Back to Journals » International Journal of Nanomedicine » Volume 15
Exosomal lncRNA ROR1-AS1 Derived from Tumor Cells Promotes Glioma Progression via Regulating miR-4686
Authors Chai Y, Wu HT, Liang CD, You CY, Xie MX, Xiao SW
Received 26 August 2020
Accepted for publication 15 October 2020
Published 10 November 2020 Volume 2020:15 Pages 8863—8872
DOI https://doi.org/10.2147/IJN.S271795
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ebrahim Mostafavi
Yang Chai, Hai-Tao Wu, Chuan-Dong Liang, Chun-Yue You, Ming-Xiang Xie, Shun-Wu Xiao
Department of Neurosurgery, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou 563000, People’s Republic of China
Correspondence: Shun-Wu Xiao
Department of Neurosurgery, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou 563000, People’s Republic of China
Email [email protected]
Objective: Glioma is one of the most common central nervous system malignant tumors, accounting for 45%– 60% of adult intracranial tumors. However, the clinical treatment of glioma is limited. It is of great significance to seek new therapeutic methods for glioma via gene therapy.
Materials and Methods: Microarray is used to identify the lncRNAs that are differentially expressed in glioma. The expression of long non-coding RNA (lncRNA) ROR1-AS1 and miR-4686 was detected by qRT-PCR. Exosomes were isolated from the supernatant of normal and cancerous cells, and TEM was used for exosomes identification. MTT assay, wound healing assay, transwell assay, and colony formation assay were used to detect the exo-ROR1-AS1 function on proliferation, migration, and invasion in glioma cells. Luciferase assay and RIP assay were used to identify the relationship between lncRNA ROR1-AS1 and miR-4686. The effect of exo-ROR1-AS1 on tumorigenesis of glioma was confirmed by the xenograft nude mice model.
Results: ROR1-AS1 was up-regulated in glioma tissues, and the high expression of ROR1-AS1 indicated a poor prognosis in glioma patients. Interestingly, ROR1-AS1 was packaged into exosomes and derived from tumor cells. Functional analysis showed exo-ROR1-AS1 promoted the progression of glioma cell lines SHG44 and U251. Furthermore, ROR1-AS1 acted as a sponge of miR-4686 and inhibited its expression. Functionally, forced expression of miR-4686 removed the promoted effects of lncRNA ROR1-AS1 on glioma development. In vivo tumorigenesis experiments showed that exo-ROR1-AS1 promoted glioma development via miR-4686 axis.
Conclusion: Our study suggested tumor cells derived exo-ROR1-AS1 promoted glioma progression by inhibiting miR-4686, which might be a potential therapeutic target for glioma clinical treatment.
Keywords: glioma, lncRNA ROR1-AS1, exosome, miR-4686, tumorigenesis
Introduction
Glioma is the most common primary tumor of the central nervous system produced by astrocytes.1 Glioma has become one of the most invasive and lethal cancers because of its high metastasis rate and drug resistance. Surgical resection, radiotherapy and chemotherapy are commonly used at present, but most of these treatments are inefficient, often leading to recurrence and eventually death.2,3 The main reason for the weak therapeutic effect is the lack of understanding of the mechanism of glioma.4 Therefore, it is vital to clarify the mechanism of glioma progression.
Tumor cells usually increase tumorigenicity by affecting normal cells in their environment, such as vascular cells, microglia, peripheral immune cells and neural premises.5 There is growing evidence that intercellular communication in tumors depends on a nano-sized lipid bilayer vesicle, including exosomes, vesicles and apoptotic bodies.6 In recent years, exosomes have been shown to mediate the interaction between glioma microenvironment and may become a new anti-tumor target in microenvironment.7 Because all kinds of cells can secrete exosomes under normal and pathological conditions, the exosomes of different cells interact with each other, forming a complex communication network.8 Information is transmitted from tumor exosomes to other tumor cells and normal stromal cells to create a microenvironment that promotes the growth and progression of tumor.9 Exosomes are associated with drug resistance, angiogenesis, evasion of immune surveillance, apoptosis and tumor metastasis.10,11 Tumor exosomes have a strong ability to regulate tumor microenvironment.
LncRNA is an RNA with a length of more than 200 bp that does not have the ability to encode a protein.12 Previous studies have shown that lncRNA is associated with the occurrence and development of cancer and tumor-related signaling pathways.13,14 The lncRNA in exosomes is easy to extract and is expected to be used in the diagnosis and treatment of cancer.15 Most of the exosome in the tumor microenvironment are secreted by tumor cells. On the one hand, exosomes derived from tumor cells use lncRNA to change normal stromal cells.16 On the other hand, exosomes from highly malignant tumor cells can regulate the physiological state and function of low malignant tumor cells through lncRNA, so that tumor cells have strong viability.17 For example, lncRNA HOTAIR is highly expressed in glioma cells, and the highly expressed HOTAIR can be loaded into the exosomes secreted by glioma cells and transferred to endothelial cells, resulting in the increase of angiogenic factor VEGFA in vascular endothelial cells, resulting in enhanced angiogenesis.18 LncRNA ROR1-AS1 was first identified in mantle cell lymphoma and acted as an oncogene by interacting with EZH2 and SUZ12.19 Following researchers found ROR1-AS1 also contributed to colorectal cancer, bladder cancer and nasopharyngeal carcinoma.19–21 However, the function of ROR1-AS1 in glioma has not yet been fully revealed. Herein, we aimed to explore the effect of lncRNA ROR1-AS1 in glioma, and further illuminate the possible underlying mechanisms.
Materials and Methods
Tissue Specimen
The surgical specimens of 30 glioma patients from Affiliated Hospital of Zunyi Medical University were collected, which were used for follow-up experimental detection. The experiment was permitted by the Ethics Review Committee of Affiliated Hospital of Zunyi Medical University and the patients signed informed consent.
Exosome Isolation and Identification
Cells were isolated from cancer and adjacent normal tissues of glioma patients as previously described.22 Briefly, each glioma tissue specimen was minced into 1 mm3 cube chunks and enzymatically dissociated to single cells. Exosomes in culture medium and several centrifugations were performed to purify exosomes. Transmission electron microscopy (TEM) was used to identify exosomes structures. Exosomes were analyzed using exosome marker protein CD63, Alix and Tsg101 via Western blot.
Animals
Animal experiments were permitted by the Animal Protection and Ethics Committee of Affiliated Hospital of Zunyi Medical University. The research was carried out based on the proposals in the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health. BALB/c nude mice (6–8 weeks) were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. (Beijing, China). For the experiment of Xenograft, U251 cells (5 × 106) were suspended in 200 μL normal saline and subcutaneously injected into the right flanks. And a dosage of 5 mg (20ul) exosomes was administered into mice via tail vein injection once every 3 days for 2 weeks. Tumor volume (mm3): V (Mm3) = S2 (Mm2) × L (Mm)/2.
Cell Culture
Cell lines were purchased from CHI Scientific, Inc (Jiangsu, China). The cells were cultured with complete medium including 89% 1640 and 10% FBS, both were purchased from Biological Industries (Beit-Haemek, Israel), and maintained in an incubator with 37°C and 5% of CO2 saturated humidity.
Cell Transfection and Treatment
The SHG44 cells were plated until the cell density reached 80% confluency of dishes to transfect. The plasmids transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA). 5 ug/mL exosomes were added into the medium of SHG44 and U251 cells every 24 h. Plasmid of ROR1-AS1 or miR-4686 mimics or small interfering RNA (si-RNA) of ROR1-AS1 were constructed by Genechem (Shanghai, China).
qRT-PCR
As for cells, RNA was isolated after 20 h of exosomes treatment. RNA extraction was performed using trizol reagent. NanoDrop 8000 (Thermo Scientific, Waltham, MA, USA) was used to detect the concentration and purity of RNA. The single-stranded cDNAs were synthesized from 1 μg of RNA. The expression of mRNAs and miRNAs were quantified by RT-PCR with SYBR Green I (Thermo Fisher Scientific, Inc). Primer list: ROR1-AS1 (F: 5′-CTGACGAAACACTGGAACTC-3′, R: 5′-GTCTGATTTGGTAGCTTGGATG-3′), miR-4686 (F: 5′- CAACCTATCTGCTGGGCTTTC‐3′, R: 5′‐TATGCTTGTTCTCGTCTCTGTGTC‐3′), U6 (F: 5′-TCGCCCTTGGCA CAGCA-3′, R: 5′-CGAACCATTCAAGTGTTGCT-3′), GADPH (F: 5′- CTCCTGCACCACCAACTGCT −3′, R: 5′-GGGCCATCCACAGTCTTCTG −3′).
Western Blot
Cells were treated with exosomes for 24 h. After RIPA cleavage, total protein was extracted and measured with BCA method. After quantitative denaturation, protein electrophoresis membrane was transferred, and blockage of membrane used 5% BSA. The first incubation and second incubation were carried out according to the operation steps. The expression of the protein was expressed by the gray value. Primary antibodies list: CD63 (ab134045, Abcam), Tsg101 (ab125011, Abcam), and Alix (ab88388, Abcam).
MTT Assay
SHG44 and U251 cells were plated in 96-well plates, and MTT was used assay to detect the cell viability. MTT (20 nmol/L; Beyotime Biotechnology, China) was added after 12 h of exosomes treatment and incubated at 37°C for 4 h. The absorbance of 450 nm was measured with 150 μL DMSO.
Luciferase Assay
PsiCHECK-2 luciferase reporter plasmid was inserted with the wildtype WT-ROR1-AS1 and Mut-ROR1-AS1 3ʹUTR sequences that contain the putative binding sites of miR-4686 in GenePharma company (Shanghai, China). HEK293 cells were co-transfected with 20 mmol/L miR-4686 mimic or miR-NC together with WT-ROR1-AS1/Mut-ROR1-AS1. Luciferase activity was measured with Dual Luciferase Reporter Assay Kit (Transgene, China) on GloMax20/20 at 48 hr after the transfection.
Colony Formation Assay
The cells were digested and the cell suspension was diluted and inoculated in a dish containing 10 mL 37°C preheated medium, and gently rotated to make the cells dispersed evenly. The cells were then cultured for 14 days. Discard the supernatant and rinse carefully with PBS for 2 times. The cells were fixed with 4% paraformaldehyde and fixed with 5 mL for 15 minutes. Then, go to the fixed solution, add appropriate amount of GIMSA to dye solution for 10 minutes and 30 minutes, and then wash off the dye solution slowly with running water and dry the air.
RNA-Binding Protein Immunoprecipitation (RIP)
RIP assay was used to determine the binding between ROR1-AS1 and miR-4686 using Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore) as in previous study.23 Briefly, U251 cells were transfected with biotinylated miR-4686 or miR-NC, and the expression of ROR1-AS1 was detected using qRT-PCR.
Statistical Analysis
Data were shown as mean±SD. Student’s t-test or one-way ANOVA was used to compare the groups. P<0.05 was considered significant.
Results
LncRNA ROR1-AS1 Was Upregulated in Glioma Tissues and Indicated a Poor Prognosis in Glioma Patients
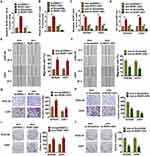
Firstly, bioinformatics was used to analyze the differentially expressed lncRNA genes in tumor tissues and adjacent tissues from glioma patients (Figure 1A and B). According to volcano plot and microarray data, a total of three significantly up-regulated lncRNAs were found to be differentially expressed between the tumor tissues and corresponding adjacent normal tissues. Among these up-regulated lncRNAs, lncRNA ROR1-AS1 was significantly higher than other lncRNAs. Suggesting ROR1-AS1 may involve in the progression of glioma. Then, the expression level of ROR1-AS1 in 30 paired glioma patients was detected, lncRNA ROR1-AS1 was upregulated in glioma cancer compared with normal tissues (Figure 1C). According to the median level of ROR1-AS1 in Figure 1A, 30 glioma patients were divided into low (n = 15) and high expression group (n = 15). Kaplan–Meier curves indicated a 5-year survival rate of glioma patients was significantly higher in low expression patients than high expression patients (Figure 1D). In addition, normal astrocytes and glioma cell lines SHG44, U251, U87 and SHG139 were cultured and found the level of ROR1-AS1 was dramatically higher in glioma cell lines than that in astrocytes (Figure 1E). Together, these data indicated that ROR1-AS1 might be involved in glioma progression.
LncRNA ROR1-AS1 Was Packaged into Exosomes
To determine the origin of ROR1-AS1 in glioma, we isolated exosomes in cancer tissues and adjacent normal tissues. TEM data showed the morphology of exosomes (Figure 2A). And exosomes markers were detected by Western blot (Figure 2B). Interestingly, ROR1-AS1 level was higher in cancer exosomes compared with normal exosomes (Figure 2C), which indicated that ROR1-AS1 could be packaged into exosomes and derived from tumor cells.
Exosomal-ROR1-AS1 Accelerated Cancer Progression of Glioma Cells
To evaluate the role of exosomal-ROR1-AS1 (exo-ROR1-AS1) in glioma development, tumor cells were transfected with ROR1-AS1 or si-ROR1-AS1 or its NC (Figure 3A), and SHG44 and U251 cells were incubated with exosomes isolated from tumor cells. ROR1-AS1 expression in isolated exosomes was detected, and ROR1-AS1 transfection induced ROR1-AS1 expression, while si-ROR1-AS1 transfection reduced ROR1-AS1 expression (Figure 3B). Then, we discovered that ROR1-AS1 expression was significantly upregulated in SHG44 and U251 cells upon incubation with exosomes with ROR1-AS1 plasmid, but not with si-ROR1-AS1 (Figure 3C). Functionally, MTT assay was used to estimate cell viability. It showed that exo-ROR1-AS1 increased cell viability, while exo-si-ROR1-AS1 decreased cell viability in SHG44 and U251 cells (Figure 3D). Furthermore, wound healing assay suggested that exo-ROR1-AS1 promoted cell migration in SHG44 and U251 cells (Figure 3E), but exo-si-ROR1-AS1 showed an opposite effect (Figure 3F). Transwell assay showed that exo-ROR1-AS1 induced cell invasion in SHG44 and U251 cells (Figure 3G), but exo-si-ROR1-AS1 showed an opposite effect (Figure 3H). In addition, overexpression of ROR1-AS1 promoted proliferation of SHG44 and U251 cells (Figure 3I), while silencing ROR1-AS1 inhibited proliferative ability (Figure 3J). Together, exo-ROR1-AS1 secreted by tumor cells promoted tumor progression in glioma cells.
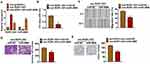
ROR1-AS1 Interacted with miR-4686
To clarify the underlying mechanism of ROR1-AS1 in glioma regulation, miRanda database was used to identify miRNA with ROR1-AS1 biding sites, which showed a strong binding between miR-4686 and ROR1-AS1 (Figure 4A). Luciferase assay showed that miR-4686 inhibited luciferase activity of WT ROR1-AS1, but not mutant ROR1-AS1 (Figure 4B). And qRT-PCR analysis showed overexpression of ROR1-AS1 significantly inhibited miR-4686 level, while si-ROR1-AS1 promoted miR-4686 expression in U251 cells (Figure 4C). Besides, exosomes from cancer cells reduced miR-4686 level, while transfection with si-ROR1-AS1 induced miR-4686 expression (Figure 4D). Further, endogenous ROR1-AS1 was enriched in biotinylated miR-4686 cells, which reveals a direct binding of ROR1-AS1 with miR-4686 (Figure 4E).
Exo-ROR1-AS1 Promoted Growth via miR-4686 in Glioma Cells
Then, miR-4686 was forced in U251 cells with the incubation of exo-ROR1-AS1 (Figure 5A). Overexpression of miR-4686 blocked the promoting effects of exo-ROR1-AS1 on cell viability, proliferation, migration and invasion in U251 cells (Figure 5B–E). Moreover, miR-4686 showed an inhibitory effect of glioma development (Supplementary Figure S1).
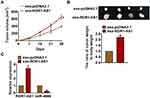
Exo-ROR1-AS1 Promoted Glioma Tumorigenesis in vivo
To further explore the effects of ROR1-AS1 in glioma, xenograft nude mice model was set up. Thirty mice were divided into two groups randomly, and U251cells were subcutaneously injected into nude mice. And a dosage of 5 mg exosomes was administered into mice via tail vein injection once every 3 days for 2 weeks. The tumor volume increased in ROR1-AS1 injection mice (Figure 6A). And the administration of exo-ROR1-AS1 promoted tumor weight (Figure 6B). In addition, isolated tumor tissues had a higher ROR1-AS1 level after injection of exoROR1-AS1 (Figure 6C). Moreover, the injection of ROR1-AS1 decreased the mRNA level of miR-4686 (Figure 6C). Taken together, ROR1-AS1 regulated the progression of glioma by inhibiting miR-4686.
Discussion
Glioma originates from glial cells or neural progenitor cells and is the most common primary tumor of the brain.24 It is reported that gliomas account for 80% of malignant primary central nervous system tumors, and its incidence is increasing year by year.25 Glioma is complex in morphology and molecular level, and shows a high degree of heterogeneity between tumors and within tumors, which brings great difficulty to the treatment of tumors. Tumor cells have the ability to communicate with other cells in the microenvironment.26 Among them, exosome is an important medium, which can be involved in the regulation of tumor migration, angiogenesis and drug resistance.27 In the present study, we found for the first time that lncRNA ROR1-AS1 could be packaged in exosomes, which targetedly inhibited the expression of miR-4686 and promoted the progression of glioma.
Exosomes are small vesicles secreted by cells to the outside, which contain signal transduction receptors, bioactive lipids, nucleic acids and proteins.28 Exosomes are involved in the occurrence and development of gliomas by regulating immune escape, proliferation, invasion and metastasis of gliomas. Domenis et al found that the exosome secreted by glioma stem cells can inhibit the proliferation and activation of T cells and the production of Th1 activating factors, resulting in immune escape.29 In addition, exosomes derived from glioma cells can secrete lncRNAs to promote the migration and invasion of glioma cells. Lang et al found lncRNA CCAT2 was enveloped into exosomes of glioma cells. Overexpression of CCAT2 could activate VEGFA and TGF β, promote angiogenesis and inhibit apoptosis.30 In addition, exo-POU3F3 from glioma cells accelerated angiogenesis and promoted glioma development.31 And in the present study, we found lncRNA ROR1-AS1 was up-regulated in glioma using microarray, and this result was confirmed by qRT-PCR assay, and high expression of ROR1-AS1 indicated a poor prognosis in glioma patients. These data suggested that ROR1-AS1 might be involved in glioma progression and act as an oncogene. A previous study has shown that lncRNA ROR1-AS1 was induced in colon cancer tissues and cell lines. Overexpression of ROR1-AS1 promoted cell proliferation and growth, and the underlying mechanism might be regulating the level of DUSP5/CDKN1A.32 And our data were similar to previous researches. Next, considering the importance of exosomes in cancer progression, we further explored the source of ROR1-AS1. Interestingly, ROR1-AS1 was packaged into exosomes and derived from tumor cells. And functional analysis showed exo-ROR1-AS1 secreted by tumor cells promoted stemness of glioma cell lines SHG44 and U251.
Accumulating evidence has clarified that lncRNAs acted as miRNA sponge, and inhibited miRNA expression, thereby modulating the gene transcription through transcription factors or modification enzymes complex.33 Silencing of lncRNA NEAT1 suppressed the migration of glioma cells. NEAT1 could have bound with miR-132, and directly inhibited miR-132 expression.34 And in the present study, we found that ROR1-AS1 acted as a sponge of miR-4686 and inhibited its expression. Functionally, forced expression of miR-4686 removed the promoted effects of lncRNA ROR1-AS1 on glioma development. In vivo tumorigenesis experiments showed that exo-ROR1-AS1 promoted glioma development via miR-4686 axis. In conclusion, this study suggested tumor cells derived exo-ROR1-AS1 promoted glioma progression by inhibiting miR-4686, which might be a potential therapeutic target for glioma clinical treatment.
Glioma is the most common intracranial malignant tumor with high morbidity and mortality, and it is easy to relapse after the operation. The exosome contains genetic information related to secretory cells, which is involved in the occurrence and development of glioma. It can be isolated from blood, urine, cerebrospinal fluid and other body fluids, which is one of the hotspots in the research of molecular biomarkers of glioma, but there are still many problems to be further explored. How does exosome transmit information between cells? Can we find its specific target to prevent it from promoting the development of glioma? The most important thing is that it is not clear which kind of exosome is the most valuable for the early diagnosis of glioma, and the value of dynamic monitoring of exosome in the prognosis of glioma patients is worthy of further study.
Conclusion
Our study suggested tumor cells derived exo-ROR1-AS1 promoted glioma progression by inhibiting miR-4686, which might be a potential therapeutic target for glioma clinical treatment.
Acknowledgement
This study was supported by the Science and Technology Support Project of Zunyi (Zunyi science and technology contract (2019) No. 79), (Zunyi science and technology contract (2019) No. 100), (Zunyi science and technology contract (2019) No. 102), (Zunyi science and technology contract (2020) No. 240), (Zunyi science and technology contract (2020) No. 241); Science and Technology Fund Project of Guizhou Provincial Health Commission in 2020: gzwjkj2020-1-107.
Disclosure
The authors declare that they have no competing interests.
References
1. Sabri F, Chiodi F, Piret J, et al. Soluble factors released by virus specific activated cytotoxic T-lymphocytes induce apoptotic death of astroglioma cell lines. Brain Pathology. 2003;13(2):165–175.
2. André-Grégoire G, Bidère N, Gavard J. Temozolomide affects Extracellular Vesicles Released by Glioblastoma Cells. Biochimie. 2018;155:11–15. doi:10.1016/j.biochi.2018.02.007
3. Friedman H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6(7):2585–2597.
4. Roth P, Weller M. Challenges to targeting epidermal growth factor receptor in glioblastoma: escape mechanisms and combinatorial treatment strategies. Neuro-Oncology. 2014;viii14–19.
5. Li S, Zhang Y, Ho S. Combination of tumour-infarction therapy and chemotherapy via the co-delivery of doxorubicin and thrombin encapsulated in tumour-targeted nanoparticles. Nat Biomed Eng. 2020.
6. Tang M. Three-dimensional bioprinted glioblastoma microenvironments model cellular dependencies and immune interactions. Cell Res. 2020.
7. Cheng J, Meng J, Zhu L, Peng Y. Exosomal noncoding RNAs in Glioma: biological functions and potential clinical applications. Mol Cancer. 2020;19(1):66. doi:10.1186/s12943-020-01189-3
8. Qian M, Wang S, Guo X. Hypoxic glioma-derived exosomes deliver microRNA-1246 to induce M2 macrophage polarization by targeting TERF2IP via the STAT3 and NF-κB pathways. Oncogene. 2020;39(2):428–442. doi:10.1038/s41388-019-0996-y
9. Ma C, Chen H, Zhang S. Exosomal and extracellular HMGB1 have opposite effects on SASH1 expression in rat astrocytes and glioma C6 cells. Biochem Biophys Res Commun. 2019;518(2):325–330. doi:10.1016/j.bbrc.2019.08.057
10. Shao H, Chung J, Lee K. Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat Commun. 2015;6(1):6999. doi:10.1038/ncomms7999
11. Garnier D, Meehan B, Kislinger T. Divergent evolution of temozolomide resistance in glioblastoma stem cells is reflected in extracellular vesicles and coupled with radiosensitization. Neuro-Oncology. 2018;20(2):236–248. doi:10.1093/neuonc/nox142
12. Nie L, Wu HJ, Hsu JM. Long non-coding RNAs: versatile master regulators of gene expression and crucial players in cancer. Am J Transl Res. 2012;4(2):127–150.
13. Hu Q. Long noncoding RNA loss in immune suppression in cancer. Pharmacol Ther. 2020;1075915.
14. Yuan L, Xu Z-Y, Ruan S-M, Mo S, Qin -J-J, Cheng X-D. Long non-coding RNAs towards precision medicine in gastric cancer: early diagnosis, treatment, and drug resistance. Mol Cancer. 2020;19(1):96. doi:10.1186/s12943-020-01219-0
15. Wang W, Han Y, Jo HA, Lee J, Song YS. Non-coding RNAs shuttled via exosomes reshape the hypoxic tumor microenvironment. J Hematol Oncol. 2020;13(1):67. doi:10.1186/s13045-020-00893-3
16. Wu J, Huang H, Huang W. Analysis of exosomal lncRNA, miRNA and mRNA expression profiles and ceRNA network construction in endometriosis. Epigenomics. 2020.
17. Guo X. Circulating Exosomal Gastric Cancer-Associated Long Noncoding RNA1 as a Biomarker for Early Detection and Monitoring Progression of Gastric Cancer: A Multiphase Study. JAMA Surg. 2020.
18. Ma X, Li Z, Li T. Long non-coding RNA HOTAIR enhances angiogenesis by induction of VEGFA expression in glioma cells and transmission to endothelial cells via glioma cell derived-extracellular vesicles. Am J Transl Res. 2017;9(11):5012–5021.
19. Hu G, Gupta SK, Troska TP, Nair A, Gupta M. Long non-coding RNA profile in mantle cell lymphoma identifies a functional lncRNA ROR1-AS1 associated with EZH2/PRC2 complex. Oncotarget. 2017;8(46):80223–80234. doi:10.18632/oncotarget.17956
20. Chen Q, Fu L. Upregulation of long non-coding RNA ROR1-AS1 promotes cell growth and migration in bladder cancer by regulation of miR-504. PLoS One. 2020;15(1):e0227568. doi:10.1371/journal.pone.0227568
21. Wu J. Long noncoding RNA ROR1-AS1 induces tumor metastasis and epithelial-mesenchymal transition by sponging miR-375 in nasopharyngeal carcinoma. Eur Rev Med Pharmacol Sci. 2020;24(1):174–180.
22. Zhang Z, Yin J, Lu C, Wei Y, Zeng A, You Y. Exosomal transfer of long non-coding RNA SBF2-AS1 enhances chemoresistance to temozolomide in glioblastoma. J Exp Clin Cancer Res. 2019;38(1):166. doi:10.1186/s13046-019-1139-6
23. Wang B, Xu L, Zhang J. LncRNA NORAD accelerates the progression and doxorubicin resistance of neuroblastoma through up-regulating HDAC8 via sponging miR-144-3p. Biomed Pharmacother. 2020;129:110268. doi:10.1016/j.biopha.2020.110268
24. Piermartiri TC. Guanosine Promotes Proliferation in Neural Stem Cells from Hippocampus and Neurogenesis in Adult Mice. Mol Neurobiol. 2020.
25. Ma L. Sensitive detection and conjoint analysis of promoter methylation by conjugated polymers for differential diagnosis and prognosis of glioma. ACS Appl Mater Interfaces. 2020.
26. Liu G. The miR-92a-2-5p in exosomes from macrophages increases liver cancer cells invasion via altering the AR/PHLPP/p-AKT/β-catenin signaling. Cell Death Differ. 2020.
27. Li J. Hypoxic Cancer-secreted Exosomal miR-182-5p Promotes Glioblastoma Angiogenesis by Targeting Kruppel-like Factor 2 and 4. Mol Cancer Res. 2020.
28. Pegtel DM, Cosmopoulos K, Thorley-Lawson DA. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107(14):6328–6333. doi:10.1073/pnas.0914843107
29. Domenis R, Cesselli D, Toffoletto B. Systemic T Cells Immunosuppression of Glioma Stem Cell-Derived Exosomes Is Mediated by Monocytic Myeloid-Derived Suppressor Cells. PLoS One. 2017;12(1):e0169932. doi:10.1371/journal.pone.0169932
30. Lang H-L, Hu G-W, Zhang B. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncol Rep. 2017;38(2):785–798. doi:10.3892/or.2017.5742
31. Lang HL Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3. Eur Rev Med Pharmacol Sci. 2017;21(5):959–972.
32. Wang XY. LncRNA ROR1-AS1 promotes colon cancer cell proliferation by suppressing the expression of DUSP5/CDKN1A. Eur Rev Med Pharmacol Sci. 2020;24(3):1116–1125.
33. Xu M, Chen X, Lin K. The long noncoding RNA SNHG1 regulates colorectal cancer cell growth through interactions with EZH2 and miR-154-5p. Mol Cancer. 2018;17(1):141. doi:10.1186/s12943-018-0894-x
34. Zhou K, Zhang C, Yao H. Knockdown of long non-coding RNA NEAT1 inhibits glioma cell migration and invasion via modulation of SOX2 targeted by miR-132. Mol Cancer. 2018;17(1):105. doi:10.1186/s12943-018-0849-2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.