Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Examination of the Relationship Between Metabolic Syndrome and Obstructive Sleep Apnea in Iranian Patients with Type 2 Diabetes: A Case–Control Study
Authors Sharifpour P, Dehvan F, Dalvand S, Ghanei Gheshlagh R
Received 11 May 2020
Accepted for publication 17 June 2020
Published 26 June 2020 Volume 2020:13 Pages 2251—2257
DOI https://doi.org/10.2147/DMSO.S260677
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Pershang Sharifpour,1 Fazel Dehvan,2 Sahar Dalvand,3 Reza Ghanei Gheshlagh2
1Student Research Committee, Kurdistan University of Medical Sciences, Sanandaj, Iran; 2Clinical Care Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran; 3Department of Epidemiology and Biostatistics, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
Correspondence: Reza Ghanei Gheshlagh
Clinical Care Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran
Tel +98 9144050284
Email [email protected]
Introduction: Obstructive sleep apnea (OSA) is a common risk factor for metabolic syndrome (MS) that increases the chance of cardiovascular disease, stroke, and mortality. Many studies have been conducted on this matter, but the results are still conflicting. The aim of the present study was to examine the relationship between metabolic syndrome (MS) and obstructive sleep apnea (OSA) in Iranian patients with type 2 diabetes (T2D).
Patients and Methods: This matched case–control study was conducted with 190 patients with T2D in Sanandaj, Iran. The data were selected using the demographic questionnaire, clinical and anthropometric measures, the Berlin Questionnaire (BQ), and the National Cholesterol Education Program’s Adult Treatment Panel III (NCEP-ATP III). Participants were divided into two groups of high risk of OSA (experimental) and low risk of OSA (control). The data were analyzed using Stata, version 14.
Results: The frequency of MS was higher in the OSA group than the control group (81.1% vs 70.5%), but the group difference was not statistically significant (p=0.127). The results of crude and age-sex adjusted logistic regression analysis revealed no significant association between OSA and the other variables under study (P> 0.05). Sensitivity analysis and external adjustment for BMI showed no significant relationship between OSA and the other variables under study (P=0.319).
Conclusion: In the present study, no significant association was found between metabolic syndrome (MS) and obstructive sleep apnea (OSA) in patients with T2D; therefore, more studies should be conducted on this subject.
Keywords: diabetes, sleep apnea, metabolic syndrome
Introduction
The high prevalence of diabetes and its complications has become a major global health issue.1 Currently, about 425 million people around the world suffer from diabetes, and this figure is projected to grow to 629 million people by 2045.2 There is an increase in the prevalence of diabetes in Iran, and it is projected that 7 million people in this country will have diabetes by 2030.3 Diabetes is accompanied by various complications, including great vessels problems (eg, cardiovascular disease), small vessels problems (eg, retinopathy), and other complications, such as skeletal, digestive, liver, and cognitive problems.4,5 It has also been shown that OSA in diabetic patients puts them at higher risk for major adverse cardiac and cerebrovascular events (MACCE) and cardiovascular mortality.6
It is a treatable chronic sleep disorder that is characterized by recurrent episodes of partial (hypopnea) or complete (apnea) collapse of the upper airway, leading to transitory episodes of hypercapnia, repeated micro-awakenings, and desaturation–reoxygenation sequences.7,8 OSA is related to poor glycemic control,9–11 high blood pressure,12 and lower quality of life.13,14 Some researchers maintain that OSA is not just a sleep disorder, but a heterogeneous metabolic disorder related to some other disorders, including fatty liver disease,15–17 hypothalamic-pituitary-adrenal (HPA) axis dysfunction, endothelial dysfunction, neurological disorders, reduced cognitive capacity, depression, elevated inflammatory markers such as C-Reactive Protein (CRP) and interleukin-6, intensification of tissue hypoxia, higher risk of systematic inflammations, heart failure, dysregulation of cardiac metabolism, dysrhythmia, cardiac conduction disturbances, atrial enlargement, and finally death.18–23
One of the disorders considered to be related to OSA is metabolic syndrome (MS) that is a collection of risk factors, including central obesity, hypertension (HTN), insulin resistance, and dyslipidemia.24,25 MS has a high prevalence throughout the world, and it is projected that half of the world’s population will be struggling with that in the coming years.26–28 According to previous reports, MS has prevalence rates of 20–40% and 29% in the world and Iran, respectively.29,30,31 MS increases the risk of cardiovascular disease by 78%, diabetes by 500%, stroke by 75%, and mortality by 35%.32–34
The relationship between OSA and MS is still debatable, and previous studies have reported conflicting results.35–37 Some studies have found an association between OSA and MS,38–47 while others have failed to find a relationship between the two variables or have found associations between OSA and some components of MS.48–50 The relationship between OSA and MS is important because the co-occurrence of these two conditions in patients with type 2 diabetes (T2D) leads to more severe diabetes complications, increased blood pressure, higher lipid levels, increased c reactive protein levels, and reduced sleep quality,37,48-51 all of which are risk factors for cardiovascular disease. Given the conflicting results on the relationship between the two variables, the goal of the present study is to examine the relationship between MS and OSA among patients with T2D attending the diabetes unit of Tohid Hospital in Sanandaj in 2019.
Patients and Methods
Study Participants
This is a matched case–control study. The participants included 190 patients with T2D who were selected using a convenience sampling method among patients attending the diabetes unit of Tohid Hospital in Sanandaj in 2019. The cases were with OSA, and the controls were without this condition. The inclusion criteria were as follows: having a medical record in the diabetes unit and the ability to communicate. The exclusion criteria included lack of interest to continue the study and incomplete questionnaires.
Data Collection
After receiving the approval of the ethics committee at Kurdistan University of Medical Sciences, the researchers attended the diabetes unit and explained the study objective and procedures to the participants and acquired their informed consents to participate in the study. For diabetic patients receiving service from the diabetes unit, medical tests are done every 3 months, and the results are recorded in medical records. Therefore, the results of the most recent lipid and blood sugar tests were extracted from the medical records and recorded in the questionnaires. In order to ensure confidentiality, medical record numbers were put on top of the questionnaires instead of participants’ real names. In addition to medical record number, demographic information, including age, gender, marital status, place of residence, insurance status, education, duration of diabetes, medications used, and anthropometric measurements, including waist circumference, height, weight, BMI, and blood pressure were recorded. Waist circumference was measured at the level of the umbilicus using an inflexible measuring tape, with the participant in a standing position and wearing light clothing. BMI was calculated by dividing weight by height square. In addition, blood pressure was measured using Beurer BC40 Wrist Blood Pressure Monitor after 5 mins resting in the sitting position.
OSA Questionnaire
Although polysomnography is often the gold standard in the diagnosis of OSA, it is not widely used due to being costly and time-consuming.43 In the present study, due to lack of access to a sleep clinic, the Berlin Questionnaire was used to screen OSA. Different instruments are available to screen sleep apnea used in different studies. After a review of the literature to find the best screening tool for sleep apnea, we decided to use the Berlin Questionnaire. In a study among Iranian patients, Amra et al (2018) compared different screening tools for sleep apnea and found that the Berlin questionnaire had higher sensitivity than the STOPBANG.52 A systematic review by Abrishami et al (2010) also indicated that the Berlin questionnaire had higher sensitivity than the STOPBAN.53 Berlin Questionnaire has 10 items assessing three domains, including snoring (items 1–5), daytime sleepiness (items 6–9), and hypertension or a high BMI (item 10).43,54 The researcher read the items for the illiterate participants and recorded their answers in the questionnaire. Based on the completed questionnaires, participants were divided into two groups of “at a high risk for OSA (cases)” and “at a low risk for OSA”. The sample included 95 patients who were matched in gender and BMI. The validity of the questionnaire in Iran has been shown by Ghanei Gheshlagh et al (2011).39 Before the main sampling, the Berlin questionnaire was administered to a sample of 20 diabetic patients in Sanandaj (who were not included in the main study) in order to examine the reliability and internal consistency (using the Cronbach’s alpha coefficient). The internal consistency of the questionnaire using the Cronbach’s alpha coefficient was found to be 0.73.
Metabolic Syndrome: Diagnostic Criteria
The National Cholesterol Education Program Adult Treatment Panel guidelines III (ATP III) was used to assess MS, according to which MS is diagnosed if at least three of the following criteria are met: hyperglycemia (fasting blood sugar level ≥110 mg/dl), dyslipidemia (triglyceride (TG) level above 150 mg/dl, high-density lipoprotein (HDL) cholesterol level below 40 mg/dl in men and below 50 mg/dl in women), obesity (waist circumference ≥102 cm in men and ≥88 in women), and blood pressure ≥130/85 mmHg.55
Data Analysis
Descriptive statistics, including frequency, frequency percentage, mean, and standard deviation, were used to describe the data. Qualitative variables were described by frequency and frequency percentage, and were compared between the OSA and control groups using the Chi-squared test. The relationship between OSA and other variables under study was examined by the crude and age-sex adjusted of odds ratio and their 95% confidence intervals using logistic regression as well as penalized logistic regression. In order to ensure the stability of the results, sensitivity analysis with additional adjustment for the MBI variable was conducted. All the analyses were performed using Stata, version 14.
Results
Participants with and without OSA were matched in gender and age (±5). There was no significant difference between the two groups in demographic variables and components of MS. Duration of diabetes was 13.2±9.1 years and 11.4±8.9 years in the experimental and control groups, respectively (p=0.183). Further details are provided in Tables 1 and 2.
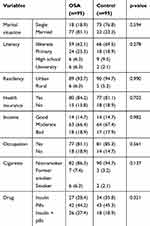 |
Table 1 Demographic Characteristics |
 |
Table 2 Components of Metabolic Syndrome |
In order to examine the association of OSA with MS and other variables under study, the logistic regression analysis was conducted, and the crude rate of odds ratio was calculated for each variable (Table 3). In the next step, variables with p-values below 0.2 were included in the multivariate regression analysis.
 |
Table 3 Association Between OSA and Other Variables Under Study Analyzed Using the Univariate Regression Analysis |
In the multivariate analysis using the crude and age-sex adjusted of odds ratio and their 95% confidence intervals, the relationship of OSA with MS and the other study variables was examined using the ordinal logistic regression and the penalized likelihood method for logistic regression. In order to ensure the stability of the results, sensitivity analysis with additional adjustment for the BMI variable was conducted. In the multivariate analysis, no significant association was found between OSA and MS with adjustment for age and sex; this was also true for the other variables present in the model. In the sensitivity analysis (with adjustment for age, gender, and BMI) no significant association was found between OSA and MS and also between OSA and the other variables present in the model (p-value >0.05) (Table 4).
 |
Table 4 Multivariable Regression Logistic |
Discussion
The results showed no significant difference between the two variables; this finding is consistent with those of studies conducted in Turkey and Greece.49,56 Chin et al (2010) also found no significant association between the severity of OSA and MS after adjusting for age and BMI.48 This finding can be attributed to certain aspects of diabetes. In other words, due to overlapping characteristics, both MS and OSA can independently lead to diabetes.
In contrast to our finding, some other studies have found a significant relationship between MS and OSA.38,40,41,45 This relationship can be attributed to intermittent hypoxia and oxidative stress due to OSA that leads to resistance to insulin, dyslipidemia, and hypertension through secretion of inflammatory cytokines, tumor necrosis factor (TNF), and interleukin 6.57–60 On the other hand, lipid deposition occurs in the pharyngeal tissue and is narrowed in the area of upper airway collapse.61 In addition, there is a strong overlap between the pathophysiology of MS and that of OSA.62 Moreover, Leon et al reported a positive association between lung function impairment and MS.63 Contracting findings from previous studies can be attributed to the use of samples with different demographic and clinical characteristics and the use of different instruments to measure MS and OSA. In the present study, no significant difference was found between diabetic patients with and without OSA in MS; this finding is in line with those of Trombetta et al (2010) and Ahbab et al (2013).50,56 Given that the study participants had diabetes, the difference between the experimental and control groups in blood glucose levels can be considered noteworthy. However, other studies have found higher levels of blood sugar,37,49 systolic blood pressure,33,64 waist circumference,54 and triglyceride in patients with OSA than those without this condition. In Drager’s study, all components of MS, except for waist circumference and HDL, were higher in patients with OSA than those without this condition.37 Wang et al (2019) also found that all components of MS, except for diastolic blood pressure and HDL, were higher in the group with OSA than the control group.17
One of the limitations of the present study was the lack of access to polysomnography as the golden standard for OSA diagnosis. In addition, patients with OSA may not be aware of their problem, and this may have affected their answers to the items of the questionnaires. Moreover, given that the study was conducted with patients with T2D, caution should be taken in generalizing the results in the general population or other populations of patients. We suggest future studies to use polysomnography to screen OSA or use it along with other tools.
Conclusion
In the present study, no significant relationship was found between metabolic syndrome (MS) and its components in patients with T2D; thus, it seems necessary to conduct more studies on this subject. In addition, medical teams should pay special attention to the presence of these two conditions, whether together or independently, in patients with diabetes, because it leads to an increased chance of morbidity and mortality in the patients.
Acknowledgments
The researchers would like to express their gratitude to the Deputy of Research of the Kurdistan University of Medical Sciences for acceptance, and approval of this research project. We also thank all the diabetic patients and staff of Sanandaj Diabetes Unit.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi:10.1038/nrendo.2017.151
2. Gościk J, Bauer W, Wawrusiewicz-Kurylonek N, et al. Efficacy of family history, genetic risk score, and physical activity in assessing the prevalence of type 2 diabetes. Polish Arch Internal Med. 2019;129(7–8):442–450. doi:10.20452/pamw.14866
3. Fathabadi J, Izaddost M, Taghavi M, Shalani B, Sadeghi S. The role of irrational health beliefs, health locus of control and health-oriented lifestyle in predicting the risk of diabetes. Payesh. 2018;17(2):169–178.
4. Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60(4):207–221. doi:10.3322/caac.20078
5. Sanz-Nogués C, Mustafa M, Burke H, O’Brien T. Knowledge, Perceptions and Concerns of Diabetes-Associated Complications Among Individuals Living with Type 1 and Type 2 Diabetes Mellitus. In: Healthcare: 2020. Multidisciplinary Digital Publishing Institute; 2020:25–37.
6. Fallahi A, Jamil DI, Karimi EB, Baghi V, Ghanei Gheshlagh R. Prevalence of obstructive sleep apnea in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metabol Syndr. 2019;13(4):2463–2468. doi:10.1016/j.dsx.2019.06.030
7. Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest. 2017;152(5):1070–1086. doi:10.1016/j.chest.2017.05.009
8. Borel AL. Sleep apnea and sleep habits: relationships with metabolic syndrome. Nutrients. 2019;11(11):2628. doi:10.3390/nu11112628
9. Tahrani AA. Obstructive sleep apnoea and vascular disease in patients with type 2 diabetes. Eur Endocrinol. 2015;11(2):81–89. doi:10.17925/EE.2015.11.02.81
10. Tahrani AA. Obstructive sleep apnoea in diabetes: does it matter? Diabetes Vasc Dis Res. 2017;14(5):454–462. doi:10.1177/1479164117714397
11. Tahrani AA, Ali A, Stevens MJ. Obstructive sleep apnoea and diabetes: an update. Curr Opin Pulm Med. 2013;19(6):631–638. doi:10.1097/MCP.0b013e3283659da5
12. Pepperell JCT, Davies RJO, Stradling JR. Systemic hypertension and obstructive sleep apnoea. Sleep Med Rev. 2002;6(3):157–173. doi:10.1053/smrv.2001.0189
13. De Backer W. Obstructive sleep apnea/hypopnea syndrome. Panminerva Med. 2013;55(2):191–195.
14. Lopes C, Esteves AM, Bittencourt LRA, Tufik S, Mello MT. Relationship between the quality of life and the severity of obstructive sleep apnea syndrome. Braz J Med Biol Res. 2008;41(10):908–913. doi:10.1590/S0100-879X2008005000036
15. Alam I, Lewis K, Stephens J, Baxter J. Obesity, metabolic syndrome and sleep apnoea: all pro-inflammatory states. Obesity Rev. 2007;8(2):119–127. doi:10.1111/j.1467-789X.2006.00269.x
16. Gileles-Hillel A, Kheirandish-Gozal L, Gozal D. Biological plausibility linking sleep apnoea and metabolic dysfunction. Nat Rev Endocrinol. 2016;12(5):290. doi:10.1038/nrendo.2016.22
17. Wang F, Xiong X, Xu H, et al. The association between obstructive sleep apnea syndrome and metabolic syndrome: a confirmatory factor analysis. Sleep Breathing. 2019;1–9.
18. Chien MY, Lee P, Tsai YF, Yang PC, Wu YT. C-reactive protein and heart rate recovery in middle-aged men with severe obstructive sleep apnea. Sleep Breathing. 2012;16(3):629–637. doi:10.1007/s11325-011-0549-2
19. Dimitri H, Ng M, Brooks AG, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm. 2012;9(3):321–327. doi:10.1016/j.hrthm.2011.10.017
20. Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62(7):569–576. doi:10.1016/j.jacc.2013.05.045
21. Gaines J, Vgontzas AN, Fernandez-Mendoza J, Bixler EO. Obstructive sleep apnea and the metabolic syndrome: the road to clinically-meaningful phenotyping, improved prognosis, and personalized treatment. Sleep Med Rev. 2018;42:211–219. doi:10.1016/j.smrv.2018.08.009
22. Heffner J, Rozenfeld Y, Kai M, Stephens E, Brown L. Prevalence of diagnosed sleep apnea among patients with Type 2 diabetes in primary care. Chest. 2012;141(6):1415–1421. doi:10.1378/chest.11-1945
23. Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105(21):2462–2464. doi:10.1161/01.CIR.0000018948.95175.03
24. Alberti K, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–1645. doi:10.1161/CIRCULATIONAHA.109.192644
25. Alberti KGM, Zimmet P, Shaw J. The metabolic syndrome - a new worldwide definition. The Lancet. 2005;366(9491):1059–1062. doi:10.1016/S0140-6736(05)67402-8
26. Batsis J, Nieto‐Martinez R, Lopez‐Jimenez F. Metabolic syndrome: from global epidemiology to individualized medicine. Clin Pharmacol Ther. 2007;82(5):509–524. doi:10.1038/sj.clpt.6100355
27. Dzherieva I, Volkova N, Panfilova N. Depressive disorders in males with metabolic syndrome. J Biomed Clin Res. 2011;4(1):46–49.
28. Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–689. doi:10.2337/diacare.24.4.683
29. Dalvand S, Niksima SH, Meshkani R, et al. Prevalence of metabolic syndrome among Iranian population: a systematic review and meta-analysis. Iran J Public Health. 2017;46(4):456–467.
30. McCullough AJ. Epidemiology of the metabolic syndrome in the USA. J Dig Dis. 2011;12(5):333–340. doi:10.1111/j.1751-2980.2010.00469.x
31. Pan WH, Yeh WT, Weng LC. Epidemiology of metabolic syndrome in Asia. Asia Pac J Clin Nutr. 2008;17:37–42.
32. Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49(4):403–414. doi:10.1016/j.jacc.2006.09.032
33. Ghanei Geshlagh R, Nourouzi-Tabrizi K, Shabani F, Zahednezhad H. The survey on relationship of metabolic syndrome and obstructive sleep apnea in oldermans with cardiovascular diseases. Azad Univ Med Sci. 2016;26(1):46–51.
34. Tanner JM, Chang TI, Harada ND, Santiago SM, Weinreb JE, Friedlander AH. Prevalence of comorbid obstructive sleep apnea and metabolic syndrome: syndrome Z and maxillofacial surgery implications. J Oral Maxillofacial Surg. 2012;70(1):179–187. doi:10.1016/j.joms.2011.01.012
35. Erdim I, Akcay T, Yilmazer R, Erdur O, Kayhan FT. Is metabolic syndrome associated with obstructive sleep apnea in obese adolescents? J Clin Sleep Med. 2015;11(12):1371–1376. doi:10.5664/jcsm.5266
36. Lam JCM, Mak JCW, Ip MSM. Obesity, obstructive sleep apnoea and metabolic syndrome. Respirology. 2012;17(2):223–236. doi:10.1111/j.1440-1843.2011.02081.x
37. Drager LF, Lopes HF, Maki-Nunes C, et al. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS One. 2010;5:8. doi:10.1371/journal.pone.0012065
38. Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25(9):735–741. doi:10.1016/j.ehj.2004.02.021
39. Ghanei Gheshlagh R, Ghoci S, Hemmati Maslak Pak M. Sleep apnea and metabolic syndrome in hemodialysis patients. J Urmia Univ Med Sci. 2011;22(4):339–345.
40. Gruber A, Horwood F, Sithole J, Ali N, Idris I. Obstructive sleep apnoea is independently associated with the metabolic syndrome but not insulin resistance state. Cardiovasc Diabetol. 2006;5(1):22. doi:10.1186/1475-2840-5-22
41. Lam JC, Lam B, Lam CL, et al. Obstructive sleep apnea and the metabolic syndrome in community-based Chinese adults in Hong Kong. Respir Med. 2006;100(6):980–987. doi:10.1016/j.rmed.2005.10.003
42. Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3(05):467–472. doi:10.5664/jcsm.26910
43. Qu XX, Esangbedo IC, Zhang XJ, et al. Obstructive sleep apnea syndrome is associated with metabolic syndrome among adolescents and youth in Beijing: data from Beijing child and adolescent metabolic syndrome study. Chin Med J. 2015;128(17):2278. doi:10.4103/0366-6999.163394
44. Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med. 2007;176(4):401–408. doi:10.1164/rccm.200703-375OC
45. Sasanabe R, Banno K, Otake K, et al. Metabolic syndrome in Japanese patients with obstructive sleep apnea syndrome. Hypertension Res. 2006;29(5):315–322. doi:10.1291/hypres.29.315
46. Savransky V, Jun J, Li J, et al. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ Res. 2008;103(10):1173–1180. doi:10.1161/CIRCRESAHA.108.178533
47. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi:10.1164/rccm.2109080
48. Chin K, Oga T, Takahashi K-I, et al. Associations between obstructive sleep apnea, metabolic syndrome, and sleep duration, as measured with an actigraph, in an urban male working population in Japan. Sleep. 2010;33(1):89–95. doi:10.1093/sleep/33.1.89
49. Papanas N, Steiropoulos P, Nena E, et al. Predictors of obstructive sleep apnea in males with metabolic syndrome. Vasc Health Risk Manage. 2010;6:281.
50. Trombetta IC, Somers VK, Maki-Nunes C, et al. Consequences of comorbid sleep apnea in the metabolic syndrome – implications for cardiovascular risk. Sleep. 2010;33(9):1193–1199. doi:10.1093/sleep/33.9.1193
51. Grassi G, Seravalle G, Quarti-Trevano F, et al. Reinforcement of the adrenergic overdrive in the metabolic syndrome complicated by obstructive sleep apnea. J Hypertens. 2010;28(6):1313–1320. doi:10.1097/HJH.0b013e328337a9fd
52. Amra B, Javani M, Soltaninejad F, et al. Comparison of Berlin questionnaire, STOP-Bang, and Epworth sleepiness scale for diagnosing obstructive sleep apnea in Persian patients. Int J Prev Med. 2018;9.
53. Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anesthesia/Journal Canadien D’anesthésie. 2010;57(5):423–438. doi:10.1007/s12630-010-9280-x
54. Cepeda FX, Virmondes L, Rodrigues S, et al. Identifying the risk of obstructive sleep apnea in metabolic syndrome patients: diagnostic accuracy of the Berlin Questionnaire. PLoS One. 2019;14(5):e0217058. doi:10.1371/journal.pone.0217058
55. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735–2752. doi:10.1161/CIRCULATIONAHA.105.169404
56. Ahbab S, Ataoğlu HE, Tuna M, et al. Neck circumference, metabolic syndrome and obstructive sleep apnea syndrome; evaluation of possible linkage. Med Sci Monitor. 2013;19:111. doi:10.12659/MSM.883776
57. Lavie P, Lavie L. Unexpected survival advantage in elderly people with moderate sleep apnoea. J Sleep Res. 2009;18(4):397–403. doi:10.1111/j.1365-2869.2009.00754.x
58. Leinum CJ, Dopp JM, Morgan BJ. Sleep‐disordered breathing and obesity: pathophysiology, complications, and treatment. Nutri Clin Pract. 2009;24(6):675–687. doi:10.1177/0884533609351532
59. Li J, Nanayakkara A, Jun J, Savransky V, Polotsky VY. Effect of deficiency in SREBP cleavage-activating protein on lipid metabolism during intermittent hypoxia. Physiol Genomics. 2007;31(2):273–280. doi:10.1152/physiolgenomics.00082.2007
60. Xu S, Wan Y, Xu M, et al. The association between obstructive sleep apnea and metabolic syndrome: a systematic review and meta-analysis. BMC Pulm Med. 2015;15(1):105. doi:10.1186/s12890-015-0102-3
61. Theorell-Haglöw J, Berne C, Janson C, Lindberg E. The role of obstructive sleep apnea in metabolic syndrome: a population-based study in women. Sleep Med. 2011;12(4):329–334. doi:10.1016/j.sleep.2010.06.014
62. Calvin AD, Albuquerque FN, Lopez-Jimenez F, Somers VK. Obstructive sleep apnea, inflammation, and the metabolic syndrome. Metab Syndr Relat Disord. 2009;7(4):271–277. doi:10.1089/met.2008.0093
63. Leone N, Courbon D, Thomas F, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179(6):509–516. doi:10.1164/rccm.200807-1195OC
64. Lin Q-C, Zhang X-B, Chen G-P, Huang D-Y, Din H-B, Tang A-Z. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome in nonobese adults. Sleep Breathing. 2012;16(2):571–578. doi:10.1007/s11325-011-0544-7
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
