Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Exercise Performance of Lowlanders with Chronic Obstructive Pulmonary Disease Acutely Exposed to 2048 m: A Randomized Cross-Over Trial
Authors Bitos K, Kuehne T, Latshang TD, Aeschbacher SS, Huber F, Flueck D, Hasler ED, Scheiwiller PM, Lichtblau M, Ulrich S , Bloch KE , Furian M
Received 8 December 2022
Accepted for publication 14 June 2023
Published 17 August 2023 Volume 2023:18 Pages 1753—1762
DOI https://doi.org/10.2147/COPD.S400816
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Konstantinos Bitos,1 Tobias Kuehne,1 Tsogyal D Latshang,1 Sayaka S Aeschbacher,1 Fabienne Huber,1 Deborah Flueck,1 Elisabeth D Hasler,1 Philipp M Scheiwiller,1 Mona Lichtblau,1 Silvia Ulrich,1 Konrad E Bloch,1 Michael Furian1,2
1University Hospital Zurich, Department of Respiratory Medicine, Zurich, Switzerland; 2Swiss University of Traditional Chinese Medicine, Research Department, Bad Zurzach, Switzerland
Correspondence: Michael Furian, University Hospital Zurich, Department of Pulmonology, Raemistrasse 100, Zurich, 8092, Switzerland, Email [email protected]
Background: Amongst the millions of travelers to high altitude worldwide are many with chronic obstructive pulmonary disease (COPD), but data regarding the effects of acute exposure to altitude on exercise performance are limited. The current study investigated how acute exposure to moderate altitude influences exercise performance in COPD patients, providing novel insights to the underlying physiological mechanisms.
Methods: Twenty-nine COPD patients, GOLD grade 2– 3, median (quartile) forced expiratory volume in 1 second (FEV1) of 60% predicted (46; 69) performed cycling incremental ramp exercise test (IET) at 490 m and after acute exposure of 2– 6 hours to 2048 m or vice versa, according to a randomized cross-over design. Exercise performance and breath-by-breath analyses of the last 30 seconds of each IET were compared between locations.
Results: At 2048 m compared to 490 m, the maximum power output (Wmax) was 77 watts (62;104) vs 88 watts (75;112), median reduction 5 watts (95% CI, 2 to 8, P< 0.05), corresponding to a median reduction of 6% (95% CI, 2 to 11, P< 0.05) compared to 490 m. The peak oxygen uptake (V’O2peak) was 70% predicted (56;86) at 2048 m vs 79% predicted (63;90) at 490 m, median reduction of 6% (95% CI, 3 to 9, P< 0.05). The oxygen saturation by pulse oximetry (SpO2) at 2048 m was reduced by 8% (95% CI, 4 to 9, P< 0.05) compared to 490 m. The minute ventilation (V’E) increased by 2.8L/min (95% CI, 0.9 to 4.2, P< 0.05) at 2048 m. The maximum heart rate and the subjective sense of dyspnea and leg fatigue did not change.
Conclusion: Lowlanders with moderate-to-severe COPD acutely exposed to 2048 m reveal small but significant reduction in cycling IET along with a reduced V’O2peak. As dyspnea perception and maximal heart rate were unchanged, the lower blood oxygenation and exaggerated ventilatory response were culprit factors for the reduced performance.
Keywords: COPD, exercise, high altitude, hypoxia, hypoxemia, cardiopulmonary exercise testing
Introduction
Chronic obstructive pulmonary disease (COPD) is a highly prevalent disease and characterized by dyspnea, chronic productive cough and progressive exercise intolerance.1 Many studies have investigated exercise performance and limiting factors in patients with COPD near sea level and showed multifactorial physiological limitations in gas exchange and ventilation,2 dynamic hyperinflation,3 deteriorated hemodynamics,3 weakness of the peripheral muscles4 and other factors.1,5 However, taking into consideration improved accessibility of mountain regions and the high COPD prevalence, then a considerable number of COPD patients travel for professional or touristic reasons to moderate altitude.
In the hypobaric hypoxic environment at high altitude, alveolar oxygen partial pressure is decreased, leading to hypoxemia and tissue hypoxia. In healthy individuals, hypoxemia leads to an increase in resting heart rate, ventilation and catecholamine excretion both at rest and during exercise. Despite these compensatory mechanisms, peak oxygen uptake (V’O2peak) remains lower compared to sea level,6 causing an altitude-related reduction of the maximum power output.
The effects of moderate altitude on exercise performance in COPD patients have not yet been conclusively investigated. At high altitude, the reduced partial pressure of oxygen may impair exercise tolerance due to induced hypoxemia, worse ventilatory equivalents and cerebral hypoxia.7,8 These exercise limitations were exclusively observed on the second day at high altitude.7–9 However, on the second day at altitude, some patients may have already suffered from acute mountain sickness (AMS) or have withdrawn from the study due to altitude-related adverse health effects.10,11 To our knowledge, no trial has yet investigated the exercise performance on the day of arrival at moderate altitude in COPD patients.
Therefore, the aim of this study was to test the hypothesis that exercise performance is reduced in patients with COPD on the day of arrival at moderate altitude. Furthermore, the aim was to quantify the decrease in exercise performance; to study the underlying physiological mechanisms and to explore predictors that could predict the altitude-related exercise intolerance in COPD.
Materials and Methods
Study Design
The current study was performed as part of a randomized cross-over study with the primary objective to investigate the effect of nocturnal oxygen therapy (NOT) on next-day 6-minute walking test (6-MWT) performance in COPD patients staying overnight at moderate altitude.11 In the main study, patients were examined once in Zurich (490 m) and twice in St. Moritz (2048 m), Switzerland. In order to minimize the carry-over and learning effect between the two ascents to 2048 m, a two-week washout period at low altitude (<800 m) was interposed. In the current analysis, we compared the exercise performance during a cycling incremental ramp exercise test (IET) from the first ascent to 2048 m, performed 2–6 hours after arrival, with those performed at 490 m. Data from the second ascent to 2048 m were only included for two patients, who had missing IET data during the first sojourn. All IET-related findings have not been published previously. The ascent to 2048 m was performed by train and car and lasted less than three hours. The below described assessments were performed at both study locations (490 m and 2048 m).
The protocol was approved by the cantonal ethics committee Zurich, Switzerland (EK-Nr. 2013–0088) and all patients provided written informed consent. The trial complied with the Declaration of Helsinki. The abstract of this paper was presented as a poster at the European Respiratory Society (ERS) International Congress in Paris in 2018. The poster’s abstract has been published in European Respiratory Journal (https://erj.ersjournals.com/content/52/suppl_62/PA2455). The main study was registered at www.ClinicalTrials.gov (NCT02143609).11 Further findings of secondary outcomes were published from this COPD study.12–14
Patients
We recruited men and women, aged 18–75 years old, with moderate-to-severe COPD, according to GOLD classification15 (GOLD 2–3, FEV1 from ≥30% to <80% predicted), both sexes, who lived at low altitude (<800 m). Exclusion criteria were hypoxemia of SpO2 <92% at 490 m, home oxygen or CPAP therapy, recent COPD exacerbation or pulmonary rehabilitation 3 months prior to the study, unstable COPD (defined as an acute change in dyspnea, cough or sputum production compared to the individual normal state), changes in their medication during the last 30 days and patients with a previous high-altitude intolerance (<2500 m) or a recent ascent to high altitude (>1500 m) in the past four weeks before the study. Furthermore, patients with severe unstable cardiovascular (eg, angina pectoris, recent myocardial infarction, obstructive sleep apnea), known neurological and psychiatric diseases, smoking more than 20 cigarettes per day, intake of medication that affects the respiratory tract (eg opioids, sedatives, etc.) and women being pregnant or breastfeeding were also excluded.11
Patients were recruited at the clinic of pulmonology of the University Hospital Zurich. Patients with an interest to participate in our study were invited for screening before the beginning of the study. A detailed medical history was taken and an insight of the patient’s medical records was completed. Moreover, a clinical examination, a lung function test, an arterial blood gas analysis, a resting electrocardiogram (ECG) and, by suspicion of obstructive sleep apnoea, an ambulatory overnight pulse oximetry11 were performed. Accordingly, in five patients in whom clinical evaluation raised the suspicion of undiagnosed sleep apnea were evaluated and were excluded if their oxygen desaturation index exceeded 15 events/h. No patients were excluded. Finally, an IET was performed.
Randomization
The sequence of altitude exposure and nocturnal treatment (placebo or NOT) was randomized. However, due to the here investigated performance of exercise testing on the day of arrival and before the first night at altitude, treatment allocation randomization does not play a role. Patients were randomized in balanced blocks of 4 and were assigned to sequences of altitude exposure and treatment (1–4) by letting them draw a sealed envelope with 1 of the following allocations: (1) assessment at 490 m first, allocated to receive placebo on first trip to 2048 m, then NOT on second trip; (2) assessment at 490 m first, allocated to receive NOT on first trip to 2048 m, then placebo on second trip; (3) allocated to receive placebo on first trip to 2048 m, then NOT on second trip, with assessment at 490 m last; and (4) allocated to receive NOT on first trip to 2048 m, then placebo on second trip, with assessment at 490 m last.11. The two ascents to 2048 m took place one after the other, so that each patient had examinations at 490 m, followed by 2048 m or vice versa, according to a randomized cross-over design.11
Study Outcomes
The primary outcome was the change of maximal power output (Wmax) at 2048 m compared to 490 m. Secondary outcomes were changes of physiological measures including SpO2 and heart rate at peak exercise and other clinical and subjective outcomes assessed by spirometry and questionnaires.
IET
IET was performed in Zurich and 2–6 hours after the arrival at 2048 m according to randomization. The patients performed a cycling IET to exhaustion using an individually established ramp protocol. In order to define the optimal individual ramp protocol, each patient performed a maximal IET at the pre-evaluation visit, before randomization. At 490 m, an exercise duration time of 8 to 12 minutes was aimed because of the known efficiency and best diagnostic use of this exercise duration, which has been established in clinical practice.16 At 2048 m, the same ramp protocol as at 490 m was used.
During the IET, ventilation and gas exchange were measured breath by breath. The Wmax, the oxygen saturation by finger-tip pulse oximetry (SpO2), the minute ventilation (V’E), the oxygen uptake (V’O2), a 12-lead ECG, the heart rate and other variables were measured continuously. For each test, identical ergometer, flowmeter and spirometry devices (Ergostik, Geratherm Respiratory GmbH) were used. The exercise test was performed according to the guidelines of the American Thoracic Society (ATS).16 Healthy reference values published by Koch et al were applied to calculate the percent predicted.17 Furthermore, dyspnea and leg fatigue were assessed directly before and after the exercise test by a Borg CR10 scale.18 The mean value of the work rate at the last 30 seconds of exercise was defined as maximal power output and all other variables were determined similarly.
After the installation of the face mask, ECG cables and pulse oximeter, the patients remained quietly seated on the ergometer for a minimum of three minutes. At the beginning of the IET, the patient started to peddle with a rate of 60 revolutions per minute (rpm). The exercise test was terminated when the peddling rate decreased to <40 rpm.
Lung Function Testing and Estimation of Maximal Voluntary Ventilation
Before each IET, lung function testing including spirometry and diffusing capacity of carbon monoxide was performed according to international guidelines.19 Furthermore, the maximal voluntary ventilation (MVV) was assessed through an appropriate maneuver. The patients had to breathe through the mouthpiece of the spirometer as deeply and quickly as possible for 10 seconds. The MVV-maneuver was performed according to the ATS and ERS guidelines.19
Statistics
We performed a per-protocol-analysis, in which the first IET of all patients with an IET at 490 m and 2048 m was included. All variables are presented as medians with inter quartile range (IQR) unless otherwise stated. The altitude effect between 490 m and 2048 m was examined using Hodges–Lehmann–Schaetzer median difference and are presented as median difference and 95% confidence interval (command cendif in STATA).20 This approach compares the individual altitude effect and calculates the median differences instead of comparing medians between 2048 and 490 m. A mixed linear regression analysis was performed to identify possible predictors for Wmax. A two-sided p-value <0.05 was considered to be statistically significant. The statistics were performed in STATA 15.1.
Results
Patient Characteristics
We screened 42 patients for eligibility, of which 32 were recruited for the current study. Three patients, who performed only one IET (either at 490 m or 2048 m) were excluded from the final analysis. Therefore, 29 patients were included in the per-protocol analysis (Figure 1). Patient characteristics are presented in Table 1.
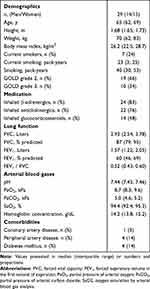 |
Table 1 Patients’ Characteristics (per-Protocol-Analysis) |
IET Results
Results from IET are presented in Table 2. We observed a statistically significant reduction in Wmax at 2048 m compared to 490 m. The median reduction in Wmax with altitude was 5 watts (95% CI, 2 to 8, P<0.05), which corresponded to a decrease of 6% (95% CI, 2 to 11, P<0.05) compared to 490 m (Figure 2). Both V’O2peak and SpO2 were also reduced, despite an increase of V’E at 2048 m of 2.8 L/min (95% CI, 0.9 to 4.2, P<0.05) (Figure 3). The increase in V’E was associated with a trending increase in MVV (95% confidence interval of 0 to 5) and unchanged ventilatory breathing reserve (MVV – maximal V’E). Furthermore, the increased V’E and lower V’O2 and V’CO2 at a lower Wmax at 2048 m resulted in an increase in ventilatory equivalents for oxygen uptake (V’E/V’O2) and for carbon dioxide output (V’E/V’CO2). We did not observe any differences in ventilatory and heart rate reserve (220 – age – maximal heart rate) between low and moderate altitude.
 |
Table 2 Results at Peak Exercise |
 |
Figure 2 Relative change of maximum power output in Watts from 490 to 2048 m. Lines represent individual patients. |
Dyspnea and leg fatigue at end-exercise were similar at both altitudes. The respiratory exchange ratio (RER) at end-exercise slightly increased at 2048 m compared to 490 m.
Results from univariate linear regression analyses are presented in Table 3. The mixed linear regression analysis confirmed an altitude-induced reduction in Wmax of 4% predicted in COPD GOLD 2 and a 7% predicted reduction in COPD GOLD grade 3 when ascending to altitude (P<0.05 between 2048 m vs 490 m; P=0.128 between GOLD grade 3 vs 2).
 |
Table 3 Mixed Linear Regression Analysis for the Work Rate Reduction, % Predicted |
Discussion
The current randomized crossover study in lowlanders with moderate-to-severe COPD revealed that acute ascent to 2048 m of altitude was associated with a slightly but significantly reduced maximal power output in cycling IET. The reduced performance was associated with a lower SpO2 and V’O2 along with a higher V’E at peak exercise at 2048 m but unchanged peak heart rate and dyspnea perception. We observed that the increase in V’E and trending increase in MVV resulted in unchanged ventilatory reserve (MVV – V’E) at 2048 m. These observations were probably explained by the lower barometric pressure and the lower airway resistance observed at high altitude as previously reported in COPD patients at higher altitude.7 The unchanged peak heart rate and dyspnea perception in both locations indicate same effort during the IET at both low and moderate altitudes. These results clarify that the reduced exercise performance at 2048 m compared to 490 m was mostly related to the hypoxemia which presumably induced myocardial hypoxia and inefficient ventilation as reflected by the increased V’E/V’O2 and V’E/V’CO2. FEV1 and DLCO measured at low altitude were identified as predictors with a negative correlation to the percentage-reduction of the exercise performance, which indicates that the altitude-related reduction of exercise performance is pronounced in patients with more severe limitation in ventilation and pulmonary gas exchange.
The exercise performance of healthy individuals, expressed in V’O2 has been suggested to linearly decrease by 1% per 100 m ascent from 1500 to 6300 m.6 The reduction in exercise performance observed in our study is close to these data from healthy individuals, despite the lower baseline exercise performance in patients with COPD.
Data from previous studies, which addressed the exercise performance of COPD patients at high altitude are scant. Eight COPD patients (FEV1 25–78% predicted) were exposed to 1920 m in 1978 by Graham and Houston)21 and showed a decrease in partial pressure of arterial oxygen (PaO2) from 8.8 to 6.8 kPa at 1920 m; the PaO2 increased to 7.4 kPa after a four-day acclimatization. Except for one patient, a light exertion on treadmill at high altitude was well tolerated by all others, but the change of the exercise performance was not quantified. However, the reduced PaO2 and spirometry suggest that being exposed to high altitude is connected to exercise limitations, a hypothesis that was confirmed by our study.
In a prior randomized study performed by our group,8 the exercise performance of 31 COPD patients (GOLD grade 2–3, mean FEV1 59% predicted) was evaluated after the first night at 1650 m by a cycling IET to exhaustion. In this study, the exercise performance was decreased by 7% at 1650 m compared to 490 m. V’E was increased and breathing reserve was reduced at peak exercise. Furthermore, maximal heart rate remained unchanged and V’O2peak was reduced. In another randomized, controlled, double-blinded trial,22 which investigated the effects of preventive treatment with acetazolamide on exercise performance in 103 patients with moderate-to-severe COPD on the day of arrival to 3100 m, a 10% reduction in Wmax was observed compared to 760 m.
Regardless of the study designs and in accordance with findings in healthy individuals, exercise was reduced with increasing altitude in COPD. Furian et al8 described a reduction in respiratory reserve at 1650 m, assuming that an additional mechanical limitation of ventilation during exercise at high altitude contributed to the decrease in maximal exercise performance, which was observed. However, in the current study, there was no significant reduction of respiratory reserve at high altitude. An etiology could be that in the current study, the MMV was measured using a breathing maneuver, although MVV is often calculated through FEV1 (MVV = 40 × FEV1). However, the measurement of MVV in COPD patients is technically difficult, depending on the daytime and the examiner performing it. In the study of Furian et al, half of the patients performed the exercise test after a 2-day period of acclimatization at 2590 m; this could also have had an influence on the ventilatory mechanism resulting in the ability to reduce respiratory reserve with maximal exercise. As previously shown, we confirmed the finding that maximal heart rate at altitude did not increase, despite exaggerated hypoxemia. This is in accordance with unchanged or even decreased maximal heart rate in healthy subjects exercising at altitude. The assumed underlying mechanisms are the protection against exaggerated myocardial hypoxia.23
In the study of Kelly et al,9 18 COPD patients (mean FEV1 42% predicted) were driven by car to a mountain Hutt at 2086 m, where they performed a 6-MWT after arrival. A decrease of 52% in 6-minute walking distance (6-MWD) compared to baseline measurements at 245 m was observed. Furthermore, 13 of the 18 patients were not able to perform the full 6-MWT and had to quit sooner. Although this study is not directly comparable to our trial, it shows a reduction of exercise performance shortly after arriving at high altitude. The discrepancy between the amount of the decrease in the exercise performance compared to our data can be explained by differences in COPD severity, assessment of exercise performance and the study design.
In summary, the observed exercise intolerance in COPD at 2048 m were mainly caused by pronounced hypoxemia and increased ventilatory equivalents without compensatory mechanisms. Compensatory mechanisms to increase the oxygen carrying capacity to the muscles such as increase in heart rate or further increase in minute ventilation by either increasing the breathing frequency or tidal volume were not observed. Whether the absence of ventilatory compensatory mechanisms was due to COPD-related airflow obstruction, interstitial lung fluid accumulation due to elevated pulmonary artery pressure13 or hypoxic pulmonary vasoconstriction, or due to elevated dynamic hyperinflation remains to be elucidated. Moreover, a study performed in the same COPD cohort indicated that hypoxemia-related cerebral hypoxia might be an exercise-limiting factor at this altitude.12
Strengths and Weaknesses
This is the first COPD study presenting results of cycling IET on the arrival day at moderate altitude. The cross-over study design and the randomization are a strength of this study. A limitation of our study is that we did not analyze the anaerobic threshold and did not perform an arterial blood gas analysis at peak exercise and therefore we do not have data about the changes in PaO2 or peak exercise lactate concentration. However, patients reached similar subjective and objective outcomes for maximal exercise exhaustion, which reassures that patients were actually maximally exhausted at both locations. Patients with very severe COPD were excluded from our study because of the high risk for medical complications at altitude. Therefore, the current data do not represent the whole population of COPD patients and are applicable only to patients with moderate-to-severe COPD.
Conclusion
This study showed that exercise performance of patients with moderate-to-severe COPD is slightly decreased on the day of arrival at 2048 m, an altitude which corresponds to the altitude of many tourist destinations. As dyspnea perception and maximal heart rate were unchanged, the lower blood oxygenation and exaggerated ventilatory response were culprit factors for the reduced performance. The observed reduction in exercise performance on the day of arrival is similar to what has been observed in healthy individuals at this altitude. Although, COPD patients have generally lower exercise capacity, they tolerated the arrival at moderate altitude well and no further medical considerations or interventions in these patients during a short-trip to moderate altitude, without staying overnight, are required. In case, patients are planning an overnight stay at moderate altitude, preventive measures such as nocturnal oxygen therapy or acetazolamide should be considered.10,11
Abbreviation
6-MWD, 6-minute walking distance; 6-MWT, 6-minute walking test; AMS, Acute mountain sickness; ATS, American Thorax Society, thoracic.org; BMI, Body mass index; CO2, Carbon dioxide; COPD, Chronic obstructive pulmonary disease; CVD, Cardiovascular disease; DLCO, Diffusion capacity of carbon monoxide; ECG, Electrocardiogram; ERS, European Respiratory Society; FEV1, Forced expiratory volume in the first second of expiration; FVC, Forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HRR, Heart rate reserve; IET, IQR; Incremental ramp exercise test, Inter quartile range; MVV, Maximal voluntary ventilation; NOT, Nocturnal oxygen therapy; O2, Oxygen; PaO2, Partial pressure of arterial O2; RER, Respiratory exchange ratio; rpm, Revolutions per minute; SpO2, Oxygen saturation by pulse oximetry; V’CO2, Output of carbon dioxide; V’E, Minute ventilation; V’E/V’CO2, Ventilatory equivalent for carbon dioxide output; V’E/V’O2, Ventilatory equivalent for oxygen uptake; V’O2, Oxygen uptake; V’O2peak, Peak oxygen uptake; Wmax, Maximal power output.
Data Sharing Statement
Anonymized data underlying this study can be requested directly from corresponding author Dr. sc. Michael Furian by qualified researchers providing an approved proposal.
Acknowledgement
The study was supported by the Swiss National Science Foundation (143875) and Lunge Zurich. Siemens Health Engineers provided some equipments for the study.
Disclosure
Prof. Dr. Silvia Ulrich reports personal fees and grants from MSD SA, Janssen SA, Orpha Swiss, Novartis, Swiss National Science Foundation, and Zurich Lung, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Vogiatzis I, Zakynthinos S. Factors limiting exercise tolerance in chronic lung diseases. Compr Physiol. 2012;2:1779–1817. doi:10.1002/cphy.c110015
2. Agusti AGN, Barbera JA, Roca J, Wagner PD, Guitart R, Rodriguez-Roisin R. Hypoxic pulmonary vasoconstriction and gas exchange during exercise in chronic obstructive pulmonary disease. Chest. 1990;97:268–275. doi:10.1378/chest.97.2.268
3. Mahler DA, Brent BN, Loke J, Zaret BL, Matthay RA. Right ventricular performance and central circulatory hemodynamics during upright exercise in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1984;130(5):722–729. doi:10.1164/arrd.1984.130.5.722
4. Debigaré R, Maltais F. The major limitation to exercise performance in COPD is lower limb muscle dysfunction. J Appl Physiol. 2008;105(2):751–753. doi:10.1152/japplphysiol.90336.2008a
5. Gallagher CG. Exercise limitation and clinical exercise testing in chronic obstructive pulmonary disease. Clin Chest Med. 1994;15(2):305–326. doi:10.1016/S0272-5231(21)01075-3
6. Wyatt FB. Physiological responses to altitude: a brief review. JEPonline. 2014;17(1):90–96.
7. Furian M, Hartmann SE, Latshang TD, et al. Exercise performance of lowlanders with COPD at 2590 m: data from a randomized trial. Respiration. 2018;95(6):422–432. doi:10.1159/000486450
8. Furian M, Flueck D, Latshang TD, et al. Exercise performance and symptoms in lowlanders with COPD ascending to moderate altitude: randomized trial. Int J Chron Obstruct Pulmon Dis. 2018;13:3529–3538. doi:10.2147/COPD.S173039
9. Kelly PT, Swanney MP, Stanton JD, Frampton C, Peters MJ, Beckert LE. Resting and exercise response to altitude in patients with chronic obstructive pulmonary disease. Aviat Space Environ Med. 2009;80(2):102–107. doi:10.3357/asem.2434.2009
10. Furian M, Mademilov M, Buergin A, et al. Acetazolamide to Prevent Adverse Altitude Effects in COPD and Healthy Adults. NEJM Evidence. 2022;1(1):EVIDoa2100006. doi:10.1056/EVIDoa2100006
11. Tan L, Latshang TD, Aeschbacher SS, et al. Effect of Nocturnal Oxygen Therapy on Nocturnal Hypoxemia and Sleep Apnea Among Patients With Chronic Obstructive Pulmonary Disease Traveling to 2048 Meters: a Randomized Clinical Trial. JAMA network open. 2020;3(6):e207940–e207940. doi:10.1001/jamanetworkopen.2020.7940
12. Gutweniger S, Latshang TD, Aeschbacher SS, et al. Effect of nocturnal oxygen therapy on exercise performance of COPD patients at 2048 m: data from a randomized clinical trial. Sci Rep. 2021;11(1):20355. doi:10.1038/s41598-021-98395-w
13. Lichtblau M, Latshang TD, Aeschbacher SS, et al. Effect of Nocturnal Oxygen Therapy on Daytime Pulmonary Hemodynamics in Patients With Chronic Obstructive Pulmonary Disease Traveling to Altitude: a Randomized Controlled Trial. Front Physiol. 2021;12:689863. doi:10.3389/fphys.2021.689863
14. Bisang M, Latshang TD, Aeschbacher SS, et al. Nocturnal Heart Rate and Cardiac Repolarization in Lowlanders With Chronic Obstructive Pulmonary Disease at High Altitude: data From a Randomized, Placebo-Controlled Trial of Nocturnal Oxygen Therapy. Clinical Trial. 2021;8:129.
15. Singh D, Agusti A, Anzueto A, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi:10.1183/13993003.00164-2019
16. Ross RM. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–277. doi:10.1164/rccm.167.2.211
17. Koch B, Schaper C, Ittermann T, et al. Reference values for cardiopulmonary exercise testing in healthy volunteers: the SHIP study. Eur Respir J. 2009;33(2):389–397. doi:10.1183/09031936.00074208
18. Borg E, Kaijser L. A comparison between three rating scales for perceived exertion and two different work tests. Scand J Med Sci Sports. 2006;16(1):57–69. doi:10.1111/j.1600-0838.2005.00448.x
19. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
20. Conroy RM. What hypotheses do “nonparametric” two-group tests actually test. Stata J. 2012;12(2):182–190. doi:10.1177/1536867X1201200202
21. Graham WG, Houston CS. Short-term adaptation to moderate altitude. Patients with chronic obstructive pulmonary disease. JAMA. 1978;240(14):1491–1494. doi:10.1001/jama.1978.03290140033017
22. Kind R, Furian M, Buergin A, et al. Effects of Acetazolamide on exercise performance in patients with COPD at high altitude. RCT. 2019;54(suppl 63):A1631.
23. Mourot L. Limitation of Maximal Heart Rate in Hypoxia: mechanisms and Clinical Importance. Review. 2018;9(972). doi:10.3389/fphys.2018.00972
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


