Back to Journals » Nature and Science of Sleep » Volume 14
Excessive Daytime Sleepiness in Parkinson’s Disease
Authors Liu H, Li J, Wang X, Huang J, Wang T, Lin Z, Xiong N
Received 17 May 2022
Accepted for publication 30 August 2022
Published 7 September 2022 Volume 2022:14 Pages 1589—1609
DOI https://doi.org/10.2147/NSS.S375098
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sarah L Appleton
Hanshu Liu,1,* Jingwen Li,1,* Xinyi Wang,1 Jinsha Huang,1 Tao Wang,1 Zhicheng Lin,2 Nian Xiong1
1Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 2Laboratory of Psychiatric Neurogenomics, McLean Hospital; Harvard Medical School, Belmont, MA, 02478, USA
*These authors contributed equally to this work
Correspondence: Nian Xiong, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, People’s Republic of China, Tel +86 13995516750, Email [email protected]
Abstract: Excessive daytime sleepiness (EDS) is one of the most common sleep disorders in Parkinson’s disease (PD). It has attracted much attention due to high morbidity, poor quality of life, increased risk for accidents, obscure mechanisms, comorbidity with PD and limited therapeutic approaches. In this review, we summarize the current literature on epidemiology of EDS in PD to address the discrepancy between subjective and objective measures and clarify the reason for the inconsistent prevalence in previous studies. Besides, we focus on the effects of commonly used antiparkinsonian drugs on EDS and related pharmacological mechanisms to provide evidence for rational clinical medication in sleepy PD patients. More importantly, degeneration of wake-promoting nuclei owing to primary neurodegenerative process of PD is the underlying pathogenesis of EDS. Accordingly, altered wake-promoting nerve nuclei and neurotransmitter systems in PD patients are highlighted to providing clues for identifying EDS-causing targets in the sleep and wake cycles. Future mechanistic studies toward this direction will hopefully advance the development of novel and specific interventions for EDS in PD patients.
Keywords: excessive daytime sleepiness, Parkinson’s disease, epidemiology, wakefulness, dopaminergic neurons, dopaminergic agents
Introduction
Parkinson’s disease (PD) is a chronic and progressive neurodegenerative disorder, with the second highest neurologic incidence, characterized pathologically by abnormal α-synuclein aggregation and significant dopaminergic neuron death in substantia nigra pars compacta (SNc).1,2 The disease manifests with various motor and non-motor symptoms. Some non-motor symptoms, including sleep disorders, depression, olfactory dysfunction, constipation and autonomic dysfunction, may precede motor deficits for decades followed by some severe negative impacts on patients’ daily life, which has attracted much attention in recent years.3,4
As one of the most common non-motor manifestations, sleep disorders affect about 90% of PD population.5 In addition, sleep disorders encompass a broad range of sleep problems, such as insomnia, rapid eye movement (REM) sleep behavior disorders (RBD) and excessive daytime sleepiness (EDS).6,7 It is of great urgency to keep a watchful eye on EDS due to its high morbidity, poor quality of life, increased risk for accidents, obscure mechanisms, comorbidity with PD and limited treatment options.
EDS refers to an irrepressible need for sleep or unintended lapses into drowsiness or sleep as a result of the inability to stay awake and alert during waking episodes of the day.8 EDS presents in 20–60% of PD patients, with an incidence increasing over time.9 Besides the differences in demographic characteristics, such a wide range of prevalence is also attributed to sample size and inconsistent assessment tools used to evaluate EDS in different studies (Table 1). A meta-analysis including 12,439 PD patients has shown that the pooled prevalence of subjective EDS in PD is 35.1%.10 Notably, all 59 studies included in this meta-analysis used a score of 10 as the cut-off value for the Epworth Sleepiness Scale (ESS) to identify EDS (positive if score > or ≥10). EDS was noted as one of the prodromal symptoms of PD.11 Previous longitudinal studies established that old adults with EDS had great likelihood to develop PD.12,13 Furthermore, researchers found that higher rate of RBD patients with EDS developed PD than those without EDS.14,15 However, other longitudinal studies reached negative conclusion that ESS >14 failed to predict conversion to neurodegenerative diseases in a RBD cohort, arguing against EDS be a reliable predictor of the development of neurodegeneration in patients with idiopathic RBD.16
 |
Table 1 The Prevalence of EDS in PD Patients |
It was estimated that 27% of PD patients reported sleep attacks at least once a day, and 19% of patients experienced sleep episodes during activities of daily living.17 Even 8% of PD patients suffered from sleep attacks while driving, which could lead to motor vehicle accidents.18 In addition to threatening the safety, EDS reduces the efficiency of daytime work and learning, affects the daily life of PD patients, and increases the burden of caregivers (someone who cares for PD patients).19
Although public understanding of EDS has gradually progressed over the past few decades, much remains unknown. Treatment options are limited due to associated adverse effects. Regarding the treatment of EDS in PD patients, some pharmacologic therapies such as modafinil and caffeine and nonpharmacologic therapies such as supplementary exposure to bright light are probably promising. However, the safety and efficacy of most therapeutic options need to be verified in a larger population.20 Therefore, the mechanisms of EDS in PD need to be elucidated in order to develop effective treatments. In this article, we summarized current literature on epidemiology of EDS in PD and compared the presence of EDS in PD patients and other populations. Moreover, the etiology, relevant risk factors and pathophysiological mechanisms of EDS in PD are also reviewed.
Epidemiology
The Measures and Related Prevalence of EDS in PD
Given the adverse effects of EDS on PD patients, it is necessary to identify EDS accurately. Currently, a number of subjective and objective measures are used to screen PD patients for their propensity for increased daytime sleepiness, the most widely used of which are ESS and multiple sleep latency test (MSLT).
ESS, a self-administered subjective scale for EDS, has been validated to be reliable in PD populations.21,22 The ESS has been shown to have a sensitivity of 93.5% and a specificity of 100% for a score of greater than 10 and have high internal consistency and test-retest reliability in healthy subjects over a period of 5 months.23,24 In addition, the ESS scores have been recommended to assess the severity of daytime sleepiness and a score of more than 16 indicates a high level of subjective sleepiness.21,25 MSLT has been extensively used to measure the severity of objective EDS by examining the ability to fall asleep.26 According to the International Classification of Sleep Societies (3rd edition) criteria, a mean sleep latency (MSL) of less than 8 minutes can be measured in patients with narcolepsy or idiopathic hypersomnia, which represents pathological sleepiness.8 As reported, the test-retest reliability of the MSLT was 0.97 in healthy volunteers over a period of 4–14 months while the sensitivity and specificity of the MSLT with MSL less than 8 minutes were 94.5% and 73.3%, respectively.23,27 The maintenance of wakefulness test (MWT) as another objective measure of EDS can examine the ability to keep awake. For individuals who are suspected of being unable to stay awake, the MWT is commonly used to assess their alertness to prevent safety issues. In addition, the MWT can also evaluate the response of patients with excessive sleepiness to treatment.28
Extensive studies showed that subjective EDS was common in PD patients in different countries. A case-control analysis in the United States found that 25% of PD patients reported daytime sleepiness with the ESS scores >10.29 A cross-sectional study in Norway revealed subjective EDS in 29% of PD patients while another cross-sectional study in the Netherlands showed that the prevalence of subjective EDS in PD patients was 29.7%.30,31 A cross-sectional study in France reported subjective sleepiness measured by ESS scores ≥10 in 33.5% of PD patients compared to 16.1% of age-matched controls.17 A cross-sectional study in China found that 22.5% of PD patients suffered from subjective EDS and a multi-center study in Japan revealed that 21.3% of PD patients had EDS.32,33
Notably, ascertaining EDS properly remains challenging due to the common comorbidity of cognitive impairment in PD patients.34 Several studies obtained clinical information from close family members or daily caregivers of PD patients who were unable to complete sleep questionnaires independently.35–37 Other studies excluded PD patients with cognitive impairment, which could lead to the underestimated prevalence of EDS.33,38,39 Fatigue is a symptom that may confound the diagnosis of EDS in PD patients due to their similar clinical manifestations.40 Besides, Valko et al found that 72.9% of PD patients with EDS simultaneously suffered from fatigue.41 Accordingly, clinicians should be careful to distinguish EDS from fatigue in PD patients. Currently, it could be an unbiased way to diagnose EDS in PD to combine subjective with objective assessments of sleepiness.42
However, some studies revealed a discrepancy between subjective and objective measures of sleepiness in PD.43,44 A study in France showed that objective sleepiness was present in 13.4% of 134 PD patients compared to 46.3% of subjective sleepiness and ESS scores had a weak negative correlation with MSLT latency.45 A case-control study in an Asian population reported no difference in objective daytime sleepiness measured by MSLT between PD patients and healthy controls (16.7% vs 12.7%, p = 0.54) but significant difference in subjective sleepiness measured by ESS of two groups (41.1% vs 19.1%, p = 0.01).46 Moreover, a recent study in Switzerland found no significant correlation between the objective sleepiness measured by MSLT and subjective sleepiness measured by ESS.42 Therefore, future investigations need to clarify a reason for the discrepancy and optimize evaluation approaches for EDS in PD population.
Comparison of EDS in PD Population and Other Populations
EDS not only occurs in PD patients, but also in patients with other neurological disorders, as well as the general population. A follow-up study showed that in PD, Alzheimer’s disease (AD) and age-matched controls, the prevalence of subjective EDS at baseline were 41%, 18% and 10%, respectively.37 Several controlled studies found that 33.5–44% of PD patients suffered from subjective sleepiness compared to 16% of general population (ie, age-matched healthy volunteers or geriatric patients without PD).17,47 In addition, higher mean ESS scores were reported in PD patients compared to healthy controls and AD patients.37,46 Similarly, a meta-analysis revealed that the prevalence and mean ESS scores of patients with essential tremor were significantly lower than those of PD patients, highlighting daytime sleepiness in PD subjects.48
There are comparisons on the presence of EDS in PD and other α-synucleinopathies including multiple system atrophy (MSA) and dementia with Lewy Bodies (DLB). A study revealed that subjective EDS was in 29% of PD patients and 28% of MSA patients compared to 2% of controls. Likewise, mean ESS scores in PD and multiple system atrophy were equally higher than those in controls.49 Another comparative study showed that 43.8% of PD patients and 61.5% of MSA patients had subjective EDS.50 Boddy et al found that subjective daytime sleepiness was also prevalent in DLB patients and PD patients with dementia with the prevalence rate of 50% and 57%, respectively, which was higher than that of PD patients without dementia.37
These epidemiological data revealed that EDS was severer and more common in α-synucleinopathies than in the age-matched general population and other neurodegenerative diseases such as AD and essential tremor, suggesting neurodegeneration due to α-synucleinopathies might play a more important role in the development of EDS than age-related neurodegeneration.
Risk Factors of EDS in PD
To date, longitudinal studies on PD have identified several risk factors of EDS, including age, gender, disease duration and severity, some nonmotor and motor symptoms, antiparkinsonian medications and nighttime sleep problems.51,52
Gender and Age
In PD patients, men appeared to experience more and severer EDS than women, and the proportion of men with EDS was significantly higher than that of men without EDS, pointing to the gender male as a risk factor for EDS in PD.53,54 In addition, extensive research revealed that patients with EDS were older than those without EDS, suggesting that aging might play another important role in the EDS development.53,55
However, it remains unclear whether obstructive sleep apnea (OSA) plays a role in the relationship of higher risk of EDS with male sex and older age in PD. As reported, OSA was common in PD patients with a prevalence of 28% and elderly men had the greater likelihood of suffering from OSA.56 Besides, OSA has been associated with severe EDS in PD patients. Similarly, another study found that PD patients with high risk of OSA had significantly higher ESS scores, compared to those with low risk of OSA.57 Therefore, the independent association of higher risk of EDS with male sex and older age in PD needs to be further clarified considering the confounding effect of OSA.
Disease Severity
Several studies demonstrated that PD patients with EDS had higher Unified PD Rating Scale scores and Hoehn and Yahr stages relative to those non-EDS PD patients, supporting the positive correlation between EDS and advancing PD.10,52 The disease severity could be also reflected by evident non-motor and motor symptoms. Previous studies assessed risk factors for EDS in PD and found several symptoms, including cognitive impairment or dementia, deteriorated autonomic function, disability, hallucination and depression, were independently associated with the development of EDS.58,59
These findings suggested the development of EDS was associated with advancing PD, consistent with the view that EDS can be a result of spreading neurodegenerative process in PD.
Disease Duration
The presence of EDS has been reported to be associated with longer disease duration of PD.60 A longitudinal study revealed that 46% of PD patients without EDS at baseline developed this symptom during 5-year follow-up.51 Likewise, another community-based cohort study assessed the development of EDS in PD patients over 8 years and found that the occurrence of EDS increased from 5.6% at baseline to 22.5% within a 4-year follow-up and 40.8% after 8 years.35 Notably, researchers found that EDS in early PD was reversible in the first few years but the symptom of daytime sleepiness became more persistent over time.35,52
Conceivably, the development of EDS can be attributed to different causes. In other words, several modifiable etiologies, such as antiparkinsonian medications and poor nighttime sleep, contribute to the emergence of reversible EDS in PD while persistent EDS can be due to impaired wake-promoting nuclei by spreading neurodegeneration of PD.52
Etiology and Pathophysiological Mechanisms of EDS in PD
Based on the high prevalence, it is crucial to find out why EDS is so common in PD patients. In fact, the etiology of EDS in PD is multifactorial. As mentioned above, antiparkinsonian medications, primary sleep disorders and impaired wake-promoting nuclei due to primary neurodegenerative lesions of PD are the extensively accepted causes of EDS.61 Moreover, genetic susceptibility is associated with an increased risk of EDS in PD. A recent cross-sectional study revealed that DQB1*06:02 (an HLA risk allele for narcolepsy)-positive PD patients were three times more likely to develop EDS than DQB1*06:02-negative PD patients and no significant differences were found in nighttime sleep between the two groups, suggesting that narcolepsy phenotype in PD patients is a cause of EDS independent of nocturnal sleep disturbances.62
Antiparkinsonian Medications
Drug therapy is necessary for PD patients to relieve motor and non-motor symptoms, of which dopaminergic drugs are the most widely used. However, extensive research revealed that almost all antiparkinsonian medications affect EDS in PD patients (Table 2).63–65
 |  |  |
Table 2 Effects of Antiparkinsonian Medications on EDS in PD Patients |
Dopaminergic therapies, including levodopa and DA agonists, can cause or aggravate daytime sleepiness, which might be attributed to their sedative effects.66 A prospective study showed that 28.4% of PD patients reported EDS after receiving dopaminergic treatment and most of these patients had an ESS score of more than 10.67 In a previous study, the mean sleep latency by the MSLT was significantly diminished (8.1±4.7min vs 11.6 ± 4min, P < 0.005) in PD patients after receiving dopaminergic treatment, suggesting that EDS could be a class effect of dopaminergic drugs.68
Similar influences on EDS in PD have been reported of DA agonists. Several studies indicated that daytime sleepiness occurred more frequently in patients on pramipexole than those on placebo or other anti-PD drugs, suggesting pramipexole could contribute to EDS.69–71 A long-term clinical trial revealed PD patients initially treated with pramipexole showed higher prevalence (57.4% vs 35.2%, P = 0.002) of daytime sleepiness together with higher ESS scores (11.3 vs 8.6, P < 0.001) than those initially treated with levodopa. Based on this finding, pramipexole might have a stronger effect on EDS relative to levodopa.72 However, compared with levodopa or DA agonist monotherapy, combination therapy with the two drugs increased the ESS scores.73
Several randomized, double-blind trials evaluated the long-term safety and tolerability of transdermal rotigotine in PD patients in the United States and Canada and noted daytime somnolence as a common adverse event, affecting 18–33% of patients on rotigotine.74–77 Nevertheless, a recent study on rotigotine in PD patients in the United States found that neither subjective nor objective sleepiness changed before and after rotigotine treatment.78 A randomized, placebo-controlled study reported that EDS occurred in 8.9–14.3% of patients on apomorphine, but not in patients on placebo, a finding supported by two other studies on apomorphine in PD.79–81 Notably, a case study reported that a PD patient suffered from somnolence episodes following increasing doses of pergolide whereas somnolence disappeared after decreasing doses of pergolide. Moreover, lower latency of sleep onset was found in PD patients taking on pergolide compared with those on placebo, suggesting that EDS is not a specific effect related to the non-ergoline class DA agonists.82
In addition to dopaminergic therapies, entacapone, a catechol-O-methyltransferase inhibitor, was shown to induce EDS in PD patients. In an open-label study, 6.5% of PD patients changing from immediate release carbidopa/levodopa to carbidopa/levodopa/entacapone reported increased sleepiness, which supported the findings in previous case reports.83–85
Notably, the risk of daytime sleepiness is related to the dose of dopaminergic medications. EDS is more common in PD patients taking higher dosages of DA agonists and levodopa, typically in the dose-increasing phase or after a period of time on a stable dosage.65,86 Two studies found that levodopa equivalent dose predicted EDS in PD patients, suggesting that EDS in PD was associated with the amount of dopaminergic drugs.49,87 In addition, decreasing dosages of dopaminergic drugs was shown to relieve subjective daytime sleepiness in PD patients in an open-label study.88 In some animals and healthy population studies, dopaminomimetic agents, including DA, levodopa and DA agonists, have been shown to induce biphasic effects on sleep and wakefulness depending on varying concentrations.89–91 Low doses enhance sleep via D2-like inhibitory autoreceptors, while high doses promote alertness and reduce slow-wave sleep and REM sleep possibly through the activation of D1-like and D2-like postsynaptic receptors. Several studies showed that dopamine D1 receptor agonist promoted wakefulness while D2 receptor agonist increased sleep.92,93 Based on relevant literature, divergent dose-dependent effects of DA agonists in healthy subjects vs PD patients may be related to the imbalance of D1 receptor and D2 receptor activity. That is to say, relatively hyperactivated D2 receptor due to the loss of D1 receptor or increased D2 receptor expression in PD could lead to sleepiness when using dopaminergic agents.94 Besides, the loss of PD-specific DA neurons responsible for promoting and maintaining wakefulness may be the contributor of EDS in PD patients. This hypothesis can also explain why EDS frequently occurs in PD patients.
It appears that some DA agonists have different effects on EDS. A cross-sectional observational study found no significant correlation between ropinirole and EDS in PD patients.95 The finding was in accordance with previous studies, suggesting that ropinirole did not contribute to sleepiness in PD.96–98 Interestingly, another study reported that patients changing from ropinirole immediate release to prolonged release showed an improvement from daytime sleepiness.99 Compared with the vague effect of ropinirole on EDS, piribedil seemed to attenuate daytime sleepiness in PD patients. One randomized controlled trial (RCT) showed a reduction in ESS scores in patients switching from pramipexole or ropinirole to equivalent dose of piribedil relative to those continuing on pramipexole or ropinirole, suggesting piribedil was potentially beneficial against EDS in PD patients.100 Such positive effects might be due to the unique antagonistic activities of piribedil at alpha-2 receptors, resulting in promoting cholinergic transmission and norepinephrine activity, which was proved to enhance awakening.101–104 Interestingly, selective D1 receptor agonist has been shown to efficiently relieve sleepiness while D2 receptor agonist had no significant effect on EDS in MPTP-induced PD macaque model.92 In addition, a recent study revealed that mevidalen, the dopamine D1 receptor positive allosteric modulator, can contribute to prolonged MSL measured by MSLT and enhance wakefulness in healthy sleep-deprived healthy volunteers.93 As previously discussed, however, most D2 receptor agonists, including pramipexole and ropinirole, could induce EDS in PD patients. Accordingly, divergent affinity for D1 and D2 receptors could result in different effects on EDS of dopaminergic drugs, which is associated with the activities of striatal neurons. Indeed, striatonigral neurons expressing D1 receptor have been demonstrated to promote wakefulness whereas striatopallidal neurons expressing D2 and A2A receptors induce sleep.105,106
Regarding amantadine and monoamine oxidase-B inhibitors, several studies revealed their advantageous effects on EDS. A recent study demonstrated a significant improvement in daytime sleepiness in patients on extended-release amantadine compared to those on placebo, suggesting amantadine had potential benefits against EDS in PD patients.107 In a 12-week open-label study, 94% of patients with EDS showed significant improvement in subjective sleepiness after adding orally disintegrating selegiline and decreasing DA agonist dosages.88 Likewise, a recent study reported that daytime somnolence was significantly improved in 45 PD patients treated with 10 mg of selegiline for 3 months, which was explained by the central excitatory effect of the selegiline metabolite amphetamine.108
A double-blind, baseline-controlled trial revealed a reduction in ESS scores in patients treated with 1 mg of rasagiline.109 However, another RCT demonstrated that 1 mg of rasagiline as an add-on to dopaminergic therapy did not change daytime sleepiness in PD patients.110 This was supported by an observational study, which showed that daytime sleepiness was significantly improved in PD patients on safinamide, the third-generation monoamine oxidase-B inhibitors, but not in patients on rasagiline.111 An open-label prospective study also reported an improvement of safinamide on daytime sleepiness, which might be related to the glutamatergic system involved in sleep-wake rhythm since safinamide could regulate the release of glutamate in some brain areas.112–114
Taking together, most anti-PD medications seemingly have dose-dependent negative effects on EDS, but some other drugs such as piribedil and selegiline may ameliorate EDS in PD patients. Therefore, it is recommended to carefully evaluate the symptom of daytime sleepiness and then select reasonable anti-PD drugs for patients.20
Other Sleep Disorders
As reported, other sleep disorders, including insomnia, RBD, OSA and restless legs syndrome (RLS), are common in PD patients and frequently coexist with EDS. A longitudinal cohort study revealed that nighttime sleep problems were present in 34% of PD patients who suffered from subjective EDS.60 Another polysomnographic study found that 69% of PD patients with objective EDS experienced OSA and RLS on overnight polysomnography.46
In fact, sleep architectures have been shown to change in PD patients and mainly manifest with nighttime sleep problems such as sleep fragmentation, difficulty in falling asleep and early awakening, which could cause EDS.51,53,115 A prospective study found that insomnia was more prevalent in PD patients with EDS than those without EDS.41 Suzuki et al found a significant difference in frequency of EDS between PD patients with and without RLS.116 Furthermore, several studies reported a positive correlation between RLS severity and EDS in PD.50,117 Notably, the relationship between EDS and RLS in PD remains an issue of debate since inconsistent results were reported.118,119 The association between poor nighttime sleep and EDS might be partially explained by the regulation of sleep homeostasis. Due to prolonged duration of wakefulness at night, somnogenic factors accumulate gradually, which leads to increased sleep pressure during the day and daytime sleepiness.120
In addition to sleep homeostasis regulation, however, it seems that a shared pathogenesis underlies the relationship between EDS and RBD in PD. Rolinski et al demonstrated that PD patients with RBD had increased frequency of EDS and higher ESS scores compared to those without RBD, implying RBD correlated to worse daytime sleepiness in PD patients.121 The association was still statistically significant after adjusted for other variables such as age and gender; similar findings were reported in other studies.122,123 As the prodromal symptoms of PD, the relationship between EDS and RBD in PD might be related to the disruption of cholinergic system within brainstem. On the one hand, the widespread degeneration within brainstem could impair the subcoeruleus complex and the pedunculopontine nucleus, which are in charge of REM atonia and awakening, respectively.124,125 On the other hand, RBD in PD could be affected by cholinergic denervation in related neocortex, limbic cortex, and thalamus those receive cholinergic input from pedunculopontine nucleus.126
Although EDS emerges as a common symptom in OSA patients, the role of OSA in EDS in PD patients remains controversial. Several studies revealed no difference in the incidence of OSA between PD patients with EDS and those without EDS.127,128 Moreover, a recent large sample study among European population showed no significant causal association between OSA and PD, implying that OSA might not be a risk factor for PD.129 However, a study in Chinese PD patients found that ESS scores were higher in PD patients with OSA than those without OSA, suggesting that OSA worsened EDS.56 This finding was consistent with another study on risk factors of OSA in PD.57 Two studies found that objective sleepiness by the MSLT was related to higher apnea hypopnea index in PD patients, while one study found no correlation between objective sleepiness and apnea hypopnea index in sleepy PD patients.45,130,131 OSA is characterized by intermittent hypoxia and sleep fragmentation, which could be responsible for EDS in PD.132 That is, OSA can trigger neuroinflammation via intermittent hypoxia and sleep fragmentation, and lead to neuronal damage in multiple wake-promoting brain regions, such as dopaminergic ventral periaqueductal gray and noradrenergic locus coeruleus, resulting in the development of EDS in PD patients.133
Collectively, sleep homeostasis regulation, as well as damaged wake-promoting brain regions may explain the relationship between primary sleep disorders and EDS in PD patients. Poor nighttime sleep can lead to compensatory EDS through the regulation of sleep homeostasis. The impairment of wake-promoting brain regions can be a shared pathogenesis of EDS and other sleep disorders in PD patients and neuroinflammation triggered by OSA can worsen such a pathological process.
Impaired Wake-Promoting Nerve Nuclei Due to PD Neurodegeneration
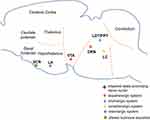
Sleep and wakefulness are regulated by multiple brain regions and neurotransmitter systems.134 Sleep or awakening condition are disturbed with neural nuclei of sleep-wake system destroyed.135 Although the pathogenesis of EDS in PD remains unknown, growing evidence suggests that degenerative changes in the structure of some brain regions involved in arousal correlated with EDS in PD (Figure 1).
Neuronal Pathology of PD
It has been well recognized that the core of pathology in PD is DA neuron death in SNc and the presence of Lewy body.136 Pathogenic mechanisms have been proposed to converge in alpha-synuclein misfolding and abnormal aggregation, impaired protein clearance, mitochondrial dysfunction, oxidative stress and neuroinflammation.2,137 The PD-specific loss of nerve cells has been reported to occur in SNc along with other brain regions, such as locus coeruleus, the dorsal motor nucleus of the vagus, pedunculopontine nucleus, the medullary reticular formation, nucleus basalis of Meynert, ventral tegmental area (VTA), dorsal raphe nucleus and thalamus.138,139 Extensive research has also shown that in addition to dopaminergic system, cholinergic, noradrenergic, serotonergic, orexinergic, glutamatergic, and adenosine pathways are also involved in the pathogenesis with PD progression.139 Several postmortem studies in PD patients revealed that histamine levels and innervation in SN increased and the expression of histamine receptors changed in SN, caudate nucleus, and putamen. Moreover, the histamine methyltransferase-mRNA in SN was found to be negatively correlated with PD disease duration, implying that altered histaminergic system might partly contribute to PD pathology.140,141 Notably, growing evidence indicates that the dopaminergic system plays a pivotal role in maintaining wakefulness. In fact, Eban-Rothschild et al demonstrated that the awake state would be suppressed to promote sleep when inhibiting the activities of VTA dopaminergic neurons, suggesting that VTA dopaminergic neurons are necessary for arousal.142 Except for VTA neurons, early experimental studies proposed that ventral periaqueductal gray matter (vPAG) dopaminergic neurons could be one neuronal population to mediate arousal.143,144 Indeed, vPAG dopaminergic neurons were found to show c-Fos immunoreactivity during wakefulness but not during sleep, and lesions of these neurons resulted in reduced wakefulness and increased sleep.145 Although vPAG dopaminergic neurons are involved in the maintenance of wakefulness, postmortem studies have not yet reported loss of wake-active ventral periaqueductal gray matter dopaminergic neurons in PD patients.139 Collectively, in the pathologic course of PD, the fiber connections among wake-promoting nerve nuclei get damaged and the wake-promoting neurotransmitters decrease.104 Accordingly, the function of wakefulness maintenance grows weakened and then leads to sleepiness.
Radiology Images on Altered Brain Areas in PD Patients with EDS
Imaging revealed changes in brain structures and function in PD patients with EDS. Matsui et al found greatly diminished fractional anisotropy values of the fornix fiber in PD patients with EDS and demonstrated the fornix fiber degeneration was related to EDS in PD patients.146 An independent magnetic resonance imaging study showed distinct gray matter atrophy in the frontal, temporal, occipital lobes, and nucleus basalis of Meynert in PD patients with EDS, compared with those without EDS and controls.147 However, a multimodal imaging study revealed an increase in grey matter volume of the bilateral hippocampus and parahippocampal gyrus, as well as amplified axial diffusivity values in the left anterior thalamic radiation and corticospinal tract. These alterations might be related to uninhibited signaling pathways or compensatory alteration due to anatomical or functional deficits in other brain regions.148 Likewise, PD patients with EDS in a resting state functional magnetic resonance imaging study displayed an increase of spontaneous neural activity in the left paracentral lobule. By contrast, reduced activity was observed in the left cerebellum and inferior frontal gyrus where functional connectivity was also decreased, suggesting neural downregulation and associated compensatory mechanisms in patients presenting EDS.149
Related Wake-Promoting Neurotransmitter Systems
Specifically, two [123I] FP-CIT single-photon emission computed tomography studies demonstrated a correlation between ESS scores and DA transporter loss in striatum, caudate nucleus and putamen in early PD. This finding suggested that the severity of EDS may relate to dopaminergic nigrostriatal degeneration at this stage.59,150 Yoo et al revealed ESS scores negatively correlated with thalamic monoamine availability (including DA, serotonin, and norepinephrine). Besides, they found decreased thalamic monoamine availability appeared to be an independent predictor of EDS in early and drug-naive PD. These findings implied that disruption of these monoaminergic systems could be a cause of EDS in PD.151 In line with this study, another human postmortem study found α-synuclein distributed across the thalamus in PD.152 In addition, the paraventricular nucleus of the thalamic was reported to play a critical role in maintaining wakefulness through projections with nucleus accumbens and with the orexinergic systems.153 Nevertheless, Wilson et al demonstrated that serotonergic dysfunction in PD was related to nocturnal sleep problems, but not to EDS.154 Similarly, another study found a substantial decline in availability of raphe serotonin transporter four years after diagnosis of PD, lacking a correlation with EDS.155
Orexin neurons in the lateral hypothalamus densely project to monoaminergic and cholinergic neurons in forebrain and brainstem and involve sleep-wake regulation and maintain arousal.156 Previous studies reported a decline both in the orexin concentration levels in ventricular CSF and in the density of orexin cells in hypothalamus.157–159 They also found orexin system degenerated with the severity of the disease in the PD patients. However, two other studies measured orexin levels in ventricular CSF of PD patients with and without objective daytime sleepiness and demonstrated lack of correlation between objective daytime sleepiness and lumbar orexin levels.160,161 Such evidence suggests that orexin be not the major pathophysiological mechanism of EDS in PD.
Collectively, impaired dopaminergic pathway appears to represent the promising pathogenesis of EDS in PD, excluding the orexinergic and serotonergic systems.59,90,154 In addition, damaged cholinergic system is also the potential pathogenesis of EDS due to its close relationship with various symptoms related to EDS.162,163
Peripheral Connections of Impaired Wake-Promoting Nuclei
Specific wake-promoting nuclei and neurotransmitter systems responsible for EDS in PD remain obscure, but EDS-related symptoms can provide clues to find out peripheral connections of impaired wake-promoting nuclei and tie EDS to specific neuroanatomical areas of PD degeneration.
Jester et al reported that PD patients with EDS experienced extra cognitive deficits in processing speed and executive control in comparison with those without EDS.164 Additionally, EDS was previously demonstrated to be a predictive factor for the future impairment of cognitive function.165 The postural instability gait difficulty (PIGD) dominant motor phenotype may give us insight into the relationship between disability and EDS, since a high PIGD score could predict the development of disability as well as EDS.51,55 Several studies revealed a positive correlation between EDS and mood measures in PD patients.52,55,166 Depression appeared significantly related to EDS while anxiety was weakly correlated with EDS, but the degree of correlation varied with subjective or objective sleepiness.
Indeed, EDS was closely related to cognitive impairment and depression in PD. These non-motor symptoms shared several common risk factors including severity of motor impairment and longer disease duration, and thus were identified as a symptom complex associated with advancing PD.167,168 Such a close relationship reinforces the notion that progressing neurodegeneration is more likely to underlie the development of EDS and related non-motor and motor manifestations in PD.58
It has been established that depression in PD was associated with the loss of serotonergic neurons projecting to the limbic regions and the disturbances of dopaminergic and noradrenergic systems in the locus coeruleus in control of alertness.169 The PIGD features in PD patients seemed to be attributed to the cholinergic dysfunction in pedunculopontine nucleus and basal forebrain, which can also lead to failure to maintain wakefulness.162,170,171 Cognitive impairment in PD has been linked to damage of thalamus and the nucleus basalis of Meynert in the substantia innominata of the basal forebrain, based on reduced gray matter volume and increased mean diffusivity in a diffusion tensor imaging study.163 Interestingly, similar damage was also reported in the two brain regions of PD patients with EDS.147,151 Since the nucleus basalis of Meynert is the primary source of cholinergic input from basal forebrain to the cortex, the relation may be explained by the disruption of cholinergic system which controls cognitive function and promote awakening.163,170,172
Circadian rhythm dysfunction has been postulated as a promising research topic due to its close correlation with nearly all non-motor manifestations, including damaged sleep and alertness in PD patients.64 As one of the sleep-wake regulations, circadian rhythm dysfunction circadian rhythmicity is dominated by the endogenic biological clock of suprachiasmatic nucleus in the anterior hypothalamus and regulated by external zeitgebers and endogenous signals. Light is the strongest external zeitgebers. In addition, physical activity, temperature and mealtime are also environmental zeitgebers. The endogenous factors of circadian rhythmicity include melatonin and age.173–175
The amplitude and amount of melatonin secretion in those with EDS decreased significantly, compared to the PD patients without EDS.38 Likewise, Breen et al found cortisol and melatonin levels in blood increased and decreased, respectively.176 Videnovic et al showed an inverse correlation between age and the amplitude/amount of melatonin secretion in PD patients, but not in healthy controls.38 These findings revealed that altered hormone might be associated with abnormal sleep in PD patients. In addition to changes in endogenous factors, many studies have observed that therapies to restore circadian rhythm, such as strong light therapy and exercise therapy, can improve EDS in PD patients.177,178
Conclusions
In summary, EDS is one of the most prevalent sleep disorders in PD population and has a negative impact on the safety and quality of these patients’ lives. Multiple factors contribute to EDS in PD patients, including the use of antiparkinsonian medications, coexistent nighttime sleep problems and primary neurodegenerative lesions of PD. Most dopaminergic drugs induce or aggravate reversible EDS in PD patients in a dose-dependent manner, while some other anti-PD drugs improve EDS, which can be attributed to different pharmacological mechanisms. Thorough summary on the effects of commonly used anti-PD drugs on EDS in this review can support the rational clinical medication in sleepy PD patients. The presence of persistent EDS was much associated with primary neurodegenerative lesions of PD. As pointed out in the review, damaged wake-promoting nerve nuclei and neurotransmitter systems including dopaminergic, cholinergic and noradrenergic systems can be the pathogenesis of EDS in PD patients. These efforts provide clues for subsequent imaging studies or mechanism research to effectively ascertain specific wake-promoting nerve nuclei responsible for EDS in PD. Future exploration toward this direction may hopefully shed light on developing effective, mechanisms-driven treatment options.
Abbreviations
AD, Alzheimer’s disease; CSF, Cerebrospinal fluid; DA, Dopamine; DLB, Dementia with Lewy Bodies; EDS, Excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; OSA, Obstructive sleep apnea; MSA, Multiple system atrophy; MSLT, Multiple sleep latency test; PD, Parkinson’s disease; PIGD, Postural instability gait difficulty; RBD, Rapid eye movement sleep behavior disorders; RCT, Randomized controlled trial; REM, Rapid eye movement; RLS, Restless legs syndrome; SNc, Substantia nigra pars compacta; VTA, ventral tegmental area; vPAG, ventral periaqueductal gray matter.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article. HL and JL contributed equally to this paper. All authors gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was funded by the National Natural Science Foundation of China (no. 81873782 and no. 82102153), 2018 Wuhan medical research project (no. WX18A10), 2019 Wuhan Huanghe Talents Program, 2020 Wuhan medical research project (no. 2020020601012303) and 2021 Hubei Youth Top-notch Talent Training Program.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15(12):1257–1272. doi:10.1016/S1474-4422(16)30230-7
2. Samii A, Nutt JG, Ransom BR. Parkinson’s disease. Lancet. 2004;363(9423):1783–1793. doi:10.1016/S0140-6736(04)16305-8
3. Postuma RB, Aarsland D, Barone P, et al. Identifying prodromal Parkinson’s disease: pre-motor disorders in Parkinson’s disease. Mov Disord. 2012;27(5):617–626. doi:10.1002/mds.24996
4. Visser M, van Rooden SM, Verbaan D, et al. A comprehensive model of health-related quality of life in Parkinson’s disease. J Neurol. 2008;255(10):1580–1587. doi:10.1007/s00415-008-0994-4
5. Stefani A, Hogl B. Sleep in Parkinson’s disease. Neuropsychopharmacology. 2020;45(1):121–128. doi:10.1038/s41386-019-0448-y
6. Lees AJ, Blackburn NA, Campbell VL. The nighttime problems of Parkinson’s disease. Clin Neuropharmacol. 1988;11(6):512–519. doi:10.1097/00002826-198812000-00004
7. Factor SA, McAlarney T, Sanchez-Ramos JR, Weiner WJ. Sleep disorders and sleep effect in Parkinson’s disease. Mov Disord. 1990;5(4):280–285. doi:10.1002/mds.870050404
8. American Academy of Sleep Medicine. International Classification of Sleep Disorders III. Darien, IL: American Academy of Sleep Medicine; 2014.
9. Chahine LM, Amara AW, Videnovic A. A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med Rev. 2017;35:33–50. doi:10.1016/j.smrv.2016.08.001
10. Feng F, Cai Y, Hou Y, et al. Excessive daytime sleepiness in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2021;85:133–140. doi:10.1016/j.parkreldis.2021.02.016
11. Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912. doi:10.1016/S0140-6736(14)61393-3
12. Abbott RD, Ross GW, White LR, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology. 2005;65(9):1442–1446. doi:10.1212/01.wnl.0000183056.89590.0d
13. Gao J, Huang X, Park Y, et al. Daytime napping, nighttime sleeping, and Parkinson disease. Am J Epidemiol. 2011;173(9):1032–1038. doi:10.1093/aje/kwq478
14. Arnulf I, Neutel D, Herlin B, et al. Sleepiness in idiopathic REM sleep behavior disorder and Parkinson disease. Sleep. 2015;38(10):1529–1535. doi:10.5665/sleep.5040
15. Zhou J, Zhang J, Lam SP, et al. Excessive daytime sleepiness predicts neurodegeneration in idiopathic REM sleep behavior disorder. Sleep. 2017;40(5):zsx041.
16. Iranzo A, Serradell M, Santamaria J. Excessive daytime sleepiness does not predict neurodegeneration in idiopathic REM sleep behavior disorder. Sleep. 2017;40(12). doi:10.1093/sleep/zsx161
17. Ferreira JJ, Desboeuf K, Galitzky M, et al. Sleep disruption, daytime somnolence and ‘sleep attacks’ in Parkinson’s disease: a clinical survey in PD patients and age-matched healthy volunteers. Eur J Neurol. 2006;13(3):209–214. doi:10.1111/j.1468-1331.2006.01262.x
18. Meindorfner C, Körner Y, Möller JC, et al. Driving in Parkinson’s disease: mobility, accidents, and sudden onset of sleep at the wheel. Mov Disord. 2005;20(7):832–842. doi:10.1002/mds.20412
19. Gooneratne NS, Weaver TE, Cater JR, et al. Functional outcomes of excessive daytime sleepiness in older adults. J Am Geriatr Soc. 2003;51(5):642–649. doi:10.1034/j.1600-0579.2003.00208.x
20. Seppi K, Ray Chaudhuri K, Coelho M, et al. Update on treatments for nonmotor symptoms of Parkinson’s disease - an evidence-based medicine review. Mov Disorders. 2019;34(2):180–198. doi:10.1002/mds.27602
21. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi:10.1093/sleep/14.6.540
22. Hagell P, Broman JE. Measurement properties and hierarchical item structure of the Epworth Sleepiness Scale in Parkinson’s disease. J Sleep Res. 2007;16(1):102–109. doi:10.1111/j.1365-2869.2007.00570.x
23. Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the Epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9(1):5–11. doi:10.1046/j.1365-2869.2000.00177.x
24. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–381. doi:10.1093/sleep/15.4.376
25. Hogl B, Arnulf I, Comella C, et al. Scales to assess sleep impairment in Parkinson’s disease: critique and recommendations. Mov Disord. 2010;25(16):2704–2716. doi:10.1002/mds.23190
26. Arand DL, Bonnet MH. The multiple sleep latency test. Handb Clin Neurol. 2019;160:393–403.
27. Zwyghuizen-Doorenbos A, Roehrs T, Schaefer M, Roth T. Test-retest reliability of the MSLT. Sleep. 1988;11(6):562–565. doi:10.1093/sleep/11.6.562
28. Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28(1):113–121. doi:10.1093/sleep/28.1.113
29. Gerbin M, Viner AS, Louis ED. Sleep in essential tremor: a comparison with normal controls and Parkinson’s disease patients. Parkinsonism Relat Disord. 2012;18(3):279–284. doi:10.1016/j.parkreldis.2011.11.004
30. Bjornara KA, Dietrichs E, Toft M. Clinical features associated with sleep disturbances in Parkinson’s disease. Clin Neurol Neurosurg. 2014;124:37–43. doi:10.1016/j.clineuro.2014.06.027
31. Louter M, van der Marck MA, Pevernagie DA, et al. Sleep matters in Parkinson’s disease: use of a priority list to assess the presence of sleep disturbances. Eur J Neurol. 2013;20(2):259–265. doi:10.1111/j.1468-1331.2012.03836.x
32. Wang G, Wan Y, Cheng Q, et al. Malnutrition and associated factors in Chinese patients with Parkinson’s disease: results from a pilot investigation. Parkinsonism Relat Disord. 2010;16(2):119–123. doi:10.1016/j.parkreldis.2009.08.009
33. Suzuki K, Miyamoto T, Miyamoto M, et al. Excessive daytime sleepiness and sleep episodes in Japanese patients with Parkinson’s disease. J Neurol Sci. 2008;271(1–2):47–52. doi:10.1016/j.jns.2008.03.008
34. Baiano C, Barone P, Trojano L, Santangelo G. Prevalence and clinical aspects of mild cognitive impairment in Parkinson’s disease: a meta-analysis. Mov Disord. 2020;35(1):45–54. doi:10.1002/mds.27902
35. Gjerstad MD, Alves G, Wentzel-Larsen T, Aarsland D, Larsen JP. Excessive daytime sleepiness in Parkinson disease: is it the drugs or the disease? Neurology. 2006;67(5):853–858. doi:10.1212/01.wnl.0000233980.25978.9d
36. Breen DP, Williams-Gray CH, Mason SL, Foltynie T, Barker RA. Excessive daytime sleepiness and its risk factors in incident Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2013;84(2):233–234. doi:10.1136/jnnp-2012-304097
37. Boddy F, Rowan EN, Lett D, et al. Subjectively reported sleep quality and excessive daytime somnolence in Parkinson’s disease with and without dementia, dementia with Lewy bodies and Alzheimer’s disease. Int J Geriatr Psychiatry. 2007;22(6):529–535. doi:10.1002/gps.1709
38. Videnovic A, Noble C, Reid KJ, et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. 2014;71(4):463–469. doi:10.1001/jamaneurol.2013.6239
39. Prudon B, Duncan GW, Khoo TK, Yarnall AJ, Anderson KN, Anderson KN. Primary sleep disorder prevalence in patients with newly diagnosed Parkinson’s disease. Mov Disord. 2014;29(2):259–262. doi:10.1002/mds.25730
40. Maestri M, Romigi A, Schirru A, et al. Excessive daytime sleepiness and fatigue in neurological disorders. Sleep Breath. 2020;24(2):413–424. doi:10.1007/s11325-019-01921-4
41. Valko PO, Waldvogel D, Weller M, et al. Fatigue and excessive daytime sleepiness in idiopathic Parkinson’s disease differently correlate with motor symptoms, depression and dopaminergic treatment. Eur J Neurol. 2010;17(12):1428–1436. doi:10.1111/j.1468-1331.2010.03063.x
42. Bargiotas P, Lachenmayer ML, Schreier DR, Mathis J, Bassetti CL. Sleepiness and sleepiness perception in patients with Parkinson’s disease: a clinical and electrophysiological study. Sleep. 2019;42(4). doi:10.1093/sleep/zsz004
43. Shen J, Barbera J, Shapiro CM. Distinguishing sleepiness and fatigue: focus on definition and measurement. Sleep Med Rev. 2006;10(1):63–76. doi:10.1016/j.smrv.2005.05.004
44. Cluydts R, De Valck E, Verstraeten E, Theys P. Daytime sleepiness and its evaluation. Sleep Med Rev. 2002;6(2):83–96. doi:10.1053/smrv.2002.0191
45. Cochen De Cock V, Bayard S, Jaussent I, et al. Daytime sleepiness in Parkinson’s disease: a reappraisal. PLoS One. 2014;9(9):e107278. doi:10.1371/journal.pone.0107278
46. Yong MH, Fook-Chong S, Pavanni R, Lim LL, Tan EK. Case control polysomnographic studies of sleep disorders in Parkinson’s disease. PLoS One. 2011;6(7):e22511. doi:10.1371/journal.pone.0022511
47. Goulart FO, Godke BA, Borges V, et al. Fatigue in a cohort of geriatric patients with and without Parkinson’s disease. Braz J Med Biol Res. 2009;42(8):771–775. doi:10.1590/S0100-879X2009000800014
48. Jimenez-Jimenez FJ, Alonso-Navarro H, Garcia-Martin E, Agundez JAG. Sleep disorders in essential tremor: systematic review and meta-analysis. Sleep. 2020;43(9). doi:10.1093/sleep/zsaa039
49. Moreno-López C, Santamaría J, Salamero M, et al. Excessive daytime sleepiness in multiple system atrophy (SLEEMSA Study). Arch Neurol. 2011;68(2). doi:10.1001/archneurol.2010.359
50. Gama RL, Tavora DG, Bomfim RC, et al. Sleep disturbances and brain MRI morphometry in Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy - a comparative study. Parkinsonism Relat Disord. 2010;16(4):275–279. doi:10.1016/j.parkreldis.2010.01.002
51. Zhu K, van Hilten JJ, Marinus J. Course and risk factors for excessive daytime sleepiness in Parkinson’s disease. Parkinsonism Relat Disord. 2016;24:34–40. doi:10.1016/j.parkreldis.2016.01.020
52. Tholfsen LK, Larsen JP, Schulz J, Tysnes OB, Gjerstad MD. Development of excessive daytime sleepiness in early Parkinson disease. Neurology. 2015;85:162–168. doi:10.1212/WNL.0000000000001737
53. Xiang YQ, Xu Q, Sun QY, et al. Clinical features and correlates of excessive daytime sleepiness in Parkinson’s disease. Front Neurol. 2019;10:121. doi:10.3389/fneur.2019.00121
54. Martinez-Martin P, Falup Pecurariu C, Odin P, et al. Gender-related differences in the burden of non-motor symptoms in Parkinson’s disease. J Neurol. 2012;259(8):1639–1647. doi:10.1007/s00415-011-6392-3
55. Amara AW, Chahine LM, Caspell-Garcia C, et al. Longitudinal assessment of excessive daytime sleepiness in early Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2017;88(8):653–662. doi:10.1136/jnnp-2016-315023
56. Shen Y, Shen Y, Dong ZF, et al. Obstructive sleep apnea in Parkinson’s disease: a study in 239 Chinese patients. Sleep Med. 2020;67:237–243. doi:10.1016/j.sleep.2019.11.1251
57. Chotinaiwattarakul W, Dayalu P, Chervin RD, Albin RL. Risk of sleep-disordered breathing in Parkinson’s disease. Sleep Breath. 2011;15(3):471–478. doi:10.1007/s11325-010-0362-3
58. Marinus J, Zhu KD, Marras C, Aarsland D, van Hilten JJ. Risk factors for non-motor symptoms in Parkinson’s disease. Lancet Neurol. 2018;17(6):559–568. doi:10.1016/S1474-4422(18)30127-3
59. Yousaf T, Pagano G, Niccolini F, Politis M. Excessive daytime sleepiness may be associated with caudate denervation in Parkinson disease. J Neurol Sci. 2018;387:220–227. doi:10.1016/j.jns.2018.02.032
60. Verbaan D, van Rooden SM, Visser M, Marinus J, van Hilten JJ. Nighttime sleep problems and daytime sleepiness in Parkinson’s disease. Mov Disorders. 2008;23(1):35–41. doi:10.1002/mds.21727
61. Happe S. Excessive daytime sleepiness and sleep disturbances in patients with neurological diseases. Drugs. 2003;63(24):2725–2737. doi:10.2165/00003495-200363240-00003
62. Adam O, Azher S, Valachovic E, Hahn A, Molho E. Narcolepsy genetic marker HLA DQB1*06:02 and excessive daytime sleepiness in Parkinson disease patients treated with dopaminergic agents. J Neurol. 2021;269:2430–2439. doi:10.1007/s00415-021-10813-1
63. Biglan KM, Holloway RG, McDermott MP, Richard IH; Parkinson Study Group C-PDI. Risk factors for somnolence, edema, and hallucinations in early Parkinson disease. Neurology. 2007;69(2):187–195. doi:10.1212/01.wnl.0000265593.34438.00
64. Videnovic A. Disturbances of sleep and alertness in Parkinson’s disease. Curr Neurol Neurosci Rep. 2018;18(6). doi:10.1007/s11910-018-0838-2
65. Zesiewicz TA, Hauser RA. Sleep attacks and dopamine agonists for Parkinson’s disease - what is currently known? CNS Drugs. 2003;17(8):593–600. doi:10.2165/00023210-200317080-00004
66. Arnulf I, Leu-Semenescu S. Sleepiness in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S101–104. doi:10.1016/S1353-8020(09)70792-8
67. Monaca C, Duhamel A, Jacquesson JM, et al. Vigilance troubles in Parkinson’s disease: a subjective and objective polysomnographic study. Sleep Med. 2006;7(5):448–453. doi:10.1016/j.sleep.2005.12.002
68. Kaynak D, Kiziltan G, Kaynak H, Benbir G, Uysal O. Sleep and sleepiness in patients with Parkinson’s disease before and after dopaminergic treatment. Eur J Neurol. 2005;12(3):199–207. doi:10.1111/j.1468-1331.2004.00971.x
69. Avorn J, Schneeweiss S, Sudarsky LR, et al. Sudden uncontrollable somnolence and medication use in Parkinson disease. Arch Neurol. 2005;62(8):1242. doi:10.1001/archneur.62.8.1242
70. Etminan M, Samii A, Takkouche B, Rochon PA. Increased risk of somnolence with the new dopamine agonists in patients with Parkinson’s disease: a meta-analysis of randomised controlled trials. Drug Saf. 2001;24(11):863–868. doi:10.2165/00002018-200124110-00007
71. Hauser RA, Schapira AH, Barone P, et al. Long-term safety and sustained efficacy of extended-release pramipexole in early and advanced Parkinson’s disease. Eur J Neurol. 2014;21(5):736–743. doi:10.1111/ene.12375
72. Study Group P; Parkinson Study Group. Pramipexole vs levodopa as initial treatment for Parkinson disease: a randomized controlled trial. Parkinson Study Group. JAMA. 2000;284(15):1931–1938. doi:10.1001/jama.284.15.1931
73. Paus S, Brecht HM, Köster J, et al. Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson’s disease. Mov Disorders. 2003;18(6):659–667. doi:10.1002/mds.10417
74. Elmer LW, Surmann E, Boroojerdi B, Jankovic J. Long-term safety and tolerability of rotigotine transdermal system in patients with early-stage idiopathic Parkinson’s disease: a prospective, open-label extension study. Parkinsonism Relat Disord. 2012;18(5):488–493. doi:10.1016/j.parkreldis.2012.01.008
75. Giladi N, Boroojerdi B, Surmann E. The safety and tolerability of rotigotine transdermal system over a 6-year period in patients with early-stage Parkinson’s disease. J Neural Transm. 2013;120(9):1321–1329. doi:10.1007/s00702-013-1001-5
76. LeWitt PA, Boroojerdi B, Surmann E, et al. Rotigotine transdermal system for long-term treatment of patients with advanced Parkinson’s disease: results of two open-label extension studies, CLEOPATRA-PD and PREFER. J Neural Transm. 2013;120(7):1069–1081. doi:10.1007/s00702-012-0925-5
77. Watts RL, Jankovic J, Waters C, et al. Randomized, blind, controlled trial of transdermal rotigotine in early Parkinson disease. Neurology. 2007;68(4):272–276. doi:10.1212/01.wnl.0000252355.79284.22
78. Liguori C, Mercuri NB, Albanese M, et al. Daytime sleepiness may be an independent symptom unrelated to sleep quality in Parkinson’s disease. J Neurol. 2019;266(3):636–641. doi:10.1007/s00415-018-09179-8
79. Pahwa R, Koller WC, Trosch RM, Sherry JH; Investigators APOS. Subcutaneous apomorphine in patients with advanced Parkinson’s disease: a dose-escalation study with randomized, double-blind, placebo-controlled crossover evaluation of a single dose. J Neurol Sci. 2007;258(1–2):137–143. doi:10.1016/j.jns.2007.03.013
80. Prashanth LK, Jaychandran R, Seetharam R, Iyer RB. Apomorphine: the initial Indian experience in relation to response tests and pumps. Ann Indian Acad Neurol. 2020;23(1):20–24. doi:10.4103/aian.AIAN_428_19
81. Hattori N, Nomoto M, Study G. Sustained efficacy of apomorphine in Japanese patients with advanced Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(8):819–823. doi:10.1016/j.parkreldis.2014.04.008
82. Jimenez-Jimenez FJ, Velasco I, De toledo M, et al. Test de latencias múltiples en un paciente con episodios de sueño inducidos por pergolida [Multiple latency test in a patient with episodes of sleep induced by pergolide]. Rev Neurol. 2002;34(12):1140–1141. Spanish.
83. Koller W, Guarnieri M, Hubble J, Rabinowicz AL, Silver D. An open-label evaluation of the tolerability and safety of Stalevo (carbidopa, levodopa and entacapone) in Parkinson’s disease patients experiencing wearing-off. J Neural Transm. 2005;112(2):221–230. doi:10.1007/s00702-004-0184-1
84. Bares M, Kanovský P, Rektor I. Excessive daytime sleepiness and ‘sleep attacks’ induced by entacapone. Fundam Clin Pharmacol. 2003;17(1):113–116. doi:10.1046/j.1472-8206.2003.00120.x
85. Tracik F, Ebersbach G. Sudden daytime sleep onset in Parkinson’s disease: polysomnographic recordings. Mov Disord. 2001;16(3):500–506.
86. O’Suilleabhain PE, Dewey JRB. Contributions of dopaminergic drugs and disease severity to daytime sleepiness in Parkinson disease. Arch Neurol. 2002;59(6):986. doi:10.1001/archneur.59.6.986
87. O’Suilleabhain PE, Dewey RB. Contributions of dopaminergic drugs and disease severity to daytime sleepiness in Parkinson disease. Arch Neurol. 2002;59(6):986–989.
88. Lyons KE, Friedman JH, Hermanowicz N, et al. Orally disintegrating selegiline in Parkinson patients with dopamine agonist-related adverse effects. Clin Neuropharmacol. 2010;33(1):5–10. doi:10.1097/WNF.0b013e3181b7926f
89. Monti JM, Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep Med Rev. 2007;11(2):113–133. doi:10.1016/j.smrv.2006.08.003
90. Rye DB, Jankovic J. Emerging views of dopamine in modulating sleep/wake state from an unlikely source: PD. Neurology. 2002;58(3):341–346. doi:10.1212/WNL.58.3.341
91. Ferreira GM, Thalamas C, Thalamas C, et al. Effect of ropinirole on sleep onset: a randomized placebo controlled study in healthy volunteers. Neurology. 2002;58(3):460–462. doi:10.1212/WNL.58.3.460
92. Hyacinthe C, Barraud Q, Tison F, Bezard E, Ghorayeb I. D1 receptor agonist improves sleep-wake parameters in experimental parkinsonism. Neurobiol Dis. 2014;63:20–24. doi:10.1016/j.nbd.2013.10.029
93. McCarthy AP, Svensson KA, Shanks E, et al. The dopamine D1 receptor positive allosteric modulator mevidalen (LY3154207) enhances wakefulness in the humanized D1 mouse and in sleep-deprived healthy male volunteers. J Pharmacol Exp Ther. 2022;380(3):143–152. doi:10.1124/jpet.121.000719
94. Kaasinen V, Vahlberg T, Stoessl AJ, Strafella AP, Antonini A. Dopamine receptors in Parkinson’s disease: a meta-analysis of imaging studies. Mov Disord. 2021;36(8):1781–1791. doi:10.1002/mds.28632
95. Kang SY, Ryu HS, Sunwoo MK, et al. Sleepiness and depression in Parkinson’s disease patients treated with ropinirole and levodopa. J Mov Disord. 2017;10(3):123–129. doi:10.14802/jmd.17048
96. Pahwa R, Stacy MA, Factor SA, et al. Ropinirole 24-hour prolonged release: randomized, controlled study in advanced Parkinson disease. Neurology. 2007;68(14):1108–1115. doi:10.1212/01.wnl.0000258660.74391.c1
97. Ray Chaudhuri K, Martinez-Martin P, Rolfe KA, et al. Improvements in nocturnal symptoms with ropinirole prolonged release in patients with advanced Parkinson’s disease. Eur J Neurol. 2012;19(1):105–113. doi:10.1111/j.1468-1331.2011.03442.x
98. Rektorova I, Balaz M, Svatova J, et al. Effects of ropinirole on nonmotor symptoms of Parkinson disease: a prospective multicenter study. Clin Neuropharmacol. 2008;31(5):261–266. doi:10.1097/WNF.0b013e31815d25ce
99. Dusek P, Busková J, Růzicka E, et al. Effects of ropinirole prolonged-release on sleep disturbances and daytime sleepiness in Parkinson disease. Clin Neuropharmacol. 2010;33(4):186–190. doi:10.1097/WNF.0b013e3181e71166
100. Eggert K, Öhlwein C, Kassubek J, et al. Influence of the nonergot dopamine agonist piribedil on vigilance in patients with Parkinson disease and excessive daytime sleepiness (PiViCog-PD): an 11-week randomized comparison trial against pramipexole and ropinirole. Clin Neuropharmacol. 2014;37(4):116–122. doi:10.1097/WNF.0000000000000041
101. Newman-Tancredi A, Cussac D, Audinot V, et al. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. II. Agonist and antagonist properties at subtypes of dopamine D(2)-like receptor and alpha(1)/alpha(2)-adrenoceptor. J Pharmacol Exp Ther. 2002;303(2):805–814. doi:10.1124/jpet.102.039875
102. Millan MJ. From the cell to the clinic: a comparative review of the partial D2/D3receptor agonist and α2-adrenoceptor antagonist, piribedil, in the treatment of Parkinson’s disease. Pharmacol Ther. 2010;128(2):229–273. doi:10.1016/j.pharmthera.2010.06.002
103. Millan MJ, Maiofiss L, Cussac D, et al. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther. 2002;303(2):791–804. doi:10.1124/jpet.102.039867
104. Weber F, Dan Y. Circuit-based interrogation of sleep control. Nature. 2016;538(7623):51–59. doi:10.1038/nature19773
105. Dong H, Chen ZK, Guo H, et al. Striatal neurons expressing dopamine D1 receptor promote wakefulness in mice. Curr Biol. 2022;32(3):600–613 e604. doi:10.1016/j.cub.2021.12.026
106. Yuan XS, Wang L, Dong H, et al. Striatal adenosine A2A receptor neurons control active-period sleep via parvalbumin neurons in external globus pallidus. Elife. 2017;6. doi:10.7554/eLife.29055
107. Mehta SH, Pahwa R, Tanner CM, Hauser RA, Johnson R. Effects of gocovri (amantadine) extended release capsules on non-motor symptoms in patients with Parkinson’s disease and dyskinesia. Neurol Ther. 2021;10(1):307–320. doi:10.1007/s40120-021-00246-3
108. Gallazzi M, Mauri M, Bianchi ML, et al. Selegiline reduces daytime sleepiness in patients with Parkinson’s disease. Brain Behav. 2021;11(5):e01880. doi:10.1002/brb3.1880
109. Schrempf W, Fauser M, Wienecke M, et al. Rasagiline improves polysomnographic sleep parameters in patients with Parkinson’s disease: a double-blind, baseline-controlled trial. Eur J Neurol. 2018;25(4):672–679. doi:10.1111/ene.13567
110. Hauser RA, Silver D, Choudhry A, et al. Randomized, controlled trial of rasagiline as an add-on to dopamine agonists in Parkinson’s disease. Mov Disord. 2014;29(8):1028–1034. doi:10.1002/mds.25877
111. Liguori C, Stefani A, Ruffini R, Mercuri NB, Pierantozzi M. Safinamide effect on sleep disturbances and daytime sleepiness in motor fluctuating Parkinson’s disease patients: a validated questionnaires-controlled study. Parkinsonism Relat Disord. 2018;57:80–81. doi:10.1016/j.parkreldis.2018.06.033
112. Santos Garcia D, Labandeira Guerra C, Yanez Bana R, et al. Safinamide improves non-motor symptoms burden in Parkinson’s disease: an open-label prospective study. Brain Sci. 2021;11(3):316. doi:10.3390/brainsci11030316
113. Pedersen NP, Ferrari L, Venner A, et al. Supramammillary glutamate neurons are a key node of the arousal system. Nat Commun. 2017;8(1):1405. doi:10.1038/s41467-017-01004-6
114. Morari M, Brugnoli A, Pisano CA, et al. Safinamide differentially modulates in vivo glutamate and GABA release in the rat hippocampus and basal ganglia. J Pharmacol Exp Ther. 2018;364(2):198–206. doi:10.1124/jpet.117.245100
115. Junho BT, Kummer A, Cardoso F, Teixeira AL, Rocha NP. Clinical predictors of excessive daytime sleepiness in patients with Parkinson’s disease. J Clin Neurol. 2018;14(4):530–536. doi:10.3988/jcn.2018.14.4.530
116. Suzuki K, Okuma Y, Uchiyama T, et al. Characterizing restless legs syndrome and leg motor restlessness in patients with Parkinson’s disease: a multicenter case-controlled study. Parkinsonism Relat Disord. 2017;44:18–22. doi:10.1016/j.parkreldis.2017.08.007
117. Verbaan D, van Rooden SM, van Hilten JJ, Rijsman RM. Prevalence and clinical profile of restless legs syndrome in Parkinson’s disease. Mov Disord. 2010;25(13):2142–2147. doi:10.1002/mds.23241
118. Gomez-Esteban JC, Zarranz JJ, Tijero B, et al. Restless legs syndrome in Parkinson’s disease. Mov Disord. 2007;22(13):1912–1916. doi:10.1002/mds.21624
119. Bhalsing K, Suresh K, Muthane UB, Pal PK. Prevalence and profile of restless legs syndrome in Parkinson’s disease and other neurodegenerative disorders: a case-control study. Parkinsonism Relat Disord. 2013;19(4):426–430. doi:10.1016/j.parkreldis.2012.12.005
120. Peng W, Wu Z, Song K, et al. Regulation of sleep homeostasis mediator adenosine by basal forebrain glutamatergic neurons. Science. 2020;369:6508. doi:10.1126/science.abb0556
121. Rolinski M, Szewczyk-Krolikowski K, Tomlinson PR, et al. REM sleep behaviour disorder is associated with worse quality of life and other non-motor features in early Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2013;85(5):560–566. doi:10.1136/jnnp-2013-306104
122. Simuni T, Caspell-Garcia C, Coffey C, et al. Correlates of excessive daytime sleepiness in de novo Parkinson’s disease: a case control study. Mov Disord. 2015;30(10):1371–1381. doi:10.1002/mds.26248
123. Zhang J-R, Chen J, Yang Z-J, et al. Rapid eye movement sleep behavior disorder symptoms correlate with domains of cognitive impairment in Parkinson’s disease. Chin Med J. 2016;129(4):379–385. doi:10.4103/0366-6999.176077
124. De Cock VC, Vidailhet M, Arnulf I. Sleep disturbances in patients with parkinsonism. Nat Clin Pract Neurol. 2008;4(5):254–266. doi:10.1038/ncpneuro0775
125. Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12(5):443–453. doi:10.1016/S1474-4422(13)70056-5
126. Kotagal V, Albin RL, Muller ML, et al. Symptoms of rapid eye movement sleep behavior disorder are associated with cholinergic denervation in Parkinson disease. Ann Neurol. 2012;71(4):560–568. doi:10.1002/ana.22691
127. Yeh N-C, Tien K-J, Yang C-M, Wang -J-J, Weng S-F. Increased risk of Parkinson’s disease in patients with obstructive sleep apnea. Medicine. 2016;95(2):e2293. doi:10.1097/MD.0000000000002293
128. Crosta F, Desideri G, Marini C. Obstructive sleep apnea syndrome in Parkinson’s disease and other parkinsonisms. Funct Neurol. 2017;32(3):137–141. doi:10.11138/FNeur/2017.32.3.137
129. Li J, Zhao L, Ding X, et al. Obstructive sleep apnea and the risk of Alzheimer’s disease and Parkinson disease: a Mendelian randomization study OSA, Alzheimer’s disease and Parkinson disease. Sleep Med. 2022;97:55–63. doi:10.1016/j.sleep.2022.06.004
130. Lelieveld IM, Müller ML, Bohnen NI, et al. The role of serotonin in sleep disordered breathing associated with Parkinson disease: a correlative [11C]DASB PET imaging study. PLoS One. 2012;7(7):e40166. doi:10.1371/journal.pone.0040166
131. Arnulf I, Konofal E, Merino-Andreu M, et al. Parkinson’s disease and sleepiness: an integral part of PD. Neurology. 2002;58(7):1019–1024. doi:10.1212/WNL.58.7.1019
132. Patel SR. Obstructive sleep apnea. Ann Intern Med. 2019;171(11):ITC81–ITC96. doi:10.7326/AITC201912030
133. Lal C, Weaver TE, Bae CJ, Strohl KP. Excessive daytime sleepiness in obstructive sleep apnea. mechanisms and clinical management. Ann Am Thorac Soc. 2021;18(5):757–768. doi:10.1513/AnnalsATS.202006-696FR
134. Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythms. 2006;21(6):482–493. doi:10.1177/0748730406294627
135. Scammell TE, Arrigoni E, Lipton JO. Neural circuitry of wakefulness and sleep. Neuron. 2017;93(4):747–765. doi:10.1016/j.neuron.2017.01.014
136. Simon DK, Tanner CM, Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med. 2020;36(1):1–12. doi:10.1016/j.cger.2019.08.002
137. Jankovic J, Tan EK. Parkinson’s disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2020;91(8):795–808. doi:10.1136/jnnp-2019-322338
138. Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinson’s disease. Mov Disorders. 2013;28(6):715–724. doi:10.1002/mds.25187
139. Giguère N, Burke Nanni S, Trudeau L-E. On cell loss and selective vulnerability of neuronal populations in Parkinson’s disease. Front Neurol. 2018;9. doi:10.3389/fneur.2018.00455
140. Anichtchik OV, Rinne JO, Kalimo H, Panula P. An altered histaminergic innervation of the substantia nigra in Parkinson’s disease. Exp Neurol. 2000;163(1):20–30. doi:10.1006/exnr.2000.7362
141. Shan L, Bossers K, Luchetti S, et al. Alterations in the histaminergic system in the substantia nigra and striatum of Parkinson’s patients: a postmortem study. Neurobiol Aging. 2012;33(7):1488 e1481–1413. doi:10.1016/j.neurobiolaging.2011.12.026
142. Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR, de Lecea L. VTA dopaminergic neurons regulate ethologically relevant sleep–wake behaviors. Nat Neurosci. 2016;19(10):1356–1366. doi:10.1038/nn.4377
143. Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68(6):1023–1042. doi:10.1016/j.neuron.2010.11.032
144. Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92(3):1087–1187. doi:10.1152/physrev.00032.2011
145. Lu J, Jhou TC, Saper CB. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci. 2006;26(1):193–202. doi:10.1523/JNEUROSCI.2244-05.2006
146. Matsui H, Nishinaka K, Oda M, et al. Disruptions of the fornix fiber in Parkinsonian patients with excessive daytime sleepiness. Parkinsonism Relat Disord. 2006;12(5):319–322. doi:10.1016/j.parkreldis.2006.01.007
147. Kato S, Watanabe H, Senda J, et al. Widespread cortical and subcortical brain atrophy in Parkinson’s disease with excessive daytime sleepiness. J Neurol. 2012;259(2):318–326. doi:10.1007/s00415-011-6187-6
148. Chondrogiorgi M, Tzarouchi LC, Zikou AK, et al. Multimodal imaging evaluation of excessive daytime sleepiness in Parkinson’s disease. Int J Neurosci. 2016;126(5):422–428. doi:10.3109/00207454.2015.1023437
149. Wen MC, Ng SY, Heng HS, et al. Neural substrates of excessive daytime sleepiness in early drug naïve Parkinson’s disease: a resting state functional MRI study. Parkinsonism Relat Disord. 2016;24:63–68. doi:10.1016/j.parkreldis.2016.01.012
150. Happe S, Baier PC, Helmschmied K, et al. Association of daytime sleepiness with nigrostriatal dopaminergic degeneration in early Parkinson’s disease. J Neurol. 2007;254(8):1037–1043. doi:10.1007/s00415-006-0483-6
151. Yoo SW, Oh YS, Ryu DW, et al. Low thalamic monoamine transporter availability is related to excessive daytime sleepiness in early Parkinson’s disease. Neurol Sci. 2020;41(5):1081–1087. doi:10.1007/s10072-019-04206-6
152. Rub U, Del Tredici K, Schultz C, et al. Parkinson’s disease: the thalamic components of the limbic loop are severely impaired by alpha-synuclein immunopositive inclusion body pathology. Neurobiol Aging. 2002;23(2):245–254. doi:10.1016/S0197-4580(01)00269-X
153. Ren S, Wang Y, Yue F, et al. The paraventricular thalamus is a critical thalamic area for wakefulness. Science. 2018;362(6413):429–434. doi:10.1126/science.aat2512
154. Wilson H, Giordano B, Turkheimer FE, Chaudhuri KR, Politis M. Serotonergic dysregulation is linked to sleep problems in Parkinson’s disease. Neuroimage Clin. 2018;18:630–637. doi:10.1016/j.nicl.2018.03.001
155. Pasquini J, Ceravolo R, Brooks DJ, Bonuccelli U, Pavese N. Progressive loss of raphe nuclei serotonin transporter in early Parkinson’s disease: a longitudinal (123) I-FP-CITSPECT study. Parkinsonism Relat Disord. 2020;77:170–175. doi:10.1016/j.parkreldis.2019.03.025
156. Kiyashchenko LI, Mileykovskiy BY, Maidment N, et al. Release of hypocretin (orexin) during waking and sleep states. JNeuroscience. 2002;22:5282–5286.
157. Drouot X, Moutereau S, Nguyen JP, et al. Low levels of ventricular CSF orexin/hypocretin in advanced PD. Neurology. 2003;61(4):540–543. doi:10.1212/01.WNL.0000078194.53210.48
158. Fronczek R, Overeem S, Lee SY, et al. Hypocretin (orexin) loss in Parkinson’s disease. Brain. 2007;130(Pt 6):1577–1585. doi:10.1093/brain/awm090
159. Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain. 2007;130(Pt 6):1586–1595. doi:10.1093/brain/awm097
160. Drouot X, Moutereau S, Lefaucheur JP, et al. Low level of ventricular CSF orexin-A is not associated with objective sleepiness in PD. Sleep Med. 2011;12(9):936–937. doi:10.1016/j.sleep.2011.08.002
161. Compta Y, Santamaria J, Ratti L, et al. Cerebrospinal hypocretin, daytime sleepiness and sleep architecture in Parkinson’s disease dementia. Brain. 2009;132(Pt 12):3308–3317. doi:10.1093/brain/awp263
162. Muller ML, Bohnen NI. Cholinergic dysfunction in Parkinson’s disease. Curr Neurol Neurosci Rep. 2013;13(9):377. doi:10.1007/s11910-013-0377-9
163. Schulz J, Pagano G, Fernandez Bonfante JA, Wilson H, Politis M. Nucleus basalis of Meynert degeneration precedes and predicts cognitive impairment in Parkinson’s disease. Brain. 2018;141(5):1501–1516. doi:10.1093/brain/awy072
164. Jester DJ, Lee S, Molinari V, Volicer L. Cognitive deficits in Parkinson’s disease with excessive daytime sleepiness: a systematic review. Aging Ment Health. 2020;24(11):1769–1780. doi:10.1080/13607863.2019.1660852
165. Goldman JG, Stebbins GT, Leung V, Tilley BC, Goetz CG. Relationships among cognitive impairment, sleep, and fatigue in Parkinson’s disease using the MDS-UPDRS. Parkinsonism Relat Disord. 2014;20(11):1135–1139. doi:10.1016/j.parkreldis.2014.08.001
166. Wen MC, Chan LL, Tan LCS, Tan EK. Mood and neural correlates of excessive daytime sleepiness in Parkinson’s disease. Acta Neurol Scand. 2017;136(2):84–96. doi:10.1111/ane.12704
167. van Rooden SM, Visser M, Verbaan D, Marinus J, van Hilten JJ. Patterns of motor and non-motor features in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2009;80(8):846–850. doi:10.1136/jnnp.2008.166629
168. van der Heeden JF, Marinus J, Martinez-Martin P, van Hilten JJ. Importance of nondopaminergic features in evaluating disease severity of Parkinson disease. Neurology. 2014;82(5):412–418. doi:10.1212/WNL.0000000000000087
169. Galts CPC, Bettio LEB, Jewett DC, et al. Depression in neurodegenerative diseases: common mechanisms and current treatment options. Neurosci Biobehav Rev. 2019;102:56–84. doi:10.1016/j.neubiorev.2019.04.002
170. Rinaldi F, Himwich HE. Alerting responses and actions of atropine and cholinergic drugs. AMA Arch Neurol Psychiatry. 1955;73(4):387–395. doi:10.1001/archneurpsyc.1955.02330100019005
171. Semba K, Fibiger HC. Organization of central cholinergic systems. Prog Brain Res. 1989;79:37–63.
172. Liu AK, Chang RC, Pearce RK, Gentleman SM. Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. 2015;129(4):527–540. doi:10.1007/s00401-015-1392-5
173. Weaver DR. The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythms. 1998;13(2):100–112. doi:10.1177/074873098128999952
174. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi:10.1146/annurev-neuro-060909-153128
175. Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol. 2020;21(2):67–84. doi:10.1038/s41580-019-0179-2
176. Breen DP, Vuono R, Nawarathna U, et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014;71(5):589–595. doi:10.1001/jamaneurol.2014.65
177. Videnovic A, Klerman EB, Wang W, et al. Timed light therapy for sleep and daytime sleepiness associated with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2017;74(4):411–418. doi:10.1001/jamaneurol.2016.5192
178. Videnovic A. Management of sleep disorders in Parkinson’s disease and multiple system atrophy. Mov Disorders. 2017;32(5):659–668. doi:10.1002/mds.26918
179. Weerkamp NJ, Tissingh G, Poels PJ, et al. Nonmotor symptoms in nursing home residents with Parkinson’s disease: prevalence and effect on quality of life. J Am Geriatr Soc. 2013;61(10):1714–1721. doi:10.1111/jgs.12458
180. Ghorayeb I, Loundou A, Auquier P, et al. A nationwide survey of excessive daytime sleepiness in Parkinson’s disease in France. Mov Disord. 2007;22(11):1567–1572. doi:10.1002/mds.21541
181. Havlikova E, van Dijk JP, Nagyova I, et al. The impact of sleep and mood disorders on quality of life in Parkinson’s disease patients. J Neurol. 2011;258(12):2222–2229. doi:10.1007/s00415-011-6098-6
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.