Back to Journals » Infection and Drug Resistance » Volume 16
Experimental Research on the Effect of Tounong San on the Immune Function of Rats with Superficial Suppurative Infection
Authors Shi Z , Liu Y , Chang X, Gao Y, Hao M, Feng S , Dong H
Received 30 May 2023
Accepted for publication 7 September 2023
Published 25 October 2023 Volume 2023:16 Pages 6807—6820
DOI https://doi.org/10.2147/IDR.S420199
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Zhiqiang Shi,1 Yu Liu,1,2 Xiaodan Chang,3 Yuan Gao,4 Min Hao,1 Shuo Feng,1 Haoyu Dong1
1Department of Traditional Chinese Medicine Surgery, College of Traditional Chinese Medicine, Inner Mongolia Medical University, Hohhot, People’s Republic of China; 2Department of Integrated Traditional Chinese and Western Medicine Surgery, College of Integrated Chinese and Western Medicine, Hunan University of Chinese Medicine, Changsha, People’s Republic of China; 3Department of Dermatology, Hohhot Second Hospital, Hohhot, People’s Republic of China; 4Department of Anorectal Diseases, Inner Mongolia Hospital of Traditional Chinese Medicine, Hohhot, People’s Republic of China
Correspondence: Yu Liu, Inner Mongolia Medical University, Jinshan Development Zone, Hohhot, Inner Mongolia, People’s Republic of China, Tel +86-153-3471-2606, Email [email protected]
Purpose: This paper aims to investigate the effect of Tounong San (TNS), a well-known traditional Chinese medicine prescription for suppurative infections, on the immune function.
Methods: A suppurative infection model was established by injecting Staphylococcus aureus into subcutaneous tissue on the backs of rats. The expressions of CD68, CD163, CD31 and MPO in abscess tissues, phagocytosis rate and reactive oxygen species (ROS) of neutrophils in blood, phagocytosis function of peritoneal macrophages, and proliferation of blood lymphocytes, expression of IL-1, IL-6, CH50, TNF-α, IFN-γ, IgG, IgM in serum were detected at different time points.
Results: On the 3rd day of medication, fibrinogen wrapped around the abscesses was visible in TNS groups, with an increase in new blood vessels and the expression of a large number of macrophages and neutrophils. On the 6th day, the pus of TNS groups diminished, and the number of new blood vessels reached its peak. On the 9th day, the abscesses disappeared in TNS groups, fewer new blood vessels, macrophages, and neutrophils were expressed, and more fibrocytes appeared and filled the original pus cavities. On the 3rd and 9th day of medication, the phagocytic rates of neutrophils and macrophages were significantly improved in TNS group, and the ROS content of neutrophils was increased on the 9th day. TNS has no effect on lymphocyte proliferation in vitro, but it can regulate the secretion of IgG and IgM by lymphocytes. TNS increases the level of IL-1, decreases the levels of IL-6 and TNF-α, and regulates the expressions of IFN-γ and CH50 in two ways.
Conclusion: TNS can form fibrinogen-wrapped pus to prevent bacterial infection from going deeper, and to improve the phagocytic function of phagocytes, the secretion of lymphocytes, and the defense function of the complement system. Therefore, it is a competitive drug for the treatment of suppurative diseases.
Keywords: Tounong San, suppurative infection, immune function, pharmacological mechanism
Introduction
Antibiotics are regarded as the drug of choice for the treatment of suppurative diseases. However, due to the emergence of super-resistant bacteria and the recurrence of suppurative infections caused by some autoimmune diseases following antibiotic treatment, clinical staff have to find new drugs capable of resolving this problem.1,2 Tounong San (TNS) is a famous prescription in traditional Chinese medicine (TCM) used to treat suppurative infectious diseases, where tounong in Mandar in Chinese means to promote abscess ulceration. This drug can constrict pus, move the focus from deep to shallow areas, thereby making abscesses to ulcerate and promoting the healing of suppurative diseases.3 Clinically, TNS can treat both common suppurative infectious diseases such as furuncle, acute suppurative mastitis, perianal abscess, and chronic osteomyelitis, as well as purulent diseases related to immune deficiency such as plasma cell mastitis and Crohn’s disease.4–7 However, the action mechanism of this drug remains unknown.
It was found in the early studies conducted by our research group that TNS increases the healing rate of local abscesses and the levels of basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) in serums in relation to rats infected by Staphylococcus aureus.8 The results of the bacteriostatic test in vitro showed that TNS produces an inhibitory effect on Staphylococcus aureus, β-hemolytic Streptococcus, and Streptococcus pneumoniae, but its bacteriostatic effect proves worse than that of Cefadroxil.9 The results of the bacteriostatic test in vivo showed that, compared to the control group, TNS can protect rats intraperitoneally injected with Staphylococcus aureus, and significantly lower the mortality of rats, with no significant difference between the protective effects of TNS and Cefadroxil.10 The experimental results above suggest that the mechanism of treating suppurative infectious diseases with TNS, which differs from the direct bactericidal effect of antibiotics, may improve the host’s immune function and therefore play a therapeutic role. As a result, the effect of TNS on the immune function of rats with suppurative infections was observed through in vitro and in vivo experiments to provide a preliminary explanation for the mechanism of this drug in the treatment of suppurative infectious diseases.
Materials and Methods
Experimental Animals
A total of 170 Wistar rats (half male and half female, SPF grade, 7 weeks old, 200 ± 10g) were purchased from SPF (Beijing) Biotechnology Co., Ltd. (SCXK (Beijing) 2016–0002). They were raised in the SPF Animal Room of the Animal Experiment Center of Inner Mongolia Medical University that was alternatively dimmed and brighten every 12 hours and had a relative humidity of between 50% and 60% and a temperature of between 23 °C and 26 °C. These rats were given unrestricted access to water and food. The use and management of experimental animals for research are in accordance with the requirements of the Ethics Committee of Inner Mongolia Medical University for the management of experimental animals and animal welfare (YKD2016007).
Experimental Drugs
Astragalus (Hebei Jixintang Pharmaceutical Co., Ltd., batch No.: 170801060), Ligusticum wallichii (Hebei Jixintang Pharmaceutical Co., Ltd., batch No.: 171001529), Chinese angelica (Anguo Lulutong Chinese Herbal Tablets Co., Ltd., batch No.: 17003004), Squama manitis (Anguo Lulutong Chinese Herbal Tablets Co., Ltd., batch No.: 170201), Spina Gleditsiae (Hebei Wanxiu Pharmaceutical Co., Ltd., batch No.: 160501cp159), and Cefadroxil capsule (CSPC Ouyi Pharmaceutical Co., Ltd., batch No.: 052181161).
Composition of TNS: 12 g of Astragalus, 6 g of Squama manitis, 9 g of Ligusticum wallichii, 4.5 g of Spina Gleditsiae, and 3 g of Squama manitis. Ultrapure water was used to heat, reflux, and extract TNS twice. The filtrate was combined and subject to rotary evaporation under reduced pressure for concentration. Then, it was frozen and dried to obtain the lyophilized powder. When used, the powder was dissolved into a solution with crude drug concentrations of 1.25 g/mL (high), 0.625 g/mL (medium), and 0.3125 g/mL (low).10 The Cefadroxil capsule (0.15 g/kg) and ultrapure water were combined to prepare an 18 mg/mL solution for reserve.10,11
Instruments and Reagents
Instruments
Flow cytometry (BD, AccuriC6), enzyme-labeled instrument (TECAN, Infinite M200), CO2 incubator (Memmert, INCO 153 med), dehydrator (Wuhan Taixing Electronics Co., Ltd., JT-12S), embedding machine (Wuhan Deli Electronics Co., Ltd., JB-P7), pathological section machine (Leica, RM2235), inverted microscope (NikonCi-S), and imaging system (NikonDS-U3).
Reagents
Fluorescein isothiocyanate (FITC) (sigma, F7250), Concanavalin A (ConA) (sigma, c2010), lipopolysaccharide (LPS) (sigma, 0000081277), ethidium bromide (EB) (sigma, MKCJ7007); Dihydrorhodamine 123 (DHR 123) (MKbio, batch No.: 2509×200098), Phorbol 12-myristate 13-acetate (PMA) (MKbio, batch No.: 2208Z20915); rat peripheral blood lymphocyte separation solution kit, RPMI-1640 culture medium, hematoxylin-eosin stain kit (Beijing Solarbio Science & Technology Co., Ltd., batch No.: 20201010, 20201013, G1420); CCK-8 kit (Dojindo Beijing Co., Ltd., batch No.: SC675), soluble starch (Tianjin Kemao Chemical Products Co., Ltd., batch No.: 20200318); rat total complement (CH50) ELISA kit, rat interleukin-6 (IL-6) ELISA kit, rat interleukin-1 (IL-1) ELISA kit, rat tumor necrosis factor α (TNF-α) ELISA Kit, rat interferon γ (IFN- γ) ELISA kit, rat immunoglobulin M (IgM) ELISA kit, rat immunoglobulin G (IgG) ELISA Kit (the above ELISA Kits are produced by Wuhan Gene Beauty Biotechnology Co., Ltd., with the batch No. GR2020-12); anti-CD31 antibody [EPR17259] (Abcam, ab182981), anti-CD163 antibody [EPR19518] (Abcam, ab182422), anti-CD16 antibody [EPR16784] (Abcam, ab198507), anti-CD68 (Xavier, GB11067), endogenous peroxidase blocker (MaxVision, SPKIT-A2), animal non-immune serum (sheep, MaxVision, SPKIT-B1), HRP-Polymer anti-Rabbit IHC Kit (MaxVision Reagent, KIT-5005), HRP-Polymer anti-Mouse/Rabbit IHC Kit (MaxVision Reagent, KIT-5010), Staphylococcus aureus (China Academy of Food and Drug Control, CMCC 26003–5a25), cultivated as a third-generation working bacterium.
Methods
Animal Modeling, Grouping, and Treatment
The back of each rat was shaved for approximately 3 cm2 in size. On the 2nd day, 1 mL of Staphylococcus aureus solution (1.8×1010/mL) was subcutaneously injected into the center of the shaved area for two consecutive days, and the rats were observed for 1 day. If there is visible redness, swelling, soreness, and fluctuation at the injection site, the model is proved to be successful.
Upon modeling, the 160 rats were randomly divided into six groups, that is, the high-, medium-, and low-dose TNS groups, Cefadroxil group, and control group, with 30 rats in each group, and the normal group, with 10 rats. The rats in all groups except the normal group were administered corresponding drugs by gavage, 10 mL/kg/d per rat, once a day, while those of the control group was administered isovolumetric saline. Their mental state, activity, local abscesses, and weight were observed daily. At the same time, on the 3rd, 6th, and 9th day of medication, 10 rats were selected at random from each group. After administering isoflurane anesthesia to the rats, macrophages were obtained from their peritoneal cavities, blood was extracted from their abdominal aortas, and skin tissues of the back abscesses were also extracted. The phagocytic rate of blood neutrophils, the generation of ROS, and the phagocytic function of peritoneal macrophages were measured using flow cytometry. Blood lymphocytes were separated, and the proliferation function of lymphocytes in vitro was detected using the CCK-8 method. In addition, the expression levels of the serum inflammatory factors and complements IL-1, IL-6, CH50, TNF-α, and IFN-γ in vitro and the secretion of IgG and IgM by lymphocytes were detected using the ELISA. Whereas the pathological conditions of abscesses was observed using the HE staining method, and the expressions of CD31, CD68, CD163, and MPO in pathological tissues were detected using the immunohistochemical method.
HE Staining
The locally abscessed skin tissues of rats stored in a neutral formalin solution were taken and stained according to the instructions of HE staining, and were observed using the Image Scope.
Immunohistochemical Staining of Abscessed Skin Tissues
The abscessed skin tissues of rats stored in 10% neutral formalin solution were taken and stained according to the manufacturer’s instructions for each detection indicator (CD31, CD68, CD163, and MPO). The positive cells appeared brownish yellow in the photos taken using the Image Scope. The expression levels of vascular proliferation, neutrophils, and macrophages were evaluated separately according to the detection indicators of CD31, MPO, CD68, and CD163.
Detection of Rat Neutrophil Phagocytosis and ROS Generation Using Flow Cytometry
Staphylococcus aureus and Escherichia coli were labeled by FITC, and the labeling rate was detected using flow cytometry. For each sample, two flow tubes were taken and each added with 100 μL of anticoagulant blood. 20 μL of 1×109/mL FITC-labeled bacterial solution were added into the experimental tube, while no such solution was added into the control tube. Both of the tubes were put into a water bath at 37 °C and kept in the dark for 30 minutes before they were removed and placed into ice for 10 minutes. Then, they were added with red blood cell lysate, rinsed using PBS, and fixed at 1% PFA before the ratio of positive neutrophils that phagocytosed the bacteria was detected using the flow cytometer.12
In the experiment to detect ROS generation by rat neutrophils, the static control tube and experimental tube were labeled for each sample. 100 μL of anticoagulant blood and 50 μL of DHR123 (30 μg/mL) (as fluorescent probe) were added into each tube, which were incubated at a 37 °C water bath for 15 minutes before they were removed and put into ice box for 5 minutes. Then, 50 μL of PMA (2.5 μg/mL) were dripped into the experimental tube, and an equal volume of PBS was dripped into the static control tube; both were incubated at 37 °C for 60 minutes in the dark. Following this, they were added with red blood cell lysate and rinsed using PBS before resuspension was conducted. Flow cytometry was used to detect the cell suspension. The mean fluorescence intensity (MFI) of DHR123 in the neutrophils of the experimental tube, which indicates the content of ROS in neutrophils, was recorded in the FLl histogram.13
Detection of Macrophage Phagocytosis Using Flow Cytometry
Rats in each group were intraperitoneally injected with 3 mL of 6% soluble starch solution to induce macrophages one day before sample collection. On the day of the experiment, 3 mL of FITC-labeled Staphylococcus aureus suspension (6.7×107/mL) was intraperitoneally injected, followed by 4 mL of PBS solution after 30 minutes. 1mL of peritoneal washing solution was sucked out, and was centrifuged to obtained macrophage, which were fixed with 1 mL of 70% ethanol. The macrophages solution was centrifugated before 1 mL of 0.5 mg/mL EB solution was added, and placed in a 37 °C water bath for 15 minutes in the dark.14 The percentage of FITC-positive macrophages phagocytosing Staphylococcus aureus was detected using a flow cytometer with the FL1 (FITC)/SSC scatter chart set.
Detection of Rat Lymphocyte Proliferation Using the CCK-8 Method
Three experimental doses (5, 10, and 20 mg/mL) were selected through the pre-experiment. The anesthetized rats were placed into 75% alcohol for 5 minutes before they were dried with sterile gauze, and had their blood extracted from their inferior vena cava. Then, according to the instructions of the lymphocyte separation kit, 1 mL of anticoagulant blood was taken, and lymphocytes were separated. The cells were suspended with 5 mL of RPMI-1640 medium (including double antibodies) containing 10% calf serum.15 The rate of viable cells was above than 95%, and the lymphocyte concentration was adjusted to 6×106/mL.
Detection of Rat Peripheral Blood Lymphocyte Proliferation
Four groups were assigned in the experiment and placed on a 96-hole culture plate (Figure 1A). TNS was dissolved in RPMI-1640 medium into solutions that each contains high-, medium-, and low-dose of drugs (10, 20, and 40 mg/mL). Finally, the absorbance value was measured with an enzyme-labeled instrument at 450 nm. See Figure 1B for details.
Detection of Rat Peripheral Blood B Lymphocyte Proliferation
Refer to Section “Detection of rat peripheral blood lymphocyte proliferation” for experimental procedures. The difference is only that in the second step of this experiment, 20 µL of B lymphocyte mitotic stimulator LPS (100 g/mL) was added, and correspondingly, the consumption of RPMI-1640 was reduced by 20 µL.
Detection of Rat Peripheral Blood T Lymphocyte Proliferation
Refer to Section “Detection of rat peripheral blood lymphocyte proliferation” for experimental procedures. The difference is only that in the second step of this experiment, 20 µL of T lymphocyte mitotic stimulator ConA (50 µg/mL) was added, and correspondingly, the consumption of RPMI-1640 was reduced by 20 µL.
Determination of Expression Levels of Rat Serum CH50, IL-1, IL-6, TNF-α, IFN- γ, IgG, and IgM Using the ELISA Method
They were conducted as instructed by the ELISA kit.
Statistical Methods
SPSS22.0 software was used for the statistical analysis of data. Counting data were analyzed by chi-square test. If the measurement data conforms to the normal distribution and variance homogeneity, a single-factor analysis of variance will be employed; otherwise, the rank sum test will be employed. The analysis is considered statistically significant when P<0.05.
Results
Macro Evaluation of Drug Effects
On the 3rd day of medication, the rats in the control group were depressed, curled up, and barely moved; their backs were covered with obvious abscesses, and most of which were diffuse abscess. In contrast, the mental state and activity of rats in the other groups were better than those in the control group; the abscesses of rats in the Cefadroxil group were localized, some rats in the high-dose group began to ulcerate, a few rats in the medium-dose group also ulcerated, and the abscesses of rats in the low-dose group were localized (Figures 2A and B).
On the 6th day of medication, the abscesses of most of the rats in the high-, medium-, and low-dose TNS groups ulcerated, and even some abscesses of the rats in the high-dose group began to heal, whereas few abscesses of the rats in the control group and the Cefadroxil group ulcerated (Figures 2A–C).
On the 9th day of medication, most of the abscesses of rats in the high- and medium-dose groups healed, each forming a small induration, some of which softened and diminished. In the Cefadroxil group, most of the abscesses did not ulcerate, but were localized and reduced in size, some of which had been softened and completely absorbed. In the control group, the abscesses were significantly larger, with unclear boundaries and minimal ulceration (Figures 2A–D).
Pathological Tissue Test Results
HE Staining Results
On the 3rd day of medication, obvious necrotic tissue and abscess formation was visible in the epidermis and dermis of rats in each group, and many inflammatory cells gathered and infiltrated. Necrotic abscess tissues were seen in most areas. However, a significant amount of fibrinogen can also be seen wrapped around the pus at the abscesses of some rats in the high- and medium-dose TNS groups (see high-power pictures in Figure 3A).
On the 6th day of medication, the pus gradually disappeared in all TNS groups, but the number of new blood vessels and fibroblasts increased. A small number of inflammatory cells infiltrated in a few areas of the dermis of the rats in the high-dose group, while diffuse and scattered inflammatory cells infiltrated the dermis of the rats in the medium- and low-dose groups. Meanwhile, tissue necrosis and more inflammatory cells were still visible in the control group, and scattering and infiltration of inflammatory cells in the dermis, as well as neovascularization and fibrocyte proliferation were observed in the Cefadroxil group. On the 9th day, the recovery of skin injury was better in each group than on the 6th day. For the rats in the high-dose TNS group, a small number of inflammatory cells were scattered and infiltrated, and fibrous tissue hyperplasia was observed in the focal area, with no other obvious pathological changes observed. Whereas fibrous tissue hyperplasia with less lymphocyte infiltration can be seen in the medium- and low-dose groups. In the control group, inflammatory cell infiltration was diffuse, and a small amount of unabsorbed pus, increased neovascularization and fibrocytes, and granuloma formation was observed. Meanwhile, in the Cefadroxil group, a small number of inflammatory cells were scattered and infiltrated in the cortex, and local fibrous tissue hyperplasia was observed locally.
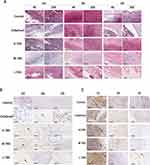
Detection of New Vessel Expression in Rats’ Back Abscess Tissues Using Immunohistochemistry
On the 3rd day of medication, CD31-labeled vascular endothelial cells began to be expressed in the high-, medium-, and low-dose TNS groups, and were positively correlated with the concentration of TNS, whereas there was almost no expression in the control group and the Cefadroxil group (Figure 3B). On the 6th day of medication, endothelial cells were expressed in most of the dermis of rats in each group. The vascular endothelial cells were highly expressed in the high-dose TNS group, while increased in expressions in the medium-dose TNS group and the Cefadroxil group, and were always lowly expressed in the control group. On the 9th day of medication, the expression level of vascular endothelial cells was higher in each group than on the 6th day.
Detection of Neutrophil Expression in Abscess Tissues Using Immunohistochemistry
On the 3rd day of medication, a large number of MPO-labeled neutrophils in the back abscess tissues of rats in each group were expressed and observed to be in brownish yellow color (Figure 3C). On the 6th day, fewer MPO-labeled neutrophils in rats’ abscess tissues in each group were expressed. On the 9th day, MPO-labeled neutrophils in the rats’ abscess tissues in each group were reduced further, some of which even disappeared.
Detection of Macrophage Expression in Rats’ Back Abscess Tissues Using Immunohistochemistry
On the 3rd day of medication, compared to the control group, CD163-labeled M2 type macrophages and CD68-labeled universal type macrophages were highly expressed to varying degrees in the high-, medium-, and low-dose TNS groups and the Cefadroxil group, with macrophages in the high-dose TNS group being the most highly expressed. On the 6th and 9th day, the number of macrophages in the high-, medium-, and low-dose TNS groups decreased gradually, whereas macrophage expression in the control group increased gradually (Figure 4).
Effect of TNS on Phagocytic Function and ROS Generation of Neutrophils in vitro
Results of Phagocytic Rate of Rats’ Neutrophils
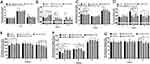
The experimental results showed that the average labeling rate of Staphylococcus aureus and Escherichia coli detected using flow cytometry was 91% and 93.5%, respectively (Figure 5A). On the 3rd day of medication, compared to the control group, the rate of positive neutrophils phagocytosing Staphylococcus aureus in the TNS groups did not differ statistically significantly (Figure 5B and E), but the rate of positive neutrophils phagocytosing Escherichia coli in the medium- and low-dose groups were statistically significant (P<0.05) (Figure 5C and F). On the 6th day of medication, compared to the control group, the rate of positive neutrophil phagocytosing two types of bacteria in each dose group did not differ statistically from the control group. On the 9th day of treatment, compared to the control group, the rate of positive neutrophil phagocytosing Staphylococcus aureus in the high-dose TNS group increased significantly (P<0.05), so did the rate of positive neutrophil phagocytosing Escherichia coli in all TNS group (P<0.05).
Results of Neutrophils ROS Generation
The content of ROS in neutrophils was expressed by the fluorescence intensity of DHR123 in neutrophils (Figure 5D and G). On the 3rd and 6th days of treatment, there was no difference between the amount of ROS generated in the TNS groups and that in the control group (P>0.05). On the 9th day of medication, compared to the control group, the high- and medium-dose groups had statistically significantly higher ROS (P<0.05).
Effect of TNS on the Phagocytic Rate of Macrophages
On the 3rd day of treatment, compared to the control group, the rate of FITC-positive macrophages in the medium-dose TNS group increased (P<0.05), with a significant difference from the Cefadroxil group (P<0.05). On the 6th day, compared to the control group, the rate of FITC-positive macrophages in the high-dose TNS group increased (P<0.05), with a significant difference from the Cefadroxil group. On the 9th day, there was no significant difference in the rate of FITC-positive macrophages between the TNS groups and the control group (P>0.05), as shown in Figure 5H and I.
Effect of TNS on CH50, IL-1, IL-6, TNF-ɑ, and IFN-γ in Rat Serum
On the 3rd day of medication, the expression levels of serum CH50 and TNF-ɑ in each TNS group were not significantly different from those of the control group (P>0.05) (Figure 6A and D); the expression level of serum IL-1 in the low-dose group was significantly higher (P<0.05), with a difference from that of the Cefadroxil group (P<0.05) (Figure 6B); the level of serum IL-6 in the medium- and low-dose groups decreased (P<0.05), with a difference from that of the Cefadroxil group (Figure 6C); the expression level of serum IFN-γ was increased in the medium-dose group (P<0.05) (Figure 6E).
On the 6th day of treatment, compared to the control group, the expression level of serum CH50 in the low-dose TNS group increased (P<0.05), and that of IL-1 in the high-dose group increased (P<0.05); serum IL-6 and IFN-γ in each TNS group with different doses had no difference in the expression level (P>0.05); serum TNF-ɑ saw a decrease in its expression level across the TNS groups with different doses (P<0.05).
On the 9th day of treatment, there was no significant difference in the expression levels of serum CH50, IL-1, and IL-6 between each of the TNS group with different doses and the control group; however, the expression level of TNF-αin the medium-dose group was significantly lower (P<0.05). Serum IFN-γ in the low-dose group saw a decrease in the expression level (P<0.05), significantly different from that of the Cefadroxil group.
Effect of TNS on the Levels of IgG and IgM in Rat Serum
On the 3rd day of medication, compared to the control group, the expression level of serum IgM in each of the TNS group with different doses increased (P<0.05), with a significant difference from that of the Cefadroxil group (Figure 6F). However, there was no difference in the expression level of serum IgG in the TNS groups with different doses (P>0.05) (Figure 6G).
On the 6th day of medication, compared to the control group, the expression level of serum IgM in the low-dose group decreased (P<0.05), with a significant difference from that of the Cefadroxil group. However, there was no significant difference in the expression level of serum IgG in the TNS groups with different doses (P>0.05).
On the 9th day of medication, compared to the control group, there was no difference in the expression level of serum IgM in the TNS groups with different doses (P>0.05). The expression level of serum IgG in the high- and low-dose groups decreased (P<0.05), with a significant difference from that of the Cefadroxil group.
Effect of TNS on Proliferation of Total Lymphocytes, B Lymphocytes, and T Lymphocytes in vitro
The results showed that under the action of TNS on total lymphocytes (Figure 7A), the joint action of TNS and LPS on B lymphocytes (Figure 7B), and the joint action of TNS and ConA on T lymphocytes (Figure 7C) for 24 hours and 48 hours, respectively, there was no significant difference in the proliferation of total lymphocytes, B lymphocytes, and T lymphocytes in vitro between the high-, medium-, and low-dose TNS groups when compared to the control group, drug group, and culture medium group (P>0.05).
Discussion
Pathogenic bacteria such as staphylococcus will invade the human body when human skin is damaged, triggering a series of immune responses of the body. In this case, macrophages settled in connective tissues will rapidly rush to the inflammatory lesion to produce the phagocytosis effect and secrete a large number of chemokines and inflammatory factors. Also, blood vessels permeability will increase, and neutrophils will migrate to phagocytose pathogenic bacteria and jointly participate in cellular immunity. The complements contributed to humoral immunity when activated through the bypass pathway, and the total complement activity was reflected by CH50. The activated complements produce anaphylactic toxins, such as C5a, which directly or indirectly causes immune inflammatory damage at the site where a large number of phagocytes gather.16 Extracellular fluid, pathogenic bacteria, phagocytes, and their remains combined at the site of an inflammatory injury to form pus. As the disease progresses, some macrophages will phagocytize the antigen and transmit it to T and B cells to sensitize and activate lymphocytes, thereby playing its specific immune function.
Cytokines are a class of small molecular proteins with broad biological activities, which are mainly produced by activated lymphocytes and monocytes/macrophages.17 IL-1 can cause fever in the hypothalamus through the effect of blood circulation, release neutrophils from the bone marrow cell bank into the blood, activate them, and enhance their ability to kill pathogenic microorganisms and migrate. Local low concentration of IL-1 mainly acts on other immune cells to stimulate their activation, proliferation, and secretion of antibodies, and attract neutrophils, thereby triggering the release of inflammatory mediators. Capable of regulating immune response, acute phase response, and hematopoiesis. IL-6 plays a vital role in the body’s anti-infection immune response. Following the onset of infection and inflammation, IL-6 will be produced first and rapidly increase in its level, with the rate of increase correlating to the severity of the infection. TNF-α is an endogenous pyrogen that can cause fever. It stimulates the phagocytosis and degranulation of neutrophils and enables the adhesion of neutrophils to endothelial cells, thereby triggering the local inflammatory reaction of the body and inducing the expression of cytokine genes such as IL-1β. IFN-γ is secreted primarily by lymphocytes and partially by activated NK cells. It can directly regulate the immune and inflammatory response of the body, and with its main biological function as a macrophage activating factor, it can also kill invasive pathogenic microorganisms.
Antibiotics mainly play a direct role in sterilization and bacteriostasis in the treatment of suppurative diseases caused by bacterial infections, and in the “game” with bacteria, antibiotics may induce resistance of bacteria.18 In TCM, the goal of the treatment is to primarily strengthen the host’s own immunity, and then employ the bactericidal and bacteriostatic function.19,20 In this study, the effects of the traditional Chinese medicine prescription TNS and Cefadroxil on the immune systems of rats with suppurative disease in soft skin tissues caused by Staphylococcus aureus infection were compared through in vitro and in vivo experiments. The results showed that on the 3rd day of the medication, the rats in the TNS groups began to develop back abscesses and ulcerations, which peaked on the 6th day, with a rate directly proportional to the concentration of TNS. The abscess that began to ulcerate gradually healed on the 9th day, but the rate of abscess ulceration in the back of rats in the Cefadroxil group was significantly lower than the rats in the TNS groups, and had significantly slower healing progress. The pathological results showed that the reason for this phenomenon was related to the formation of wrapped fibrous tissues around infected areas of the rats’ backs in the TNS groups, which constrained pus and prevented the infection from spreading to deeper tissues. The phagocytosis test of neutrophils and macrophages showed that TNS can increase the phagocytic rate of rats’ phagocytes. Therefore, it can be inferred that TNS can promote the proliferation of fibrin in the infected area, improve the phagocytic rate of phagocytes, cause the infected area to produce a large amount of thick pus, prevent the flow of pus from entering into deeper tissues, and increase the internal pressure of the pus cavity, causing the pus to ulcerate from the weak skin. On the 3rd day of medication, TNS may increase the level of IL-1 secretion, promote the release of neutrophils into the blood, improve its ability to kill pathogenic microorganisms and migration, and inhibit the release of excessive IL-6 inflammatory mediators to reduce and regulate the early inflammatory response. On the 6th day of medication, TNS may reduce TNF-α by increasing the levels of IL-1 and CH50 in the serum level, regulate the continuous exudation of neutrophils, alleviate inflammatory response, and promote tissue repair. On the 9th day of medication, TNS can inhibit the level of TNF-α released, alleviate inflammatory responses, and promote tissue repair. IgM is the first antibody to appear after the body is infected. On the 3rd day of medication, TNS can increase the levels of IgM and IFN-γ in the serum, improve bactericidal function, regulate phagocytosis, and stimulate the activation of mononuclear macrophages. On the 6th day of medication, most of the abscesses in the TNS groups ulcerated and pus was discharged. At the same time, the inflammatory response was alleviated, and the amount of IgM produced was decreased. For IFN-γ, the effect continued, and the level was still high, which is consistent with the results of the macrophage phagocytosis test and IL-1 detection. On the 9th day of medication, the inflammation in the other groups was relatively obvious, whereas it was relatively mild in the TNS groups, and the pathogenic bacteria were mostly phagocytosed. Possibly due to the afore mentioned reasons, IgG and IFN-γ were recovered earlier, and had a relatively lower level. The CCK-8 method was used to detect the proliferation of rat lymphocytes. It was found that TNS does not promote the proliferation of peripheral blood lymphocytes in vitro.
Conclusion
TNS can improve the phagocytic function of phagocytes, form a localized fibrin network in the infected area, and regulate the secretion function of lymphocytes and the complement system to promote the abscess to rupture, accelerate the healing of abscesses, and enhance the immune function of rats with suppurative diseases in soft tissues on the body surface. However, due to the complex components and the characteristics of multiple targets and links, this experiment may not demonstrate the specific action mechanism of TNS, which we will improve in future studies.
Abbreviations
TNS, Tounong San; ROS, reactive oxygen species; TCM, traditional Chinese medicine; bFGF, basic fibroblast growth factor; EGF, Epidermal Growth Factor; IL, interleukin; TNF-α, tumor necrosis factor α; IFN-γ, interferon γ; MFI, mean fluorescence intensity; ConA, Concanavalin A; LPS, lipopolysaccharide; EB, ethidium bromide; DHR 123, Dihydrorhodamine 123; PMA, Phorbol 12-myristate 13-acetate; MPO, myeloperoxidase.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
The use and management of experimental animals for research are in accordance with the requirements of the Ethics Committee of Inner Mongolia Medical University for the management of experimental animals and animal welfare (YKD2016007).
Acknowledgments
The authors are grateful to SHUANGZHI (SHUANGZHI Co., Ltd., Hohhot, China) for providing help in part date analysis.
Funding
This study was financially supported by the National Natural Science Foundation of China (82205127, 81660792).
Disclosure
The authors report no conflicts of interest in this work.
References
1. David MZ, Daum RS. Treatment of Staphylococcus aureus Infections. Curr Top Microbiol Immunol. 2017;409:325–383. doi:10.1007/82_2017_42
2. Wong FLT, Chan LC, Prince A, et al. Staphylococcus aureus adaptive evolution: recent insights on how immune evasion, immunometabolic subversion and host genetics impact vaccine development. Front Cell Infect Microbiol. 2022;12:1060810. doi:10.3389/fcimb.2022.1060810
3. Chen HF. Traditional Chinese Surgery.
4. Gu H, Chen HJ, Chen CZ, Zhu BY. The effect of Tounong-san on wound of low perianal abscess to severe fire poison type. Henan Trad Chin Med. 2014;34(4):687–688. doi:10.16367/j.issn.1003-5028.2014.04.048
5. Tan WX, Li GS. Clinical effect of Tounong-san on adult chronic osteomyelitis. Inner Mongolia J Trad Chin Med. 2020;39(2):36–37. doi:10.16040/j.cnki.cn15-1101.2020.02.024
6. Zhao LL, Yu YH, Zhan J, Hu YD, Shi ZY. Yanghe Decoction and Tounongsan in the treatment of 59 cases of plasma cell mastitis. Zhejiang J Tradit Chin Med. 2020;55(2):107. doi:10.13633/j.cnki.zjtcm.2020.02.017
7. Hu ZP, Yang MY, Ye QB, et al. Tou nong san attenuates inflammation in TNBS-IBD model by inhibiting NF-κB signaling pathway. Evid Based Complementary Altern Med. 2018;2018:6929307. doi:10.1155/2018/6929307
8. Hao FM, Zhao QS, Ren X, et al. Animals experimental research on mechanism of righting and expelling Toxin in Tuofa. Chin Arch Trad Chin Med. 2010;28(7):1418–1420. doi:10.13193/j.archtcm.2010.07.76.haofm.018
9. Chang XD, Liu Y, Dong HY, Shi ZQ. Comparative research on main components and in vitro bacteriostasis of different extracts of Tounong-san. Trad Chin Drug Res Clin Pharmacol. 2020;31(11):1277–1281. doi:10.19378/j.issn.1003-9783.2020.11.003
10. Shi ZQ, Chang XD, Liu Y, Dang Y, Jiao YQ, Shi JP. Research on the bacteriostasis of different extracts of Tounong Powder. China J Trad Chin Med Pharm. 2020;35(11):5804–5806.
11. Setiawan LTK, Nugraha J, Lestari P, et al. Effect of African Leaf (Vernonia Amygdalina) to IL-6 and IL-10 Level on Staphylococcus aureus Infection. Indonesian J Trop Infect Dis. 2019;7(4):69. doi:10.20473/ijtid.v7i4.9654
12. Boero E, Brinkman I, Juliet T, et al. Use of flow cytometry to evaluate phagocytosis of Staphylococcus aureus by Human Neutrophils. Front Immunol. 2021;12:635825. doi:10.3389/fimmu.2021.635825
13. Kaundal U, Khullar A, Leishangthem B, et al. The effect of methotrexate on neutrophil reactive oxygen species and CD177 expression in rheumatoid arthritis. Clin Exp Rheumatol. 2021;39(3):479–486. doi:10.55563/clinexprheumatol/4h5onh
14. Yan Q, Ahn SH, Fowler VG Jr. macrophage phagocytosis assay of Staphylococcus aureus by flow cytometry. Bio-Protocol. 2015;5(4). doi:10.21769/bioprotoc.1406
15. Mirenisha Y, Zulihumaer A, Munisa D, Cong YY, Palida A. Effects of polysaccharide from Fomes Ames on immune function of murine splenic lymphocytes. J Food Safety Qua. 2018;9(16):4369–4374. doi:10.3969/j.issn.2095-0381.2018.16.029
16. Prame KK, Nicholls AJ, Wong CHY. Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018;371(3):551–565. doi:10.1007/s00441-017-2753-2
17. Shi ZQ, Bao XF, Li YK, Zhang XP, Xiao C. Effect of Tounong powder on chemotactic factor C5a and LTB4 in superficial purulent inflammation. J Changchun Univ Chin Med. 2017;33(5).703–705.
18. Chin KW, Michelle THL, Vijitra LI, Ma NL. An overview of antibiotic and antibiotic resistance. Environ Adv. 2023;11:100331. doi:10.1016/j.envadv.2022.100331
19. Wang X, Chen J, Yang F, et al. Two kinds of traditional Chinese medicine prescriptions reduce thymic inflammation levels and improve humoral immunity of finishing pigs. Front Vet Sci. 2022;9:929112. doi:10.3389/fvets.2022.929112
20. Luo C, Yu H, Yang T, et al. Data mining and systematic pharmacology to reveal the mechanisms of traditional Chinese medicine in recurrent respiratory tract infections’ treatment. Evid Based Complementary Altern Med. 2020;2020:8979713. doi:10.1155/2020/8979713
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.