Back to Journals » Orthopedic Research and Reviews » Volume 15
Exogenous Crosslinking of Tendons as a Strategy for Mechanical Augmentation and Repair: A Narrative Review
Authors Fofiu A , Tripon RG , Băţagă T, Chirilă TV
Received 12 May 2023
Accepted for publication 9 August 2023
Published 21 August 2023 Volume 2023:15 Pages 165—173
DOI https://doi.org/10.2147/ORR.S421106
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Clark Hung
Alexandru Fofiu,1,2 Robert G Tripon,3 Tiberiu Băţagă,4 Traian V Chirilă2,5– 8
1Department of Orthopedics-Traumatology, Emergency County Hospital Bistriţa, Bistriţa Năsăud, Romania; 2School of Medicine, George Emil Palade University of Medicine, Pharmacy, Science, and Technology, Târgu Mureş, Romania; 3Department of Ophthalmology, George Emil Palade University of Medicine, Pharmacy, Science, and Technology, Târgu Mureş, Romania; 4Department of Orthopedics-Traumatology, George Emil Palade University of Medicine, Pharmacy, Science, and Technology, Târgu Mureş, Romania; 5Department of Research, Queensland Eye Institute, South Brisbane, QLD, Australia; 6School of Chemistry and Physics, Queensland University of Technology, Brisbane, QLD, Australia; 7Australian Institute of Bioengineering and Nanotechnology, University of Queensland, St Lucia, QLD, Australia; 8School of Molecular Science, University of Western Australia, Crawley, WA, Australia
Correspondence: Traian V Chirilă, Email [email protected]
Abstract: Collagens constitute a family of triple-helical proteins with a high level of structural polymorphism and a broad diversity of structural and chemical characteristics. Collagens are designed to form supporting aggregates in the extracellular spaces of our body, but they can be isolated from animal sources and processed to become available as biomaterials with wide applications in biomedicine and bioengineering. Collagens can be conveniently modified chemically, and their propensity for participating in crosslinking reactions is an important feature. While the crosslinking promoted by a variety of agents provides a range of collagen-based products, there has been minor interest for therapies based on the crosslinking of collagen while located within living connective tissues, known as exogenous crosslinking. Currently, there is only one such treatment in ocular therapeutics (for keratoconus), and another two in development, all based on mechanical augmentation of tissues due to ultraviolet (UV)-induced crosslinking. As seen in this review, there was some interest to employ exogenous crosslinking in order to reinforce mechanically the lax tendons with an aim to arrest tear propagation, stabilize the tissue, and facilitate the healing. Here we reviewed in details both the early stages and the actual status of the experimental research dedicated to the topic. Many results have not been encouraging, however there is sufficient evidence that tendons can be mechanically reinforced by chemical or photochemical exogenous crosslinking. We also compare the exogenous crosslinking using chemical agents, which was predominant in the literature reviewed, to that promoted by UV radiation, which was rather neglected but might have some advantages.
Keywords: collagen, crosslinking, tendon, chemical agents, ultraviolet radiation, mechanical properties
Introduction
Natural collagen, as a non-living material from human or animal sources, is a biomaterial prominently employed in tissue engineering, regenerative medicine, dentistry, and pharmaceutical applications. The formation of covalent crosslinks between structural moieties at various hierarchical levels in collagen induces additional molecular stability leading to augmentation of mechanical properties of the collagen-based materials. As discussed in some recent reviews,1–4 chemical or physical crosslinking techniques have become a common tool for reinforcing mechanically the engineered collagen scaffolds, implants, or devices and enhancing their resistance to enzymatic degradation.
The crosslinking of native collagen while located in the body is a completely different issue, which purports two types of processes, endogenous and exogenous. The endogenous natural enzyme-driven crosslinking of living collagen is fundamental to the maturation and healing processes in our organism, and to the development and function of connective tissues. Lysyl oxidase (LOX) is the enzyme responsible for this fundamental process that is basically outside our control. The endogenous nonenzymatic crosslinking between adjacent collagen fibrils stabilizes the network and is believed to be mediated by macromolecules such as proteoglycans and fibronectin. Another endogenous nonenzymatic crosslinking is due to aging, and is driven by a process triggered by the advanced glycation end-products (known as AGEs). It induces brittleness, less resistance to damage, and impaired matrix remodelling of the connective tissues, and is therefore considered deleterious, despite leading to enhanced stiffness of tissular collagen.
The exogenous crosslinking of native collagen has likely been inspired from the crosslinking of engineered collagen-based materials, and is becoming a potentially therapeutic strategy for treating disorders of the connective tissue. It implies the direct exposure at specific anatomic locations of the living collagenous tissues to suitable chemical, photochemical, enzymatic, or thermal processes, resulting in structural modifications of the collagen macromolecules, which are accompanied by the expected or intended changes in properties. In other words, the crosslinks are introduced on purpose in order to modulate favorably the mechanical and physiological properties of the native tissue. However, it is essential to identify and overcome the biological and technical challenges to the translation of crosslinking methodology into a practicable clinical treatment.
To date, a successful application of an exogenous crosslinking process has been the photochemical crosslinking of the corneal collagen in the eye,5–7 which is currently the routine treatment for keratoconus, an ophthalmic condition that can lead to severe visual disability if left untreated. By irradiating the cornea with ultraviolet (UV)-A rays (wavelengths 315–400 nm, photon energies ~3 to ~ 4 eV), in the presence of a photoinitiator, the progression of keratoconus is arrested due to the stiffening of corneal collagen induced by a radiation-induced crosslinking reaction. We have proposed to extend the method to the treatment of eyelid laxity (eg, floppy eyelid syndrome), and have shown8,9 in ex-vivo animal (ovine) tissue that the UV-A-induced crosslinking of tarsal collagen led to increased mechanical strength and stiffening of the tissue. We also demonstrated that the mechanical properties of ex-vivo porcine aortic peripheral collagen (residing in tunica adventitia of the vessel) were significantly enhanced following irradiation with UV-A rays, and proposed the process as a potential method for preventing the rupture of abdominal aortic aneurysms.10 In a subsequent study,11 the aortic specimens were subjected to in-vitro collagenolysis and then subjected to mechanical evaluation, with or without irradiation. We found not only that crosslinking would be beneficial to the degenerated wall, but also that the earlier the irradiation the more resistant to rupture will be the wall.
In this overview, we analyzed the literature published on the use of exogenous collagen crosslinking in tendons. We expound on the ability of crosslinking to arrest propagation of partial tears by augmenting mechanical properties, to impart stabilization to the tissue, and thus to contribute to the healing of injured or lax tendons. The crosslinking technologies applied for the fabrication of collagen-based tissue-engineered scaffolds and devices to repair torn tendons or ligaments are not a topic of the present review.
Chemical Crosslinking of Tendons
Some decades ago, Haut12 reported that the quantitative reduction of natural crosslinks in the collagen of experimental animals subjected to a lathyritic diet was associated with the decrease of mechanical strength and elastic modulus of the rat-tail tendons. Dietary lathyrogens (eg, β-aminopropionitrile) are known to inhibit the activity of LOX,13,14 such as impairing the covalent crosslinking, but not the biosynthesis of collagen, in the body. Notwithstanding this relevant finding, the idea to crosslink the tendinous tissue in order to make it stronger took another two decades to emerge.
The first study attempting to establish the importance of exogenous collagen crosslinking related to tendons was published by Zhao et al15 at the Mayo Clinic in Rochester, MN. Considering the problems associated with the suture–tendon interface (recurrent tissue rupture, suture rupture, or suture failure due to low pullout resistance), the investigators suggested the injection of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC, see structure in Figure 1) as a crosslinking agent into the interface of 24 ex-vivo canine flexor digitorum tendons. A tendon specimen that was pliable became a rigid rod after 1 h in 10% aqueous EDC. Mechanical evaluation showed an increase in ultimate strength but no significant change in stiffness. All samples failed the suture pullout tests, which did not hamper the investigators’ optimism. In experiments reported later by the same group,16 the sutures were impregnated with EDC, such avoiding injection but using the same tendon model. However, the results were similar to those reported previously.15 All specimens failed the suture cutting tests. The assessment of cell viability indicated a favorable effect on the numbers of viable cells (cultured tenocytes). It is not clear whether EDC was first adsorbed onto the suture and after that released into the tendon’s interfacial region. As the chemical nature of the sutures was not disclosed, it is difficult to ascertain which of the physisorption or chemisorption processes governed formation of a coating.
 |
Figure 1 Chemical structures of agents commonly used for crosslinking the collagenous tissues. |
Another pioneering work was carried out by Magnusson’s group,17 then at the Institute of Sports Medicine in Copenhagen, who investigated the mechanical effects induced by the crosslinking of rat-tail tendon with glutaraldehyde (GA, Figure 1) at two structural levels of the tendon hierarchy, the fascicles and the fibrils. The reaction was conducted at 4°C in diluted aqueous medium for 24 h. Mechanical evaluation was performed using atomic force microscopy (AFM) in three different procedures for tendon fibrils, and uniaxial tensile test for fascicles. In fibrils, the crosslinking induced a substantial increase of mechanical characteristics, especially of Young’s modulus (YM), an indirect measure of stiffness. Significant increases in YM, yield stress, and yield strain were also measured for the tendon fascicles. The authors concluded that crosslinking is important in providing additional strength to tendons. They also noted that fibril strength induced by crosslinking appears to surpass that induced in fascicles, which is important considering that the fibril is the primary load-bearing unit of a tendon, although the fascicles are hierarchically the largest.
To our knowledge, the next relevant body of work on tendon crosslinking has been carried out at ETH Zürich, in Snedeker’s Orthopedic Biomechanics Laboratory.18–23 The investigators’ aim was to modulate in situ collagen crosslinks in order to improve tendon healing by arresting progression of tearing. More precisely, they envisaged that the mechanical enhancement of tendons by exogenous collagen crosslinking may have such results as:18 (a) an improvement of suture retention; (b) reinforcing shoulder joint stability by stabilization of capsular ligaments; (c) to arrest progression of partial rotator cuff tendon tears; or (d) to reinforce the marginal regions of partial tears, such restoring a favorable strain distribution and rendering smaller tears manageable surgically. In their first experiments, genipin (GP, Figure 1) was used as a crosslinking agent, which is a non-toxic natural substance isolated from plants of genera Genipa and Gardenia. Samples of both rat-tail tendon and equine digital flexor tendon that were treated with GP showed an increase in their YM (stiffness), maximal tensile stress, and toughness. Genipin treatment was also applied in an in vitro model (equine tendon) of partial tendon tear/injury, when specimens of intact tendon, partially torn tendon, and GP-crosslinked tendon (3-day incubation) were compared. The crosslinked tendon recovered much of the mechanical integrity lost in the torn specimens. In a subsequent study,19 the effects of two different chemical crosslinkers, GP and methylglyoxal (MG, Figure 1), were compared in rat-tail tendon fascicles (RTTF) and equine superficial digital flexor tendon (ESDFT) specimens by evaluation in a uniaxial mechanical tester. Beside direct measurements, two in-vitro models were also employed for evaluation (ESDFT load-bearing model, and tear propagation under cyclic loading to failure model). It was found that GP enhanced the strength and stiffness in both tendons, while MG detrimentally affected the ESDFT, which was tentatively attributed to a certain hierarchical order of crosslink formation. Some other parameters (toughness, yield force, hysteresis, and cyclic relaxation) displayed conflicting values. In the investigators’ conclusion, GP showed the most potential for arresting tear progression and improving mechanical performance of treated tendons.
To further promote GP as a suitable chemical agent for exogenous crosslinking of the tendinous tissue, the ETH group performed extensive quantitative evaluation of putative associated cytotoxicity.20 Based on cell viability, metabolic activity and motility assays, gene expression analysis, differential scanning calorimetry, and uniaxial mechanical testing, they have concluded that the mechanical augmentation through GP-induced crosslinking can be implemented at the compromise of allowing some level of cytotoxicity, a situation that can be improved post-crosslinking by cell survival techniques. GP was further evaluated in the fresh ovine shoulder infraspinatus tendon, an established ex-vivo model for rotator cuff tendon tears.21 The objective of the study was to see whether crosslinking can improve the suture pullout strength in the case of either simple single-loop sutures or modified Mason-Allen suture bridge technique stitch patterns. A total of 32 tendons were used and 142 suture pullout tests were carried out for both suture techniques. Two sets of tendons were incubated in a buffer containing 20 mmol/L GP at 36°C, for 4 h or 24 h, and following the treatment all specimens (including untreated controls) were evaluated uniaxially in a mechanical tester. For single-loop sutures, only the 24-h treatment with GP led to an increase of the maximum pullout force (73 N vs 56 N), while the shorter treatment did not have any effect. For the Mason-Allen stitch patterns, crosslinking did not show any benefit. It was concluded that, in the case of single-loop sutures, the exogenous crosslinking with GP presents clinical relevance at the suture–tendon interface by improving the resistance to pullout, which may help with the repair of rotator cuff tears. Publication of this study attracted both interest and some criticism.22 Thus, the study did not discuss why the stitching configuration has an effect on the crosslinking outcome. It neither provided an indication on the clinical utility, safety and efficacy, or level of healing. Perhaps the most useful suggestion22 was to implement a delivery system for GP at the crosslinking site.
The relevance of suture–tendon interface was further investigated in a more recent study from the ETH group.23 Twenty-five ex-vivo bovine superficial digital flexor tendons were processed into the following sets: (a) healthy tendons stitched with polyethylene sutures (single loop); (b) healthy tendons stitched with GP-coated sutures; (c) specimens degraded by in-vitro collagenolysis and stitched with normal sutures; and (d) degraded tendons stitched with GP-coated sutures. Mechanical evaluation was performed 24 h after suturing. The coating with GP improved with statistical significance the suture pullout parameters, both force to failure (N) and work to failure (mJ), in the healthy tendons, while in those degraded it was only the force to failure that improved. Clearly the presence of genipin in the coating enhanced regionally the crosslinking of surrounding tendinous tissue where the sutures were confined. As acknowledged by ETH investigators, the coating used for sutures in this study was actually developed24 by Hedman et al at the University of Kentucky, as a sustained release system for the crosslinking agent. It consists of a complex mixture of GP, poly(lactic-co-glycolic acid), and poly(ethylene glycol) dissolved in acetone and dimethyl sulfoxide. It was shown that the resulting layer is a diffusion type system for the controlled release of an active agent (GP) from a biodegradable polymer matrix, and this was checked both in vitro and ex vivo (equine tendons).24 The tendons coming in contact with the GP that resides on, or is released from the sutures, displayed enhanced ultimate tensile stress, load to failure, and stiffness. In a later study,25 the group investigated the intratendinous injection of GP in ex-vivo bovine superficial digital flexor tendons, either healthy or degenerated (by in-vitro collagenolysis). The injectable solution consisted of 80 mmol/L GP in a solution of buffer and dimethyl sulfoxide. The specimens were cyclically loaded for 500 cycles and then loaded to failure. While no significant benefit of injection was noticed in the healthy tendons, the degraded specimens showed significant increases in ultimate force, ultimate stress, elastic modulus, work to failure, and strain to failure. It was concluded that a degenerated tendon can be mechanically recovered by injection with GP and the procedure was suggested to treat tendinopathies. In their most recent work,26 the ETH team investigated the benefit of GP-coated sutures in human tendons. Human biceps long head tendons were harvested at operation from 25 patients. Polyethylene sutures, either normal or GP-coated, were inserted in two sets of tendons. Mechanical testing showed that the coated sutures were associated with higher forces to failure, but the other properties were not affected. Cell viability was a function of the distance from the suture, with cytotoxicity manifested up to about 3 mm to suture, and diminishing farther to normality.
Benefits of crosslinking tendons with GP were also contemplated for a different reason. Maher’s group at the Hospital for Special Surgery in New York have investigated the protective effects against damage to tendon grafts caused by gamma-ray sterilization.27 The use of donor tendon allografts to repair the rupture of anterior cruciate ligament requires their exposure to high doses of gamma radiation resulting in damage to the graft material. As the damage is mainly mechanical (about 50% loss of properties) the enhancement of mechanical properties will offer an indirect protection. In the study,27 bovine and human patella tendons were incubated with GP for durations up to 12 h. While YM increased 2.4-fold in the bovine tendons, there was no significant effect of crosslinking in the human tendons. The increase in elastic moduli in the gamma-irradiated tendons were statistically insignificant both in animal and human specimens. It was surmised that the benefits of GP in human allografts may depend on specific aging aspects.
Currently, the exogenous crosslinking of connective tissues using chemical crosslinking agents is regarded as a promising method to treat injured tissues, tendons among them, and GP as a preferred agent thanks mainly to its low cytotoxicity and effective performance. Very recently, a system for the sustained local delivery of GP has been developed28 by von Recum et al at Case Western Reserve University. They proposed cyclodextrins as carriers, and compared them with non-cyclic dextran. The cyclodextrins are molecules that possess cavities, and it was expected that the relatively small molecule of GP may display molecular affinity for certain cavity sizes. The investigators demonstrated the sustained release of GP from β-cyclodextrin, and its active participation to the crosslinking reaction in the rat-tail tendons. Mechanical testing showed statistically significant enhancements of certain ultimate properties (strength, energy, and strain to failure) following incubation with GP-cyclodextrin composites. The tendons treated with GP-dextrin (no cavities) showed less mechanical augmentation, but still superior to the controls (empty β-cyclodextrin cavities, no GP). It was also found that GP improved resistance to collagenase. A cytotoxicity assay indicated that the protective effects must be balanced with a certain level of acceptable cytotoxicity.
Results of Chemical Crosslinking
Table 1 contains a synopsis of the results published so far on the exogenous crosslinking of tendons using chemical crosslinking agents. Only data supported by statistical significance are included, and only those results are presented that showed actual mechanical augmentation of the tendinous tissue subjected to crosslinking. For uniformity, the results were re-calculated into percentage enhancement of the respective mechanical property.
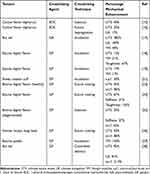 |
Table 1 Exogenous Crosslinking of Tendons Using Chemical Agents: a Selective Summary of Published Results |
Photochemical Crosslinking of Tendons
An ideal and safe procedure to achieve exogenous crosslinking in living connective tissues shall avoid the use of chemicals. Even GP itself displays a certain level of toxicity, and a compromise has to be usually secured. As shown in our introduction, the UV-A radiation proved to be effective and safe in treating corneal disorders and in other medical procedures currently at various investigative stages of development. UV-A radiation can be delivered conveniently and the only chemicals involved are innocuous photoinitiators such as vitamin B2 (riboflavin). The generation of crosslinks by photochemistry is mediated by free radicals, which are sufficiently reactive as to directly involve the amino residues of collagen chains, and therefore no extraneous chemical structures are incorporated in the crosslinkages.
Interestingly, there are recent reports on the reinforcement of musculoskeletal tissues different from tendons using photochemically induced exogenous crosslinking. Vasilikos et al at Ulm University in Germany have investigated the photochemical crosslinking of intervertebral disc (IVD) employing the system UV-A radiation/riboflavin, at an irradiance of 3 mW/cm2. They have shown29 that the photocrosslinking of IVD led to reduced annular delamination, increase of the peel strength, and a significant increase of Young’s modulus. In another study,30 the subject was the shoulder’s glenohumeral joint capsule; its laxity is one of the main lesions leading to shoulder instability. Human capsular specimens (~1 mm3 each) were harvested during shoulder surgery, and exposed to UV-A (365 nm) for 30 minutes after incubation in riboflavin. There was a clear enhancement of the Young’s modulus. Due to very small sample size, the authors had to use AFM in order to measure the moduli. UV-A irradiation increased capsule stiffness without affecting structural organization or inducing cell death.
The use of UV regions different from UV-A have been reported for crosslinking a tendon, however. For reasons not clearly explained, collagen isolated from the rat-tail tendon was exposed to UV-C radiation (wavelength 254 nm, energy 4.9 eV) at an irradiance of 4.4 mW/cm2 for very long durations (2–8 hours).31 Such radiation is associated with a photon energy higher than that of UV-A (365 nm, energy 3.4 eV), and is known to be harmful to biological matter, especially over a lengthy exposure. We suppose that the study was related to the sterilization with UV-C of collagen-based implant materials. As expected, the effects of irradiation on mechanical properties were detrimental, as the ultimate strength, ultimate strain, and YM all decreased. Considering the hazard of irradiating living tissue with UV-C rays, such studies are irrelevant for our review, even if some have episodically reported mechanical augmentation.32
In an earlier study,33 rat-tail tendon specimens were irradiated with either visible light (> 370 nm) or UV (> 295 nm) regions in the presence of methylene blue as a photosensitizer. Chemical analysis of the products suggested that collagen crosslinking processes took place. Diffraction studies showed that the photo-induced crosslinking did not affect the molecular packing arrangement of the native collagen. However, this study did not investigate the effect on the mechanical properties.
To date, Snedeker’s group have published19 the only report on investigating the exogenous crosslinking of tendinous collagen by exposure to UV-A radiation aiming at a medical application, in a study where two chemical crosslinking agents (MG and GP) were also included for comparison, as aforementioned. Strips of either RTTF or ESDFT have been soaked in diluted aqueous riboflavin for 5 minutes and then each exposed to UV-A radiation (wavelength 375 nm) at an irradiance of 4.4 mW/cm2 for 30 minutes. The radiation was generated by a UV diode source and delivered at a distance of 2.5 cm from the samples. While the irradiated rat-tail tendon specimens showed enhanced mechanical properties (yield/ultimate strength and strain, stiffness, and toughness), similar to or even higher than those induced by GP, the UV irradiation did not have any effect of the properties of the equine tendon strips. GP was the only agent that induced mechanical enhancement in both kinds of tendons. It is probable that for irradiation of the equine tendon a higher irradiance should have been employed.
Discussion
Tendons can become lax (loose) in two situations: over time due to repetitive or excessive force (associated with overuse, or with poor biomechanics), or suddenly due to trauma. Part of their elasticity is lost when stretched beyond their normal capacity, therefore the lax tendons do not recoil to the initial length after the application of force is stopped. Perhaps a more suitable term for a lax tendon would be stretched or elongated. Together with ligamentous laxity, the tendinous laxity contributes to instability in the joints, which increases the risk for permanent functional impairment.
It is disputable whether the artificial exogenous crosslinking could be effective in regenerating an overstretched and permanently elongated tendon, while not yet ruptured. In principle, the mechanical stabilization of the tissue can be achieved by crosslinking, which may arrest the ability for further stretching. The crosslinking cannot achieve, however, the recovery of the initial contractility level, ie, the shortening back to the initial length. This can happen only in two situations: (a) through the actin-mediated activity of the tenocytes that can generate traction forces against an extracellular matrix able to contract the lax tendons; and (b) if the collagen crosslinking reaction as such has a contractile effect (shrinkage) on the collagen. The former issue (a) is related with the tendon laxity resulting from loss of collagen tension and disrupting cytoskeletal tensional homeostasis in tenocytes, and also inducing catabolic changes such as upregulation of collagenase expression and apoptosis. However, an actin-based cell contraction mechanism can re-establish the tensional homeostasis in the lax tendons. This mechanism has been extensively studied by Arnoczky’s group at Michigan State University.34–36 However, we do not know whether it will be active in a tendon previously subjected to the process of chemical or photochemical exogenous crosslinking, and therefore affected by certain structural modifications.
When considering the latter issue (b), we are not aware of any reported evidence that the crosslinking of collagen is associated with the shrinkage of its matrix, which would enable a contractile effect on stretched connective tissues. Otherwise, it is well known that the exposure of collagen in general, and tendinous collagen in particular, to temperatures exceeding ~60°C, leads to its shrinkage,37–41 a process that has actually been suggested as a therapy for glenohumeral instability, based on experiments with human joint capsule or bovine extensor tendons at temperatures over 60°C.42,43 On another note, the hyperthermic treatment for tendinopathies, which is carried out at temperatures around 42°C, results in enhanced contraction rates in the lax tendons, but seems to be based on a shrinking mechanism not yet elucidated.44,45 Such thermal shrinking processes are physical in nature and are not related to chemical reactions leading to crosslinking.
Looking at existing and proposed methods of treatment for tendinopathies, and at the repair strategies based on bioengineering (eg, biomaterials, tissue engineering, and cell-based therapies), as presented in a number of seminal reviews,46–51 one can see that a methodology based on collagen crosslinking has never been mentioned as a viable alternative. This clearly implies that there is a need for more experimental work to be done for raising the legitimate interest of orthopedic specialists.
Conclusion
There is not much interest currently in developing therapies for tendinopathies based on the exogenous crosslinking of tendon collagen, although topical reports emerge episodically from some research centers. Certain reports have provided evidence for the mechanical augmentation of crosslinked tendons, but in many cases the results may be seen as tenuous. Further research work is obviously needed in order to develop such therapies. With regards to safety, it is worth considering the replacement of chemical crosslinking with UV radiation-induced crosslinking.
Abbreviations
AGE, advanced glycation end-product; ATM, atomic force microscopy; EDC, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride; GA, glutaraldehyde; GP, genipin; IVD, intervertebral disc; LOX, lysyl oxidase; MG, methylglyoxal; RTT, rat-tail tendon; SDFT, superficial digital flexor tendon; UE, ultimate elongation (strain); UTS, ultimate tensile stress; UV, ultraviolet; YM, Young’s modulus.
Funding
This project did not receive any specific grant from funding agencies in the public or commercial sector.
Disclosure
The authors declare no potential conflicts of interest or any financial interests that are relevant to the content, authorship, or publication of this article.
References
1. Parenteau-Bareil R, Gauvin R, Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials. 2010;3(3):1863–1887. doi:10.3390/ma3031863
2. Gu L, Shan T, Ma Y, Tay FR, Niu L. Novel biomedical applications of crosslinked collagen. Trends Biotechnol. 2019;37(5):464–491. doi:10.1016/j.tibtech.2018.10.007
3. Meyer M. Processing of collagen based biomaterials and the resulting materials properties. BioMed Eng OnLine. 2019;18(1):24.
4. Nair M, Best SM, Cameron RE. Crosslinking collagen constructs: achieving cellular selectivity through modifications of physical and chemical properties. Appl Sci. 2020;10(19):6911. doi:10.3390/app10196911
5. Sorkin N, Varssano D. Corneal collagen crosslinking: a systematic review. Ophthalmologica. 2014;332(1):10–27. doi:10.1159/000357979
6. Randleman JB, Khandelwal SS, Hafezi F. Corneal cross-linking. Surv Ophthalmol. 2015;60(6):509–523. doi:10.1016/j.survophthal.2015.04.002
7. Lim L, Lim EWL. A review of corneal collagen cross-linking – current trends in practice applications. Open Ophthalmol J. 2018;12(suppl. 1):181–213. doi:10.2174/1874364101812010181
8. Smith TM, Suzuki S, Cronin BG, et al. Photochemically induced crosslinking of tarsal collagen as a treatment for eyelid laxity: assessing potentiality in animal tissue. Ophthal Plast Reconstr Surg. 2018;34(5):477–482. doi:10.1097/IOP.0000000000001063
9. Smith TM, Suzuki S, Sabat N, Rayner CL, Harkin DG, Chirila TV. Further investigations on the crosslinking of tarsal collagen as a treatment for eyelid laxity: optimizing the procedure in animal tissue. Ophthal Plast Reconstr Surg. 2019;35(6):600–603. doi:10.1097/IOP.0000000000001413
10. Chirila TV, Suzuki S. Photocrosslinking of adventitial collagen in the porcine abdominal aorta: a preliminary approach to a strategy for prevention of aneurysmal rupture. Designs. 2022;6(1):5. doi:10.3390/designs6010005
11. Chirila TV, Suzuki S. Effects of ultraviolet-A radiation on enzymatically degraded tunica adventitia of the porcine abdominal aorta. Biomed Mater Dev. 2023. doi:10.1007/s44174-023-00080-1
12. Haut RC. The effect of a lathyritic diet on the sensitivity of tendon to strain rate. J Biomech Eng. 1985;107(2):166–174. doi:10.1115/1.3138537
13. Tanzer ML. Cross-linking of collagen. Science. 1973;180(4086):561–566. doi:10.1126/science.180.4086.561
14. Tanzer ML. Experimental lathyrism. Int Rev Connect Tissue Res. 1965;3:91–112.
15. Zhao C, Sun Y-L, Zobitz ME, An K-N, Amadio PC. Enhancing the strength of the tendon-suture interface using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride and cyanoacrylate. J Hand Surg Am. 2007;32(5):606–611. doi:10.1016/j.jhsa.2007.03.004
16. Thoreson AR, Hiwatari R, An K-N, Amadio PC, Zhao C. The effect of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide suture coating on tendon repair strength and cell viability in a canine model. J Hand Surg Am. 2015;40(10):1986–1991. doi:10.1016/j.jhsa.2015.06.117
17. Hansen P, Hassenkam T, Brüggebusch Svensson R, et al. Glutaraldehyde cross-linking of tendon—mechanical effects at the level of the tendon fascicle and fibril. Connect Tissue Res. 2009;50(4):211–222. doi:10.1080/03008200802610040
18. Fessel G, Gerber C, Snedeker JG. Potential of collagen cross-linking therapies to mediate tendon mechanical properties. J Shoulder Elbow Surg. 2012;21(2):209–217. doi:10.1016/j.jse.2011.10.002
19. Fessel G, Wernli J, Li Y, Gerber C, Snedeker JG. Exogenous collagen cross-linking recovers tendon functional integrity in an experimental model of partial tear. J Orthop Res. 2012;30(6):973–981. doi:10.1002/jor.22014
20. Fessel G, Cadby J, Wunderli S, van Weeren R, Snedeker JG. Dose- and time-dependent effects of genipin crosslinking on cell viability and tissue mechanics – toward clinical application for tendon repair. Acta Biomater. 2014;10(5):1897–1906. doi:10.1016/j.actbio.2013.12.048
21. Camenzind RS, Wieser K, Fessel G, Meyer DC, Snedeker JG. Tendon collagen crosslinking offers potential to improve suture pullout in rotator cuff repair: an ex vivo sheep study. Clin Orthop Relat Res. 2016;474(8):1778–1785. doi:10.1007/s11999-016-4838-8
22. Abboud JA. CORR Insights®: tendon collagen crosslinking offers potential to improve suture pullout in rotator cuff repair: an ex vivo sheep study. Clin Orthop Relat Res. 2016;474(8):1786–1787. doi:10.1007/s11999-016-4914-0
23. Camenzind RS, Tondelli TO, Götschi T, Holenstein C, Snedeker JG. Can genipin-coated sutures deliver a collagen crosslinking agent to improve suture pullout in degenerated tendon? An ex vivo animal study. Clin Orthop Relat Res. 2018;476(5):1104–1113. doi:10.1007/s11999.0000000000000247
24. Sundararaj S, Slusarewicz P, Brown M, Hedman T. Genipin crosslinker releasing sutures for improving the mechanical/repair strength of damaged connective tissue. J Biomed Mater Res Part B Appl Biomater. 2017;105B(8):2199–2205. doi:10.1002/jbm.b.33753
25. Tondelli T, Götschi T, Camenzind RS, Snedeker JG, Awad HA. Assessing the effects of intratendinous genipin injections: mechanical augmentation and spatial distribution in an ex vivo degenerative tendon model. PLoS One. 2020;15(4):e0231619. doi:10.1371/journal.pone.0231619
26. Götschi T, Scheibler AG, Jaeger P, et al. Improved suture pullout through genipin-coated sutures in human biceps tendons with spatially confined changes in cell viability. Clin Biomech. 2023;103:105907. doi:10.1016/j.clinbiomech.2023.105907
27. Ng KW, Wanivenhaus F, Chen T, et al. Differential cross-linking and radio-protective effects of genipin on mature bovine and human patella tendons. Cell Tissue Bank. 2013;14:21–32. doi:10.1007/s10561-012-9295-3
28. Rivera-Delgado E, Learn GD, Kizek DJ, Kashyap T, Lai EJ, von Recum HA. A polymeric delivery system enables controlled release of genipin for spatially-confined in situ crosslinking of injured connective tissues. J Pharm Sci. 2021;110(2):815–823. doi:10.1016/j.xphs.2020.09.044
29. Vasilikos I, Teixeira GQ, Seitz A, et al. Can UVA-light-activated riboflavin-induced collagen crosslinking be transferred from ophthalmology to spine surgery? A feasibility study on bovine intervertebral disc. PLoS One. 2021;16(6):e0252672. doi:10.1371/journal.pone.0252672
30. Cornette P, Jaabar IL, Dupres V, et al. Impact of collagen crosslinking on dislocated human shoulder capsule—Effect on structural and mechanical properties. Int J Mol Sci. 2022;23(4):2297.
31. Sionkowska A, Wess T. Mechanical properties of UV irradiated rat tail tendon (RTT) collagen. Int J Biol Macromol. 2004;34(1–2):9–12. doi:10.1016/j.ijbiomac.2003.10.001
32. Cornwell KG, Lei P, Andreadis ST, Pins GD. Crosslinking of discrete self-assembled collagen threads: effects on mechanical strength and cell-matrix interactions. J Biomed Mater Res. 2007;80A(2):362–371. doi:10.1002/jbm.a.30893
33. Ramshaw JAM, Stephens LJ, Tulloch PA. Methylene blue sensitized photo-oxidation of collagen fibrils. Biochim Biophys Acta Prot Struct Molec Enzymol. 1994;1206(2):225–230. doi:10.1016/0167-4838(94)90212-7
34. Gardner K, Lavagnino M, Egerbacher M, Arnoczky SP. Re-establishment of cytoskeletal tensional homeostasis in lax tendons occurs through an actin-mediated cellular contraction of the extracellular matrix. J Orthop Res. 2012;30(11):1695–1701. doi:10.1002/jor.22131
35. Lavagnino M, Gardner K, Arnoczky SP. Age-related changes in the cellular, mechanical, and contractile properties of rat tail tendons. Connect Tissue Res. 2013;54(1):70–75. doi:10.3109/03008207.2012.744973
36. Lavagnino M, Brooks AE, Oslapas AN, Gardner KL, Arnoczky SP. Crimp length decreases in lax tendons due to cytoskeletal tension, but is restored with tensional homeostasis. J Orthop Res. 2017;35(3):573–579. doi:10.1002/jor.23489
37. Weir CE. Rate of shrinkage of tendon collagen: heat, entropy, and free energy of activation of the shrinkage of untreated tendon; effect of acid, salt, pickle, and tannage on the activation of tendon collagen. J Res Natl Bur Stand. 1949;42(1):17–32. doi:10.6028/jres.042.002
38. Weir CE, Carter J. Rate of shrinkage of tendon collagen: further effects of tannage and liquid environment on the activation constants of shrinkage. J Res Natl Bur Stand. 1950;44(6):599–609. doi:10.6028/jres.044.054
39. Banga I, Baló J, Szabó D. Contraction and relaxation of collagen fibres. Nature. 1954;174(4434):788–789. doi:10.1038/174788a0
40. Wright NT, Humphrey JD. Denaturation of collagen via heating: an irreversible rate process. Annu Rev Biomed Eng. 2002;4(1):109–128. doi:10.1146/annurev.bioeng.4.101001.131546
41. Rossmann C, Garrett-Mayer E, Rattay F, Haemmerich D. Dynamics of tissue shrinkage during ablative temperature exposures. Physiol Meas. 2014;35(1):55–67. doi:10.1088/0967-3334/35/1/55
42. Hayashi K, Thabit G III, Massa KL, et al. The effect of thermal heating on the length and histologic properties of the glenohumeral joint capsule. Am J Sports Med. 1997;25(1):107–112. doi:10.1177/036354659702500121
43. Wall MS, Deng XH, Torzilli PA, Doty SB, O’Brien SJ, Warren RF. Thermal modification of collagen. J Shoulder Elbow Surg. 1999;8(4):339–344. doi:10.1016/S1058-2746(99)90157-X
44. Giombini A, Di Cesare A, Safran MR, Ciatti R, Maffuli N. Short-term effectiveness of hyperthermia for supraspinatus tendinopathy in athletes. A short-term randomized controlled study. Am J Sports Med. 2006;34(8):1247–1253. doi:10.1177/0363546506287827
45. Lavagnino M, Malek K, Gardner KL, Arnoczky SP. Thermal energy enhances cell-mediated contraction of lax rat tail tendon fascicles following exercise. Muscles Ligaments Tendons J. 2015;5(01):51–55. doi:10.32098/mltj.01.2015.11
46. James R, Kesturu G, Balian G, Chhabra B. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg. 2008;33A(1):102–112. doi:10.1016/j.jhsa.2007.09.007
47. Riley G. Tendinopathy—from basic science to treatment. Nature Clin Pract Rheumatol. 2008;4(2):82–89. doi:10.1038/ncprheum0700
48. Andres BM, Murrell GAC. Treatment of tendinopathy. What works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466(7):1539–1554. doi:10.1007/s11999-008-0260-1
49. Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14(1):47–71. doi:10.1146/annurev-bioeng-071811-150122
50. Alshomer F, Chaves C, Kalaskar DM. Advances in tendon and ligament tissue engineering: materials perspective. J Mater. 2018;2018:17.
51. Steinmann S, Pfeiffer CG, Brochhausen C, Docheva D. Spectrum of tendon pathologies: triggers, trails and end-state. Int J Mol Sci. 2020;21(3):844. doi:10.3390/ijms21030844
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
