribing (IP) includes inappropriate prescription (overprescription, misprescription) and omission of prescription (underprescription).1,2 IP is closely linked to drug-related problems (DRPs). A DRP is defined as “an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes.”3 DRP is a significant problem for patients and the health-care system. Studies have shown that DRPs in the pediatric population are a major concern.4–8 The prevalence of DRPs in pediatrics is 21%–45%.4–8 DRPs linked to IP in pediatric units can lead to adverse drug events (ADEs). ADEs can have serious consequences in a frail population like children.9 These consequences can be medical (morbidity and mortality), medicolegal, or economic.9–13 ADEs progress to serious complications or death three times as frequently in children as in adults.9,14,15 Prescribing in pediatrics is a complex process. Children differ from adults in many ways, such as pharmacokinetics, which can affect the efficacy and safety of drugs.16 This complexity of prescribing is reinforced by frequent off-label drug use in pediatrics.17 For all these reasons, drug prescribing should be given special attention in pediatrics.
To improve prescribing quality and facilitate ADE management, many health professionals have developed and validated several lists of IPs using consensus methods.18 Many tools are related to the elderly population.18–20 Using these tools has helped to reduce the occurrence of IPs in the health management of elders.21 In pediatrics, IP tools called POPI (pediatrics: omission of prescriptions and inappropriate prescription)22–24 and PIPc (potential IP in children)25 have been constructed. These tools were developed according to health problems and prescriptions frequently encountered in European context.22–25 However, their universal use as tools to detect IP can be reduce. Indeed, the variability of clinical and therapeutic practice throughout the world can make it difficult to make optimal and relevant using of IP tools outside their design context. For this reason, many lists of IPs have been developed throughout the world.18,26,27 Some studies carried out in pediatric units in Côte d’Ivoire28–30 showed many DRPs that IP tools in pediatrics (POPI, POPI-UK, PIPc)22–25 could not help to detect. For optimal use, a list of IPs must be established by consensus. It must also be related to DRPs encountered in a local context. This study aimed to develop and validate by expert consensus, a list of IP after prescription review in pediatric units in Abidjan, Côte d’Ivoire.
Materials and Methods
Study Design and Population
We used a method similar to Abrogoua et al.30 A cross-sectional study was conducted in four teaching hospitals of Abidjan: Angré, Cocody, Treichville, and Yopougon). The population comprised inpatients and outpatients aged 1 month to 15 years followed in pediatric units from March 2019 to July 2020 (16 months).
The prescription review was carried out based on patients’ medical records and prescriptions. When information was not in the medical record, the pharmacist sought it directly from the physician. The review was performed by a clinical pharmacist according to the prescription-review algorithm of Calop.31 Our prescription review first looked into pathophysiological characteristics (eg, age, weight, kidney function) and comorbidities (eg, diabetes) that may have affected drug therapy. We then checked for medication not indicated or untreated indications. Various critical points were also checked: pathophysiological contraindications, underdosing or overdosing, drug interactions, and drugs with narrow therapeutic range. Finally, we researched drugs justifying particular clinical or biological monitoring.31 The information collected for the prescription review was based on the classification of DRPs outlined by the French Society of Clinical Pharmacy.32 This classification did not allow the DRPs detected to be prioritized. We thus included all DRPs detected.
The prescription review was performed using the Dictionnaire Vidal 2019,33 a practical guide of therapeutic recommendations (Vidal Recos 2016),34 a thesaurus of drug–drug interactions developed by the Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM) France,35 therapeutic recommendations of Côte d’Ivoire’s national health authority,36 and recommendations of international health authorities or scientific societies where national recommendations did not exist.
Recommendations that were both backed up by evidence and published after 2000 (priority given to the most recent) were chosen. These came from health organizations (Ivorian Health Ministry), World Health Organization, ANSM France, Haute Autorité de Santé (France), National Institute for Health and Care Excellence (UK), European Medicines Agency, Ivorian Society of Pneumophthisiology, French Society of Otorhinolaryngology, French Society of Pediatrics, American Academy of Pediatrics, North America Society for Pediatric Gastroenterology, Italian Pediatric Society, Italian Society of Gastroenterology, Canadian Pain Society, a French-speaking group of pediatric hepatogastroenterology and nutrition, European Society for Pediatric Gastroenterology, Hepatology, and Nutrition, and College of Infectious and Tropical Diseases. The Medline database was used for viewing publications (meta-analyses and systematic reviews) related to therapy effectiveness and safety.
Development of IP List after Prescription Review
Items on the IP list were developed from DRPs detected by the clinical pharmacist after prescription review. All the DRPs detected were summarized in a qualitative list. This list was divided into inappropriate prescription and omission of prescription. Inappropriate prescription covered noncompliance with recommendations or contraindication, “medication not indicated, overdosing, underdosing, drug–drug interactions, and inappropriate administration. The omissions of prescription were regarded as untreated indications.
This list of IPs was submitted for validation to experts during the consensus survey.
Selection and Recruitment of Experts
Our experts comprised a clinical pharmacist and pediatricians working at the six major public hospitals in Côte d’Ivoire: the four teaching hospitals of Abidjan, the teaching hospital of Bouaké (second-largest city of Côte d’Ivoire), l’Hôpital Mère–Enfant of Bingerville (district of Abidjan), and the Military Hospital of Abidjan. The list of pediatricians from each pediatric unit in the selected hospitals was sent to the principal investigator by their unit heads. A letter requesting participation in the survey as an expert was sent to health professionals. Experts who agreed to participate declared no conflict of interest that could interfere with ratings and opinions issued.
Consensus Survey
A two-round Delphi survey37 was executed to retain items for the final IP list in pediatrics in Côte d’Ivoire. An Access database file using the parameters “IP,” “rationale,” (justifying the inappropriateness of prescription or omission of prescription), and “recommendations and/or therapeutic alternative” (related to each IP) was sent to the experts by email. Experts were asked to rate their level of agreement on IP only. The level of agreement was gauged using a six-point Likert scale: 0, no opinion; 1, strongly disagree; 2, disagree; 3, neither agree nor disagree; 4, agree; 5, strongly agree.38 Experts were able to consult all documents used to identify IP. They were encouraged to make suggestions about IP and provide appropriate references. Justifications of the nonvalidity of items were required for a score <4/5.23
For the first round, we retained items with agreement >70% of experts who had given a non-zero rating. Agreement meant a rating of 4 or 5. Expert suggestions related to proposed items were analyzed by two evaluators (clinical pharmacists) not participating in the validation. These were taken into account for the second round if they gad been referenced and/or were deemed suitable. For each IP proposition, answers in the first round and median scores of the entire panel were gathered. For the second round, experts had to confirm or refute their results knowing the panel’s global answer. Selection criteria was different from the first round, with an agreement required of >80% of experts (ratings of 0 excluded).
Data Analysis
Data analysis was performed using GraphPad Prism and R version 4.0.2. Variables are summarized as cases with percentages for categorical variables and means and 95% CIs for continuous variables. During each Delphi round and for each item, median agreement rating (with range), number of experts with an explicit opinion (ratings of 0 excluded), and percentage of these who rated an item as 4 or 5 were evaluated.
To study the impact of missing data on the results of the second round, a sensitivity analysis was performed. This compared the final results that would probably have been obtained if the number of participating experts had remained the same between the first and the second round, assuming that the participants’ answers did not change between the two rounds. To do that, we replaced experts’ missing answers from the second round with their answer in the first round.23
Ethics Approval
This study was conducted with the approval of medical scientific departments (MSDs) of hospitals (Angré Teaching Hospital, Cocody Teaching Hospital, Treichville Teaching Hospital, and Yopougon Teaching Hospital) under the authorization of pediatric unit heads. According our national health system, the MSD is a hospital’s local ethics committee (institutional review board) and gives authorization for studies conducted at teaching hospital after analysis of research protocols and verifying compliance with ethics and patient data protection (anonymity, confidentiality, and oral consent of patient’s adult referent). The study was carried out in accordance with the National Research Ethics Committee’s recommendations, which were in line with the Declaration of Helsinki.39 According to these national recommendations, the approval of MSDs of teaching hospitals for this descriptive noninterventional study was alone required. National Research and Ethics Committee approval is not required for prescription-review studies in teaching hospitals in Côte d’Ivoire.
Results
Development of IP List
There were 267 prescription lines relating to 881 patients. These DRPs corresponded to a list of 54 distinct IP selected for Delphi survey (Figure 1). The qualitative list of IP (corresponding to the DRPs detected) consisted of inappropriate prescriptions (85%) and omission of prescription (15%). Inappropriate prescriptions mainly comprised noncompliance with recommendations or contraindications (41%) and drug–drug interactions; 20%) (Table 1).
 |
Table 1 Qualitative list of IP corresponding to drug-related problems |
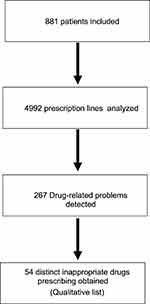 |
Figure 1 Diagram of development of qualitative list of IP from prescription review. |
Delphi Survey and Professional Characteristics of Experts
In sum, 36 health professionals were called upon, and among these 35 agreed to participate, with 23 taking part in the first round of Delphi survey for a 65.7% (23 of 35) participation rate (Figure 2).
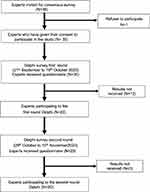 |
Figure 2 Chronological sequence of the Delphi surveys. |
A majority of these experts worked at Cocody (39%) and Treichville (17%) Teaching Hospitals. Experts were pediatricians (96%) and a clinical pharmacist (4%) with a mean 13.4 years of professional seniority (4–35 years), and 48% were academic and clinical practitioners (Table 2).
 |
Table 2 Professional characteristics of experts participating in the Delphi surveys |
Twenty experts took part in the second round of the Delphi survey for an 86.9% (20 of 23) participation rate (Figure 2). Durations of the first and second rounds were 26 and 22 days, respectively (Figure 2).
General Characteristics of Data Survey
In the first round, the number of experts giving a non-zero rating for each IP was 15–23 with a median of 22 (IQR 25%–75%, 20.75–23). A median of one expert with “no opinion” per item was noted. Only one item was evaluated by fewer than 18 of 23 (78%) experts. The mean agreement rating was 4.43/5 (95% CI 4.39–4.48). For the second round, the 20 experts (86.9%) participated again. The number of experts giving a non-zero rating for each IP fluctuated between 17 and 20, with a median of 20 (IQR 25%–75%, 17–20). Mean agreement was 4.6/5 (95% CI 4.56–4.64), higher than the rating from the first round (p<0.001).
Agreement Data
First Round
A total of 54 IP types were proposed for scoring in the first round. All propositions (n=54) obtained agreement of 70% of experts who had responded with a non-zero rating (Table 3). These propositions were maintained, and among them 13 (24%) were reworded after the panel’s suggestions (Table 3).
 |  |  |  |
Second Round
At the end of the second round, 96.3% (52 of 54) of the submitted IPs were retained for the final list. These IPs obtained an agreement level in >80% of experts expressing a non-zero rating. The final list contained 44 inappropriate prescriptions and eight omissions of prescription. Thirty items (57.7%) on the final list obtained agreement of all experts giving a non-zero rating (Table 3).
Sensitivity Analysis
According to the sensitivity analysis, if the participation rate for the second round was similar to the first one, 53 IPs (instead of 52) would have obtained a rate of experts who answered 4 or 5 of >80%.
Discussion
This study on the validation of a list of IPs in pediatrics after prescription review is the first to be carried out in Côte d’Ivoire. The methodology used for developing this list was quite original. It differed from those adopted for the development of the POPI22,23 and PIPc tools.25 In fact, the choice of items for these tools was made from a literature search on recommendations for the management of health problems related to local pediatric contexts. In our study, clinical pharmacy was used to elaborate a list of IP. Indeed, IPs were identified after a prescription review of patients seen in pediatrics unit. The final IP list obtained by this methodology does not claim to be exhaustive. However, drawing inspiration from DRPs encountered in the Ivorian context, it can be a valuable aid in the detection of IP during prescription review in pediatric units in Côte d’Ivoire. The qualitative list of IP (corresponding to the detected DRPs) mainly comprised noncompliance with recommendations or contraindications (41%) and drug–drug interactions (20%). Other studies in Côte d’Ivoire concluded that DRPs is linked to noncompliance with recommendations or contraindications after prescription review in clinical units.30,40 The lack of written therapeutic protocols would have favored health‑care gaps.
The Delphi method was the consensus tool used in our study. This method is frequently used for validation of tools for detecting IP in the geriatric and pediatric fields.18,41 In pediatrics, only the POPI-UK tool was developed without Delphi method. The authors of this tool did not refer to a panel of experts:24 one researcher compared POPI items to UK national guidelines.
In our study, the low number of rounds was chosen to avoid a high dropout of experts. Most experts on our panel were pediatricians (96%). In validating the POPI tool, a panel has included as many physicians as pharmacists.22 In our study, we wished to include mainly professionals with experience in clinical practice and/or drug prescription in pediatrics. For this reason, we selected mainly pediatricians working in teaching hospitals in Côte d’Ivoire.
The participation rate of the experts in the second round was 86.9%. This rate was higher than that observed during validation of the latest version of the POPI tool (86.9% vs 70%).23 It was, however, lower than that observed for validation of the PIMCheck tool (potentially inappropriate medication—patients in the internal medicine unit; 86.9% vs 100%).38
In our study, it was possible for experts not to give an explicit opinion by choosing the 0 rating. A rating of 0 was chosen when experts felt they did not have sufficient hindsight or the necessary information to make an objective judgment. This rating method, also used for validation of the PIMCheck tool,38 seemed relevant to us. Indeed, it makes it possible to reduce nonobjective ratings that may bias the final result. The number of experts who gave a non-zero rating for each proposal was 16–23 over the two Delphi rounds. To our knowledge, there is no consensus on the minimum number of panelists to include for the validity of a Delphi method.18,41 A literature review on tools for detecting IP reported that the number of experts participating in the Delphi method ranged from four to 57, with a median of 15.18 In our study, the number of experts who gave an explicit opinion (0 excluded) was not very different from that of experts who participated in Delphi validation of POPI and PIPc tools. Indeed, 20 and 14 experts participated, respectively, in the first and second rounds of a Delphi survey for the latest POPI tool.23 For the PIPc tool,25 15 experts participated in the two round.
Mean agreement ratings of our experts were 4.43/5 (95% CI 4.39–4.48) and 4.6/5 (95% CI 4.56–4.64), respectively, during the first and second Delphi rounds (p<0.001). These results were close to those reported during PIMcheck-tool validation. Indeed, in that study, mean agreement ratings were 4.32/5 and 4.35/5, respectively, during the first and second Delphi rounds.38
The agreement rates necessary for the validation of items were >70% of experts for the first round and 80% for the second round. These rates were lower than those used for validation of the latest version of POPI tool (75% and 85%),23 but higher than those used for validation of the first POPI22 and PIMCheck tool38 (65% and 75%). In our study, the high levels of agreement for IP acceptance made it possible to obtain a final list with a strong consensus.
Of the IP items retained for the second round, 24% were reworded after suggestions made by the experts. These were reformed or clarified to make the description of IP more explicit. Only two items in the second round were discarded from the final list. Those IPs related to prescription of salbutamol for treatment of a first case of acute bronchiolitis in infants <12 months of age and the prescription of phenobarbital at a daily dose of 20 mg/kg for treatment of repeated convulsions in severe malaria. Regarding the prescription of salbutamol in the management of bronchiolitis, the experts who disagreed with this item believed the use of salbutamol had no negative impact on the clinical state of the infant in their common medical practice. As for the prescription of phenobarbital at a dosage of 20 mg/kg, the experts who disagreed stated that in their common practice, phenobarbital at a daily dosage of 20 mg/kg in newborns and some infants had not resulted in increased cases of respiratory distress.
The final list obtained after consensus validation comprised 52 items, lower than the latest version of POPI (52 vs 103).23 However it was higher than than for PIPc (52 vs 12).25
The final list validated in this study was organized by category of IP (inappropriate and omission). The structure thus adopted was close to the POPI tool’s organization.22,23 Indeed, in the POPI tool, the IP was divided into the same two categories, classified according to the main physiological systems.22,23
Some IP descriptions in our validated list were similar to those contained in POPI or PIPc. For example, “Prescription of domperidone for treatment of vomiting in children under 12 years of age” was listed as inappropriate. In the latest version of POPI, the prescription of domperidone is considered inappropriate.23 However, several IP types validated in our study are not found in the POPI and PIPc tools, including omission of prescription of oral zinc for the management of acute diarrhea in children not receiving therapeutic zinc-containing foods. Diarrhea guidelines in the European context do not recommend prescription of zinc for children aged <5 years.42 According to one literature review, zinc supplementation may be beneficial in situations where the prevalence of zinc deficiency or malnutrition is high.43 This would explain the recommendation of zinc supplementation by Ivorian guidelines for management of diarrhea in children.
Also, unlike the POPI23 and PIPc25 tools, the IP list validated in our study included items related to such pathologies as malaria, severe acute malnutrition, and tetanus. In fact, these are almost nonexistent in the the European clinical context, but are still managed in Côte d’Ivoire. Many IP types related to these pathologies exist. These IP types can compromise the achievement of treatment aims or increase the risk of ADEs, with potentially serious consequences for patients.
All these factors justify the need to design IP lists specific to local clinical and therapeutic contexts.
Strengths and Limitations
The Delphi method used for validation of an IP list is a robust method commonly used for the validation of tools for detection of inappropriate prescriptions and omission of prescriptions. The development of an IP list from prescription reviews in pediatric units in Côte d’Ivoire makes its use more practical in this country. Nevertheless, the problem of its use outside the Ivorian context does not really arise, taking into account the pathologies common in pediatric practice in sub-Saharan Africa. However, updating this list requires a routine prescription review in pediatric units and the possible consideration of new treatment recommendations.
Implications for Clinical Practice and Research
This study resulted in the composition of an IP list in a pediatric context. Specific to the Ivorian therapeutic context, this list is more practical and judicious in identifying DRPs in a local setting. Using this list in routine clinical pharmacy activities should contribute to reducing IP incidence and consequences in drug management in pediatric units.
A prescription review to assess the appropriateness of prescribing can be time-consuming and difficult. Our IP list can be applied quickly and easily in clinical activity in pediatrics to identify inappropriate prescription and omission of prescription.
The list validated in our study could be helpful for researchers in estimating IP incidence in the pediatric field in Côte d’Ivoire. This could help in evaluating prescription quality, factors, and cost associated with IP, as has been proposed for STOPP/START and Beers criteria.44–48
We also showed how the Delphi method can be used to develop consensus guidelines for practitioners working in the same therapeutic and clinical contexts.
The methodology used for developing our IP list can be adopted by other pharmacists to elaborate and validate new IP lists adapted to various medical fields and specific to their clinical and therapeutic context.
Conclusion
Our study enabled the validation of an IP list after prescription review in pediatric units in Abidjan. The validation carried out by the Delphi process over two rounds saw the participation of experts who were mainly pediatricians practicing in Côte d’Ivoire. In sum, 52 of the 54 IP types submitted for evaluation were retained on the final list, with agreement found among >80% of the experts who gave non-zero ratings in the second round of the Delphi survey. This list of explicit criteria should help detect DRP and optimize drug prescriptions in pediatric units in Côte d’Ivoire.
Acknowledgments
The authors would like to thank every expert for sharing their knowledge:
Acquah Patrick Olivier (pediatrician), Akoun Charles (pediatrician), Amon-Tanoh Dick Flore (pediatrician), Assamoi Hermance (pediatrician), Asse Kouadio Vincent (pediatrician), Cardenat Melissa (pediatrician), Dainguy Marie-Evelyne (pediatrician), Demenouan Hyacinthe, Djoman Isabelle (pediatrician), Gilbernaire Enderson (pediatrician), Gnamien Charlotte (pediatrician), Marius Gro Bi (pediatrician), Kamenan Alexis (clinical pharmacist), Mea Assande Tanoh (pediatrician), Micondo Kouamé Hervé (pediatrician), Nassirou Faissal (pediatrician), Oka Berethe (pediatrician), Ouattara Aby (pediatrician), Ouattara Joseph (pediatrician), Sinde Kapeu (pediatrician), Yao Guy (pediatrician), Yeboua Roland (pediatrician), and Zobo Konan Nathalie (pediatrician).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. UCLA division of geriatric medicine. Arch Intern Med. 1991;151(9):1825–1832.
2. Spinewine A, Schmader KE, Barber N, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet. 2007;370(9582):173–184. doi:10.1016/S0140-6736(07)61091-5
3. Pharmaceutical Care Network Europe Foundation. PCNE classification for Drug related problems. (revised 01-05-06) V5.01. Available from: http://www.pcne.org/upload/files/16_PCNE_classification_V5.01.pdf.
4. Rashed AN, Neubert A, Tomlin S, et al. Epidemiology and potential associated risk factors of drug-related problems in hospitalised children in the United Kingdom and Saudi Arabia. Eur J Clin Pharmacol. 2012;68(12):1657–1666. doi:10.1007/s00228-012-1302-x
5. Easton KL, Chapman CB, Brien JA. Frequency and characteristics of hospital admissions associated with drug-related problems in paediatrics. Br J Clin Pharmacol. 2004;57(5):611–615. doi:10.1111/j.1365-2125.2003.02052.x
6. Rashed AN, Neubert A, Alhamdan H, et al. Drug-related problems found in children attending an emergency department in Saudi Arabia and in the United Kingdom. Int J Clin Pharm. 2013;35(3):327–331. doi:10.1007/s11096-013-9758-z
7. Rashed AN, Wilton L, Lo CC, Kwong BY, Leung S, Wong IC. Epidemiology and potential risk factors of drug-related problems in Hong Kong paediatric wards. Br J Clin Pharmacol. 2014;77(5):873–879. doi:10.1111/bcp.12270
8. Birarra MK, Heye TB, Shibeshi W. Assessment of drug-related problems in pediatric ward of Zewditu Memorial Referral Hospital, Addis Ababa, Ethiopia. Int J Clin Pharm. 2017;39(5):1039–1046. doi:10.1007/s11096-017-0504-9
9. Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285(16):2114–2120. doi:10.1001/jama.285.16.2114
10. Oshikoya KA, Chukwura H, Njokanma OF, Senbanjo IO, Ojo I. Incidence and cost estimate of treating pediatric adverse drug reactions in Lagos, Nigeria. Sao Paulo Med J. 2011;129(3):153–164. doi:10.1590/S1516-31802011000300006
11. Ghaleb MA, Barber N, Franklin BD, Wong ICK. The incidence and nature of prescribing and medication administration errors in pediatric inpatients. Arch Dis Child. 2010;95(2):113–118. doi:10.1136/adc.2009.158485
12. Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277(4):301–306. doi:10.1001/jama.1997.03540280039031
13. Kongkaew C, Hann M, Mandal J, et al. Risk factors for hospital admissions associated with adverse drug events. Pharmacotherapy. 2013;33(8):827–837. doi:10.1002/phar.1287
14. Cowley E, Williams R, Cousins D. Medication errors in children: a descriptive summary of medication error reports submitted to the United States pharmacopeia. Curr Ther Res. 2001;62(9):
15. Fortescue EB, Kaushal R, Landrigan CP, et al. Prioritizing strategies for preventing medication errors and adverse drug events in pediatric inpatients. Pediatrics. 2003;111(4):722–729. doi:10.1542/peds.111.4.722
16. Kearns GL, Abdel-Rahman SM, Alander SW. Developmental pharmacology drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167. doi:10.1056/NEJMra035092
17. Shah SS, Hall M, Goodman DM, et al. Off-label drug use in hospitalized children. Arch Pediatr Adolesc Med. 2007;161(3):282–290. doi:10.1001/archpedi.161.3.282
18. Desnoyer A, Guignard B, Lang PO, Desmeules J, Vogt-Ferrier N, Bonnabry P. Prescriptions médicamenteuses potentiellement inappropriées en gériatrie: quels outils utiliser pour les détecter? Presse Med. 2016;45(11):957–970. doi:10.1016/j.lpm.2016.06.033
19. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–631. doi:10.1111/j.1532-5415.2012.03923.x
20. American Geriatrics Society Beers Criteria® Update Expert Panel. American geriatrics society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–694. doi:10.1111/jgs.15767
21. Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89(6):845–854. doi:10.1038/clpt.2011.44
22. Prot-Labarthe S, Weil T, Nguyen NPK, et al. Validation par consensus d’un outil d’identification de prescriptions inappropriées en pédiatrie (POPI). Arch Pediatr. 2016;23(5):481–490. doi:10.1016/j.arcped.2016.02.010
23. Sadozai L, Sable S, Le Roux E, et al. International consensus validation of the POPI tool (Pediatrics: omission of Prescriptions and Inappropriate prescriptions) to identify inappropriate prescribing in pediatrics. PLoS One. 2020;15(10):e0240105. doi:10.1371/journal.pone.0240105
24. Corrick F, Choonara I, Conroy S, Sammons H. Modifying a Paediatric Rational Prescribing Tool (POPI) for use in the UK. Healthc Basel Switz. 2019;7(1):33.
25. Barry E, O’Brien K, Moriarty F, et al. PIPc study: development of indicators of potentially inappropriate prescribing in children (PIPc) in primary care using a modified Delphi technique. BMJ Open. 2016;6(9):e012079. doi:10.1136/bmjopen-2016-012079
26. Laroche ML, Charmes JP, Merle L. Potentially inappropriate medications in the elderly: a French consensus panel list. Eur J Clin Pharmacol. 2007;63(8):725–731. doi:10.1007/s00228-007-0324-2
27. Rognstad S, Brekke M, Fetveit A, Spigset O, Wyller TB, Straand J. The Norwegian general practice (NORGEP) criteria for assessing potentially inappropriate prescriptions to elderly patients. A modified Delphi study. Scand J Prim Health Care. 2009;27(3):153–159. doi:10.1080/02813430902992215
28. Abrogoua DP, Konan KC, Doffou E. Assessment of the relevance of pharmacist interventions in the therapeutic management of malaria in a pediatric unit in Cote d’Ivoire. J Pharm Health Serv Res. 2016;7(4):233–239. doi:10.1111/jphs.12146
29. Abrogoua DP, Koffi NO, Doffou E. Interventions pharmaceutiques sur les prescriptions d’antibiotiques en consultations externes de pédiatrie d’un centre hospitalier universitaire de Côte d’Ivoire [Pharmaceutical interventions on antibiotic prescriptions in pediatric outpatient clinics at a university hospital in Côte d'Ivoire]. Ann Pharm Fr. 2016;74(5):380–388. French. doi:10.1016/j.pharma.2015.12.004
30. Abrogoua DP, Békégnran CP, Gro BM, Doffou E, Folquet MA. Assessment of a clinical pharmacy activity in a pediatric inpatient department in Cote D’ivoire. J Basic Clin Pharm. 2017;8(1):15–18.
31. Calop J, Fernandez B. Algorithme De Validation De L’ordonnance. Pharmacie Clinique Et Thérapeutique[Prescription Validation Algorithm. Clinical and Therapeutic Pharmacy].
32. Conort O, Bedouch P, Juste M, et al. Validation d’un instrument de codification des interventions en pharmacie clinique [Validation of a coding instrument for clinical pharmacy interventions. J Pharm Clin. 2004;23(3):141–147. French.
33. Vidal, editor. Vidal 2019: Le dictionnaire[Vidal 2019: The Dictionary].
34. Charles C. Vidal recos 2016, recommandations en pratique - 185 stratégies thérapeutiques[Vidal recos 2016, recommendations in practice - 185 therapeutic strategies].
35. ANSM. Thesaurus des interactions médicamenteuses [Drug Interaction Thesaurus]; 2019. Available from: https://www.ansm.sante.fr/var/ansm_site/storage/original/application/0002510e4ab3a9c13793a1fdc0d4c955.pdf.
36. Ministère de la santé et de la lutte contre le Sida [Ministry of Health and the Fight against AIDS]. Recueil des protocoles thérapeutiques nationaux de prise en charge des pathologies Compendium of national therapeutic protocols for the management of pathologies]; 2019. Available from: www.activitepharma-ci.org.
37. Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. Am J Public Health. 1984;74(9):979–983. doi:10.2105/AJPH.74.9.979
38. Desnoyer A, Blanc A-L, Pourcher V, et al. PIM-check: development of an international prescription screening checklist designed by a Delphi method for internal medicine patients. BMJ Open. 2017;7(7):e016070. doi:10.1136/bmjopen-2017-016070
39. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
40. Abrogoua DP, Doffou E, Akroman M, Soro L, Amonkou A. Assessment of a clinical pharmacy activity in an intensive care unit in Côte d’Ivoire. Eur J clin pharm. 2016;18(1):36–42.
41. Corrick F, Conroy S, Sammons H, Choonara I. Paediatric rational prescribing: a systematic review of assessment tools. Int J Environ Res Public Health. 2020;17(5):1473. doi:10.3390/ijerph17051473
42. Guarino A, Lo Vecchio A, Dias JA, et al. Universal recommendations for the management of acute diarrhea in nonmalnourished children. J Pediatr Gastroenterol Nutr. 2018;67(5):586–593. doi:10.1097/MPG.0000000000002053
43. Lazzerini M, Wanzira H. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev. 2016;12(12):CD005436.
44. Hill-Taylor B, Sketris I, Hayden J, Byrne S, O’Sullivan D, Christie R. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38(5):360–372. doi:10.1111/jcpt.12059
45. Van der Hooft CS, Jong GW, Dieleman JP, et al. Inappropriate drug prescribing in older adults: the updated 2002 Beers criteria—a population-based cohort study. Br J Clin Pharmacol. 2005;60(2):137–144. doi:10.1111/j.1365-2125.2005.02391.x
46. Stevens MB, Hastings SN, Powers J, et al. Enhancing the Quality of Prescribing Practices for older veterans discharged from the Emergency Department (EQUiPPED): preliminary results from enhancing quality of prescribing practices for older veterans discharged from the emergency department, a novel multicomponent interdisciplinary quality improvement initiative. J Am Geriatr Soc. 2015;63:1025–1029.
47. Bradley MC, Fahey T, Cahir C, et al. Potentially inappropriate prescribing and cost outcomes for older people: a cross-sectional study using the Northern Ireland Enhanced Prescribing Database. Eur J Clin Pharmacol. 2012;68(10):1425–1433. doi:10.1007/s00228-012-1249-y
48. Bongue B, Laroche ML, Gutton S, et al. Potentially inappropriate drug prescription in the elderly in France: a population-based study from the French National Insurance Healthcare system. Eur J Clin Pharmacol. 2011;67(12):1291–1299. doi:10.1007/s00228-011-1077-5
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
