Back to Journals » Journal of Inflammation Research » Volume 15
Exosomes Derived hsa-miR-4669 as a Novel Biomarker for Early Predicting the Response of Subcutaneous Immunotherapy in Pediatric Allergic Rhinitis
Authors Jiang S, Xie S, Fan R, Tang Q, Zhang H, Wang F, Xie S, Gao K, Zhang J, Xie Z, Jiang W
Received 2 July 2022
Accepted for publication 25 August 2022
Published 3 September 2022 Volume 2022:15 Pages 5063—5074
DOI https://doi.org/10.2147/JIR.S379414
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Sijie Jiang,1– 3 Shaobing Xie,1– 3 Ruohao Fan,1– 3 Qingping Tang,4 Hua Zhang,1– 3 Fengjun Wang,1– 3 Shumin Xie,1– 3 Kelei Gao,1– 3 Junyi Zhang,1– 3 Zhihai Xie,1– 3 Weihong Jiang1– 3
1Department of Otolaryngology Head and Neck Surgery, Xiangya Hospital of Central South University, Changsha, People’s Republic of China; 2Hunan Province Key Laboratory of Otolaryngology Critical Diseases, Xiangya Hospital of Central South University, Changsha, People’s Republic of China; 3National Clinical Research Center for Geriatric Disorders, Xiangya Hospital of Central South University, Changsha, People’s Republic of China; 4Department of Rehabilitation, Brain Hospital of Hunan Province, Hunan University of Chinese Medicine, Changsha, People’s Republic of China
Correspondence: Weihong Jiang, Department of Otolaryngology Head and Neck Surgery, Xiangya Hospital of Central South University, Changsha, People’s Republic of China, Tel +86-731-8975-3045, Email [email protected]
Purpose: Subcutaneous immunotherapy (SCIT) is an effective treatment for pediatric allergic rhinitis (AR), but its efficacy fluctuates among individuals. This study aims to identify the profile of serum exosomes derived microRNAs (miRNAs) and evaluate their capacities to early predict SCIT efficacy in pediatric AR.
Patients and Methods: High-throughput sequencing was applied to identify the miRNA of serum exosomes in AR children. GO enrichment and KEGG pathway analysis were performed to enrich the biological annotations of target mRNAs of miRNAs. Then we validated differentially expressed miRNAs in two independent cohorts by RT-qPCR. Logistic regression and receiver operating characteristic curve (ROC) were applied to evaluate the abilities of identified miRNAs in predicting the efficacy of SCIT in AR children.
Results: A total of 812 miRNAs were detected in the serum exosomes, including 16 upregulated and 14 downregulated. Differentially expressed genes are enriched in the biological process of developmental process and regulation of cellular process, and gathered in pathways such as the signaling pathways regulating pluripotency of stem cells and the Wnt signaling pathway. In the first validation cohort, hsa-miR-4669 (P=0.009) and hsa-miR-4686 (P=0.032) were significantly downregulated in the effective group than the ineffective group, while hsa-miR-3196 (P=0.015) was upregulated. In the second cohort, hsa-miR-4669 level (P< 0.0001) was downregulated in the effective group than the ineffective group. In addition, logistic regression revealed that hsa-miR-4669 level was correlated with the visual analogue scale (r=0.323, P=0.001) and total nasal symptoms score (r=0.269, P =0.007). ROC curve highlighted that hsa-miR-4669 level exhibited a reliable accuracy in predicting SCIT efficacy in pediatric AR (AUC=0.785).
Conclusion: Serum exosomes derived miRNA were associated with the efficacy of SCIT. Serum exosomes derived hsa-miR-4669 might serve as a novel biomarker for early predicting the response of SCIT in AR children.
Keywords: allergic rhinitis, subcutaneous immunotherapy, miRNA, exosome, children
Introduction
Allergic rhinitis (AR) is a common allergic disease caused by exposure to aeroallergens.1,2 The prevalence rate of AR varies around the world and increased dramatically in recent years. According to longitudinal studies, AR mostly occurs in childhood, and the prevalence increases with the growth of age.3,4 Several epidemiological questionnaires in China showed that the self-reported prevalence of AR in children ranged from 18.10% to 49.68%.5–7 Meanwhile, a previous research reported that 42% of AR children accompanied with asthma.8 AR has become a global public health problem with huge government sanitary investment, and it also brought heavy financial and mental burdens to patients.2,9 Currently, AR therapies consist of allergen avoidance, antiallergic agents, allergen immunotherapy (AIT), and surgery.1 Mainly performed subcutaneously (SCIT) or sublingually (SLIT), AIT is the only approach to alert the disease process and is generally recommended for allergic patients who respond poorly to pharmacotherapy.10,11 SCIT exhibited better performance in symptom control and adherence, especially in children.12–14 However, there still were some patients who did not respond well to SCIT, and AR children were at higher risk of adverse reactions during SCIT.15–17 Therefore, a trustworthy biomarker to predict the clinical efficacy of SCIT was crucial.18 Although previous publications discovered that IgG4 antibodies, serum periostin, and specific IgE/total-IgE ratio were associated with SCIT responses and contributed to the immune tolerance formation.19–21 However, it is still insufficient because of their inadequate sensitivities and specificities, and constructing an early SCIT response forecasting system is ongoing.
Exosomes, extracellular vesicles with sizes of 40–100 nm, are endocytically originated and released by cells and widely dissolved in human body fluids, including serum.22 Exosomes containing proteins and small RNAs such as microRNAs (miRNAs), act as crucial signaling mediators in transcellular material transportation and intercellular communication.23–25 Previous studies showed that tumor exosomes regulated immune response via blocking dendritic cell maturation and decreasing Th1 cell differentiation.26 Exosomal lncRNA GAS5 inhibited EZH2 and T-bet expression and mediated the helper T cell differentiation in AR.27 These investigations indicated that the exosomes regulate immune response by transferring essential signaling molecules. MiRNA silences target genes at the post-transcriptional level and down-regulates the expression of target genes. It has been reported that miRNAs played crucial roles in the immune microenvironment,28,29 suggesting that they might participate in the occurrence and development of AR. However, little is known about the role of exosome-derived miRNAs in SCIT response of AR patients.
Thus, we hypothesized that miRNAs derived from serum exosomes might be involved in the immune response to SCIT in pediatric AR. To test this hypothesis, we profiled the exosomal miRNAs by RNA sequencing, and validated the potential differential expressed miRNAs in two independent cohorts. The capacity of miRNA for predicting SCTI response was assessed with logistic regression and receiver operating characteristic (ROC) curve. The flowchart of this study was displayed in Figure 1.
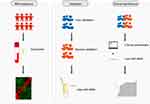 |
Figure 1 Graphic workflow of this study, including miRNA sequencing, validation and clinical significance. |
Materials and Methods
Participants
AR children (age≥6 and ≤14 years old) who have been receiving SCIT (Novo-Helisen-Depot allergen extracts, Allergopharma, Reinbek, Germany) for more than 1 year were included in our study. All patients were diagnosed with HDM-induced AR, referring to the ARIA guidelines.1 The participant’s inclusion and exclusion criteria, immunotherapy schedules and efficacy assessment strategy were carried out as our previous study described.30 Eight patients were allocated to the effective group (n=4) and ineffective group (n=4) for sequencing. The validation was conducted with two independent cohorts. Fifty-two children were assigned to the first validation cohort from January 2019 to March 2019, including 26 children in the effective group and 26 in the ineffective group. Another cohort recruited 50 children between June 2019 and August 2019, with 25 in the effective group and 25 in the ineffective group. This study complied with the Declaration of Helsinki, and was approved by the Medical Ethics Committee of Xiangya Hospital of Central South University (No.201901713). Guardians of all children were informed and consented before collecting information and clinical samples.
Serum Specimen Collection and Exosome RNA Isolation
All serum specimens were obtained before the onset of SCIT. We collected 5mL peripheral blood from every participant and kept it at room temperature for 1 hour. All samples were centrifuged at 3000 g/min for 10 min. We separated the supernatants and stored them at −80°C for further experiments. The serum exosome isolation was conducted with miRCURY Exosome Serum/Plasma Kit (Qiagen, No.76603, Hilden, Germany), and RNAs were extracted with miRNeasy Micro Kit (Qiagen, No.217084, Hilden, Germany), following the manufacturer’s instructions. The miRNA cel-miR-39-3p was used as an external reference.
Exosome Identification
Isolated exosome sediments were resuspended. The exosomes were identified by transmission electron microscopy (TEM). The exosome suspension (10μL) was put on a copper grid, and then 2% phosphotungstic acid was added to the suspension. The equipment used for analyzing exosomes mounted on the grid was HITACHI H-7650 (Hitachi Limited, Tokyo, Japan). The particle size of the exosome was detected with Zetasizer Ultra (Malvern, Inc., Shanghai, China).
Library Construction and miRNA Sequencing
Library construction and miRNAs sequencing were performed by Aksomics Inc (Shanghai, China). The total RNAs were extracted. The integrity examination of total RNA was conducted by agarose gel electrophoresis. Further RNA quantifying and quality testing has proceeded with NanoDrop ND-1000. MiRNA library was constructed with NEB Multiplex Small RNA Library Prep Set for Illumina (Illumina, USA) based on the manufacturer’s instructions. The quality of sequencing libraries was tested with Agilent 2100 Bioanalyzer. Libraries were mixed and degenerated into single-stranded DNA with 0.1M NaOH. The mixed samples were sequenced 50 cycles with Illumina NextSeq 500 (Illumina, USA).
Differentially Expressed miRNAs Identification and Data Analysis
Raw sequencing data underwent the quality control process to assess the data usability. Then data was pre-processed to produce the trimmed data aligned with the reference genome (STAR version 2.5.2b). MiRNAs were included in the statistical analysis when the mean of counts per million reads ≥ 1. Differential expressions of miRNAs were calculated with the edgeR package (version 3.20.9) in the statistical R program (version 3.5.0). MiRNAs were selected as differentially expressed genes with a fold change (FC) ≥1.5 or FC ≤0.67 and P<0.05 between groups.
Target Gene Prediction
The targeted mRNAs of the differentially expressed miRNAs were predicted with miRDB (http://www.mirdb.org) and Targetscan version 7.2, and were illustrated with Cytoscape version 2.8.2.
Gene Ontology (GO) Enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis
The GO enrichment analysis was performed in Gene Ontology (geneontology.org). The biological process (BP), cellular component (CC), and molecular function (MF) of the predicted target mRNA of identified miRNAs were enriched. The KEGG pathway analysis enriched the predicted target genes pathway with the KEGG database (www.genome.jp/kegg).
Real-Time Quantification
The qRT-PCR was performed to validate the miRNAs identified by sequencing. Six miRNAs were selected, including the top three upregulated and three downregulated miRNAs. All primers were compounded by RiboBio (Shanghai, China). The cDNA synthesis was performed with total RNA and Mir-X miRNA First-Strand Synthesis Kit (Takara Bio USA, Inc.) following the instructions. The qRT-PCR reaction was performed with PerfectStart® Green qPCR SuperMix (TransGene Biotech, Beijing, China) on the ABI 7500HT qRT-PCR system (Applied Biosystems, Foster City, CA). The reaction procedure was 94°C for 30s, followed by 45 cycles of 94°C for 5s, 55°C for 15s, and 72°C for 10s. The comparative miRNA expressed level was normalized to the external control cel-miR-39 and calculated according to the 2−ΔΔCT method.31
Statistical Analyses
Numerical data were described as the mean ± SEM, and t-test or Mann–Whitney U-test was used for difference comparison. Categorical data were expressed as frequencies and percentages, and the Chi-square test or Fisher’s exact test was applied. Logistic regression and ROC curve were used for effectiveness evaluation. All statistical analyses in this study were carried out using GraphPad Prism 7.0 software, and the difference was considered statistically significant when the P-value was less than 0.05.
Results
Demographic Characteristics of Study Participants
The first validation cohort consisted of 26 effective children and 26 ineffective children who finished 1-year following up, and 25 paired subjects were included in the second cohort. The clinical parameters of enrolled participant from two cohorts were presented in Tables 1 and 2, respectively. There was no statistical difference in gender, age, BMI, concomitant diseases, baseline disease severity, and allergen species between the two groups in both validation cohorts.
 |
Table 1 The Demographic Character of the First Validation Group |
 |
Table 2 The Demographic Character of the Second Validation Group |
Exosomal miRNA Sequencing Profile in Serum of Pediatric AR Patients
Serum exosomes were identified by TEM, and particle diameter detection. Consistent with previous studies, our results indicated that the exosomes were small cup-shaped bilayer membrane vesicle structures (Figure 2A and B).
 |
Figure 2 Identification of serum exosomes. (A) The morphology of serum-derived exosomes was observed by TEM. Scale bar= 100 nm. (B) Particle diameter detection of exosomes. |
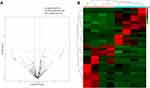
A total of 812 miRNAs were detected by sequencing, and 30 were identified as differentially expressed. Sixteen miRNAs were significantly upregulated in the effective group, while 14 miRNAs were downregulated (Figure 3A and B). Table 3 summarized the differentially expressed miRNAs.
 |
Table 3 Differentially Expressed miRNAs Between the Effective and Ineffective Groups |
GO and KEGG Pathway Analysis of miRNAs Profile
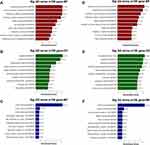
To better understand high-level functions and utilities of the identified serum exosomal miRNAss, we conducted GO enrichment and KEGG Pathway analysis among the target genes of detected miRNAs. The top 10 enriched GO terms were profiled in Figure 4. The developmental process [GO:0032502] and regulation of cellular process [GO:0050794] in BP, cytoplasm [GO:0005737] and cell periphery [GO:0071944] in CC, and protein binding [GO:0005515] in MF were identified as the most enriched GO terms. Figure 5 manifested that the detected serum exosomal miRNAs enriched in pathways such as the signaling pathways regulating pluripotency of stem cells [hsa04550] and the Wnt signaling pathway [hsa04310] etc.
 |
Figure 5 KEGG pathway analysis of the target mRNAs of the differentially expressed miRNAs. (A) Upregulated miRNAs and (B) downregulated miRNAs. |
Validation of Differential Expressed miRNAs
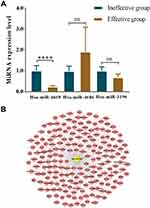
The top three upregulated and the downregulated miRNAs were selected for further validation according to the FC-value. In the first experimental cohort, we found that hsa-miR-4669 (P=0.009) and hsa-miR-4686 (P=0.032) levels were significantly downregulated in the effective group, while hsa-miR-3196 (P=0.015) concentration was upregulated (Figure 6). Moreover, we further verified the results with a second cohort. Hsa-miR-4669 (P<0.0001) level was proven to be significantly downregulated in the effective group (Figure 7A). The target genes of hsa-miR-4669 were predicted, and 34 genes were found to exhibit the potentials of binding hsa-miR-4669 (Figure 7B).
Altered Hsa-miR-4669 Predicted the Efficacy of SCIT and Was Correlated with Disease Severity
Correlation analysis was applied to explore the relationships between hsa-miR-4669 level and clinical parameters in AR. The results indicated that the VAS (r=0.323, P=0.001) and TNSS (r=0.269, P =0.007) were correlated with hsa-miR-4669 level (Figure 8). We further performed a logistic regression and found that hsa-miR-4669 level was an independent factor associated with SCIT efficacy in AR children. The ROC curve (Figure S1) indicated that exosomes derived hsa-miR-4669 was a potential biomarker with promising accuracy and reliability in predicting the efficacy of SCIT (AUC=0.785).
 |
Figure 8 Altered hsa-miR-4669 was correlated with disease severity. Correlation between exosomes derived hsa-miR-4669 level and (A) VAS and (B) TNSS. |
Discussion
In this study, we profiled serum exosomal miRNAs in pediatric AR patients before SCIT and then enriched the function and pathways of target genes of identified miRNAs. We further validated the differential expressed miRNAs with two cohorts and evaluated their correlation with clinical parameters and efficiency of SCIT. Our results corroborated that the exosomal miRNAs possessed predictive value of SCIT efficacy in pediatric AR patients and might be involved in the mechanisms of SCIT-induced immune tolerance formation.
Exosomes were proved to function in various diseases, such as acute myocardial infarction,32 breast cancer,32 and asthma.24 MiRNAs can be transferred by exosomes, and generally function by binding target mRNA transcripts and regulating the cellular response pathways.33 EV-derived miRNAs were reported to be differentially expressed between chronic rhinosinusitis and healthy controls in nasal lavage fluid, suggesting that they might be involved in the pathomechanism of upper airway inflammatory diseases.34 In the present study, we sequenced the serum exosomal miRNAs in pediatric AR patients under SCIT. The results indicated that exosomal miRNAs were differentially expressed between the effective and ineffective groups. The potential target genes of detected miRNAs enriching in GO terms such as developmental process, cytoplasm, and protein binding, and might participate in the Signaling pathways regulating pluripotency of stem cells and the Wnt signaling pathway, etc. The stem cells and the Wnt signaling pathway have been described as important components in allergic diseases.35,36 Therefore, it was reasonable to believe that the exosomal miRNAs were involved in the immune response to SCIT in pediatric AR.
Although previous publications discovered that IgG4 antibodies, serum periostin, and specific IgE/total-IgE ratio were associated with SCIT responses and contributed to the immune tolerance formation,19–21 these biomarkers were still unavailable in clinical application because of their deficiency in sensitivity and specificity. To address this predicament, we focused on the serum-derived exosomal miRNAs. Zhou et al37 observed that the miR-511-3p could modulate macrophage polarization and alleviate inflammation response in allergen-induced lung disease. Zeng et al38 found that the miR-181a and miR-155 were involved in AR development by regulating the differentiation of regulatory T cells. In this study, sequencing and cohort verification results demonstrated that hsa-miR-4669 level was significantly decreased in the serum exosomes in AR children who responded well to SCIT. A prior study found that hsa-miR-4669 polymorphism was associated with the susceptibility of ischemic stroke in a Korean population, and positively correlated with the baseline disease severity.39 Through GO analysis, the target genes of hsa-miR-4669 were enriched in acute myeloid leukemia[hsa05221], cholesterol metabolism[hsa04979], and cell adhesion molecules (CAMs)[hsa04514]. Immune cells play nonredundant roles in immune pathomechanism,40 and CAMs are strongly associated with immune cell migration and chemotaxis.41 Meanwhile, CAMs participated in airway allergic inflammation by involving the progression of epithelial cell adhesion. Thus, we assumed that serum exosomes might influence the body’s response to SCIT by transferring miRNAs such as hsa-miR-4669 and participating in immune tolerance formation. A high-level hsa-miR-4669 baseline might contribute to a failure to form immune tolerance and indicate an unsatisfactory response to SCIT in pediatric AR patients.
Overall, this study emphasizes the significance of serum exosomal miRNAs in SCIT and strengthens the existing understanding of miRNAs in AR. Meanwhile, miRNAs are promising in the immunotherapy area, as agents of sustained-release preparations or molecular therapy targets.42 Nevertheless, there are still some limitations in this present study. First, the sample size of this study was limited to some extent. Second, this study was not furthered in disease mechanism exploration. Last, the relatively short follow-up period may undermine the credibility of the conclusion.
Conclusion
In conclusion, this study suggested that serum exosomal hsa-miR-4669 was an expectant predictive biomarker of SCIT efficacy in AR children. Despite its exploratory nature, this study offers valuable insights into the relationship between exosomal miRNAs and AR. It demonstrates that exosomal miRNAs possess a fruitful area for further exploring and lays the groundwork for future molecularly targeted therapy research.
Acknowledgments
We gratefully acknowledge all sample donors who participated in this study.
Funding
This work was supported by the National Natural Science Foundation of China (No.82171118) and the Provincial Natural Science Foundation of Hunan (2022JJ30327).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Brozek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140(4):950–958. doi:10.1016/j.jaci.2017.03.050
2. Wise SK, Lin SY, Toskala E, et al. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol. 2018;8(2):108–352. doi:10.1002/alr.22073
3. Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr. 2016;16:133. doi:10.1186/s12887-016-0673-z
4. Kulig M, Klettke U, Wahn V, Forster J, Bauer CP, Wahn U. Development of seasonal allergic rhinitis during the first 7 years of life. J Allergy Clin Immunol. 2000;106(5):832–839. doi:10.1067/mai.2000.110098
5. Tong H, Gao L, Deng Y, et al. Prevalence of allergic rhinitis and associated risk factors in 6 to 12 years school children from Wuhan in central China: a cross-sectional study. Am J Rhinol Allergy. 2020;34(5):632–641. doi:10.1177/1945892420920499
6. Zhang YM, Zhang J, Liu SL, et al. Prevalence and associated risk factors of allergic rhinitis in preschool children in Beijing. Laryngoscope. 2013;123(1):28–35. doi:10.1002/lary.23573
7. Chen Y, Zhu J, Lyu J, et al. Association of maternal prepregnancy weight and gestational weight gain with children’s allergic diseases. JAMA Network Open. 2020;3(9):e2015643. doi:10.1001/jamanetworkopen.2020.15643
8. Dixon AE. Rhinosinusitis and asthma: the missing link. Curr Opin Pulm Med. 2009;15(1):19–24. doi:10.1097/MCP.0b013e32831da87e
9. Avdeeva KS, Reitsma S, Fokkens WJ. Direct and indirect costs of allergic and non-allergic rhinitis in the Netherlands. Allergy. 2020;75(11):2993–2996. doi:10.1111/all.14457
10. Roberts G, Pfaar O, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73(4):765–798. doi:10.1111/all.13317
11. Globinska A, Boonpiyathad T, Satitsuksanoa P, et al. Mechanisms of allergen-specific immunotherapy: diverse mechanisms of immune tolerance to allergens. Ann Allergy, Asthma Immunol. 2018;121(3):306–312. doi:10.1016/j.anai.2018.06.026
12. Liu Z, Lu H, Feng X, Hu L, Wang J, Yu H. Predictive methods for efficacy of house dust mite subcutaneous immunotherapy in allergic rhinitis patients: a prospective study in a Chinese population. Int Forum Allergy Rhinol. 2020;10(3):314–319. doi:10.1002/alr.22508
13. Zissler UM, Schmidt-Weber CB. Predicting success of allergen-specific immunotherapy. Front Immunol. 2020;11:1826. doi:10.3389/fimmu.2020.01826
14. Liu W, Zeng Q, He C, et al. Compliance, efficacy, and safety of subcutaneous and sublingual immunotherapy in children with allergic rhinitis. Pediatr Allergy Immunol. 2021;32(1):86–91. doi:10.1111/pai.13332
15. Endaryanto A, Nugraha RA. Indonesia-based study of the clinical and cost-saving benefits of subcutaneous allergen immunotherapy for children with allergic rhinitis in private practice. Cells. 2021;10(7):1841. doi:10.3390/cells10071841
16. Vogelberg C, Brüggenjürgen B, Richter H, Jutel M. Real-world adherence and evidence of subcutaneous and sublingual immunotherapy in grass and tree pollen-induced allergic rhinitis and asthma. Patient Prefer Adherence. 2020;14:817–827. doi:10.2147/ppa.S242957
17. Zhang W, Deng Y, Tong H, et al. Adverse reactions to subcutaneous immunotherapy in patients with allergic rhinitis, a real-world study. Eur Archiv Oto-Rhino-Laryngol. 2021;278(11):4353–4360. doi:10.1007/s00405-021-06736-2
18. Pfaar O, Agache I, de Blay F, et al. Perspectives in allergen immunotherapy: 2019 and beyond. Allergy. 2019;74(Suppl 108):3–25. doi:10.1111/all.14077
19. Shamji MH, Kappen J, Abubakar-Waziri H, et al. Nasal allergen-neutralizing IgG4 antibodies block IgE-mediated responses: novel biomarker of subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2019;143(3):1067–1076. doi:10.1016/j.jaci.2018.09.039
20. Hoshino M, Akitsu K, Kubota K, Ohtawa J. Serum periostin as a biomarker for predicting clinical response to house dust mite sublingual immunotherapy in allergic rhinitis. J Allergy Clin Immunol Pract. 2021;9(5):1864–1870. doi:10.1016/j.jaip.2020.11.046
21. Liu W, Zeng Q, Luo R. Predictors for short-term efficacy of allergen-specific sublingual immunotherapy in children with allergic rhinitis. Mediators Inflamm. 2020;2020:1847061. doi:10.1155/2020/1847061
22. Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release. 2015;219:278–294. doi:10.1016/j.jconrel.2015.06.029
23. Wang FW, Cao CH, Han K, et al. APC-activated long noncoding RNA inhibits colorectal carcinoma pathogenesis through reduction of exosome production. J Clin Invest. 2019;129(2):727–743. doi:10.1172/JCI122478
24. Zhang M, Yu Q, Tang W, et al. Epithelial exosomal contactin-1 promotes monocyte-derived dendritic cells-dominant T cell responses in asthma. J Allergy Clin Immunol. 2021;148:1545–1558. doi:10.1016/j.jaci.2021.04.025
25. Colao IL, Corteling R, Bracewell D, Wall I. Manufacturing exosomes: a promising therapeutic platform. Trends Mol Med. 2018;24(3):242–256. doi:10.1016/j.molmed.2018.01.006
26. Ning Y, Shen K, Wu Q, et al. Tumor exosomes block dendritic cells maturation to decrease the T cell immune response. Immunol Lett. 2018;199:36–43. doi:10.1016/j.imlet.2018.05.002
27. Zhu X, Wang X, Wang Y, Zhao Y. Exosomal long non-coding RNA GAS5 suppresses Th1 differentiation and promotes Th2 differentiation via downregulating EZH2 and T-bet in allergic rhinitis. Mol Immunol. 2020;118:30–39. doi:10.1016/j.molimm.2019.11.009
28. Lou Q, Liu R, Yang X, et al. miR-448 targets IDO1 and regulates CD8(+) T cell response in human colon cancer. J Immunother Cancer. 2019;7(1):210. doi:10.1186/s40425-019-0691-0
29. Wang QM, Lian GY, Song Y, Huang YF, Gong Y. LncRNA MALAT1 promotes tumorigenesis and immune escape of diffuse large B cell lymphoma by sponging miR-195. Life Sci. 2019;231:116335. doi:10.1016/j.lfs.2019.03.040
30. Xie S, Fan R, Tang Q, et al. Identification of robust biomarkers for early predicting efficacy of subcutaneous immunotherapy in children with house dust mite-induced allergic rhinitis by multiple cytokine profiling. Origin Res. 2022:12. doi:10.3389/fimmu.2021.805404
31. Lowe B, Avila HA, Bloom FR, Gleeson M, Kusser W. Quantitation of gene expression in neural precursors by reverse-transcription polymerase chain reaction using self-quenched, fluorogenic primers. Anal Biochem. 2003;315(1):95–105. doi:10.1016/s0003-2697(02)00695-4
32. Moradi-Chaleshtori M, Bandehpour M, Heidari N, Mohammadi-Yeganeh S, Mahmoud Hashemi S. Exosome-mediated miR-33 transfer induces M1 polarization in mouse macrophages and exerts antitumor effect in 4T1 breast cancer cell line. Int Immunopharmacol. 2021;90:107198. doi:10.1016/j.intimp.2020.107198
33. Correia de Sousa M, Gjorgjieva M, Dolicka D, Sobolewski C, Foti M. Deciphering miRNAs’ action through miRNA editing. Int J Mol Sci. 2019;20(24):6249. doi:10.3390/ijms20246249
34. Cha S, Seo EH, Lee SH, et al. MicroRNA expression in extracellular vesicles from nasal lavage fluid in chronic rhinosinusitis. Biomedicines. 2021;9(5):471. doi:10.3390/biomedicines9050471
35. Hammad H, Lambrecht BN. Wnt and Hippo pathways in regulatory T cells: a NOTCH above in asthma. Nat Immunol. 2020;21(11):1313–1314. doi:10.1038/s41590-020-0797-z
36. Daltro SRT, Meira CS, Santos IP, Ribeiro Dos Santos R, Soares MBP. Mesenchymal stem cells and atopic dermatitis: a review. Front Cell Dev Biol. 2020;8:326. doi:10.3389/fcell.2020.00326
37. Zhou Y, Do DC, Ishmael FT, et al. Mannose receptor modulates macrophage polarization and allergic inflammation through miR-511-3p. J Allergy Clin Immunol. 2018;141(1):350–364.e8. doi:10.1016/j.jaci.2017.04.049
38. Zeng Q, Liu W, Luo R, Lu G. MicroRNA-181a and microRNA-155 are involved in the regulation of the differentiation and function of regulatory T cells in allergic rhinitis children. Pediatr Allergy Immunol. 2019;30(4):434–442. doi:10.1111/pai.13038
39. Hong SJ, Kim SK, Yun DH, Chon J, Park HJ. Association between MicroRNA-4669 polymorphism and ischemic stroke in a Korean population. Dis Markers. 2019;2019:7238319. doi:10.1155/2019/7238319
40. Miyake K, Shibata S, Yoshikawa S, Karasuyama H. Basophils and their effector molecules in allergic disorders. Allergy. 2021;76(6):1693–1706. doi:10.1111/all.14662
41. Kalm F, Mansouri L, Russom A, Lundahl J, Nopp A. Adhesion molecule cross-linking and cytokine exposure modulate IgE- and non-IgE-dependent basophil activation. Immunology. 2021;162(1):92–104. doi:10.1111/imm.13268
42. Liu Y, Sha J, Meng C, Zhu D. The role of small extracellular vesicles and MicroRNAs in the diagnosis and treatment of allergic rhinitis and nasal polyps. Mediators Inflamm. 2022;2022:4428617. doi:10.1155/2022/4428617
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.