Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Evidence to Date: Clinical Utility of Tremelimumab in the Treatment of Unresectable Hepatocellular Carcinoma
Authors Ahmed Z, Lee SS, Victor DW 3rd, Kodali S
Received 17 April 2023
Accepted for publication 7 October 2023
Published 27 October 2023 Volume 2023:10 Pages 1911—1922
DOI https://doi.org/10.2147/JHC.S395080
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr David Gerber
Zunirah Ahmed,1 Sunyoung S Lee,2 David W Victor 3rd,1,3 Sudha Kodali1,3
1Department of Medicine, Division of Gastroenterology and Hepatology, Houston Methodist Hospital, Houston, TX, USA; 2Department of Gastrointestinal (GI) Medical Oncology, the University of Texas MD Anderson Cancer Center, Houston, TX, USA; 3Sherrie and Alan Conover Center for Liver Disease and Transplantation, Houston Methodist Hospital, Houston, TX, USA
Correspondence: David W Victor, 3 rd, Sherrie and Alan Conover Center for Liver Disease and Transplantation, Houston Methodist Hospital, 6445 Main, Suite OPC 22.001, Houston, TX, 77030, USA, Email [email protected]
Abstract: Hepatocellular carcinoma (HCC) is a leading cause of cancer-related deaths worldwide and is associated with significant health care costs and burden. Management of HCC is guided based on the Barcelona Clinic Liver Cancer (BCLC) system and includes liver transplantation, surgical resection, and liver-directed and systemic therapies. In recent years, there have been significant advancements in understanding the immunogenicity of HCC and this has led to approval of different targeted agents as well as immunotherapy for advanced HCC. Tremelimumab is a cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) blocking antibody and has recently been approved in combination with durvalumab (a programmed death-ligand 1 [PDL1] inhibitor) as first-line therapy for advanced (Barcelona Clinic Liver Cancer Stage C) HCC. In this article, we review the different available systemic therapies for advanced HCC with special focus on the clinical utility of tremelimumab for the treatment of unresectable HCC.
Keywords: unresectable hepatocellular carcinoma, immunotherapy, CTLA4 antibody, targeted therapy
Introduction
Hepatocellular carcinoma (HCC) accounts for 85–90% of primary liver cancers1 and is associated with significant health care cost and burden. It is emerging as one of the leading causes of cancer-related deaths worldwide and is currently estimated to be the sixth most common cause of cancer-related deaths across the globe.2 If these rising trends in incidence continue, it is projected that HCC will become the third leading cause of cancer-related death by 2030.3 The majority of HCC cases (70–90%) develop in patients with chronic hepatic inflammation and cirrhosis;4 however, de novo disease in non-cirrhotic patients can also occur.5 Metabolic dysfunction-associated steatotic liver disease (MASLD) is largely replacing chronic viral hepatitis (Hepatitis B and C) as an important risk factor for development of HCC.6,7
HCC management is based on its stage at presentation and Barcelona Clinic Liver Cancer (BCLC) system has been frequently adopted in clinical practice as an effective means of guiding therapy and for prognostication.8 The treatment options available for HCC include liver transplantation, surgical resection, liver-directed therapy (locoregional therapy directed to the liver), systemic therapy (including immunotherapy), and combinations thereof. Liver transplantation, radiofrequency and microwave ablation, and resection are the only curative options and preferred modalities in patients with early-stage tumors given better outcomes.9 Resection is a first-line treatment for HCC, but patients may be unresectable due to tumor location or burden, or advanced cirrhosis.10 Patients with intermediate-stage disease are candidates for liver-directed therapies like transarterial chemoembolization (TACE) and transarterial radioembolization (TARE). Systemic therapies are primarily reserved for patients with advanced disease.11 Prognosis of untreated HCC is poor, with a 3-year survival rate of 12.7% and median survival of 9 months.12
Tumor progression in HCC is complex and is mediated by liver antigen tolerance, HCC-dependent immune tolerance, and chronic inflammation-dependent immune suppression.13 Liver antigen tolerance is determined by inhibitory interleukin 10 (IL-10) released by Kupffer cells and tumor growth factor beta (TGF-β) released by Kupffer cells and liver sinusoidal endothelial cells (LSEC). There is also upregulation of immune checkpoints via programmed death-ligand 1 or 2 (PD-L1 or PD-L2) in hepatic stellate cells, LSEC, and intrahepatic leucocytes. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) also acts on T regulatory cells (Tregs) to downregulate the immune response.13 These mechanisms help protect the liver from autoimmune damage by blocking activation of effector T cells under physiological conditions. However, in HCC, these mechanisms turn out to be detrimental by contributing to the antigen immune escape in cancer-bearing hosts. Chronic hepatic inflammation promotes Treg activity14 in addition to secretion of IL-1015 and TGF- β,16 which results in T cell exhaustion and inhibition of antigen presentation.17
The treatment landscape for unresectable HCC has evolved in recent years as multiple agents, including both targeted therapy and immunotherapy agents, have been approved. The recognition of immunogenicity in pathogenesis of HCC has brought to attention the potential role of immune checkpoint inhibitors to meet the unmet needs of patients with advanced and metastatic disease. Immune checkpoint inhibitors have exhibited favorable toxicity profiles and encouraging efficacy data when compared to multikinase inhibitors and are a growing area of research interest.18,19 The response to these novel agents may be affected by the etiology of liver disease. For example, the IMBRAVE trial highlighted that anti-PD1 and PD-L1 therapies were not very effective in patients with HCC from non-viral etiologies, with median overall survival (OS) of 17 months in comparison to 24 and 19 months for hepatitis C and B, respectively.20 Another recent trial comparing the efficacy of lenvatinib versus atezolizumab plus bevacizumab in patients with MASLD showed that median progression-free survival (PFS) was 8 months in the lenvatinib group and 5.1 months in the atezolizumab/bevacizumab combination. In the non-MASLD population, PFS was 8.1 months and 6.7 months for lenvatinib and atezolizumab/bevacizumab, respectively.21 Recent studies have evaluated the use of immune checkpoint inhibitors in downstaging tumors for resection22,23 or liver transplantation.24
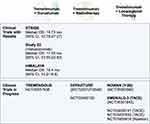
With the approval of newer agents, the BCLC staging system has evolved. Table 1 highlights the different first- and second-line agents currently approved for HCC. Figure 1 outlines the management algorithm for advanced HCC.
 |
Table 1 First- and Second-Line Agents for Advanced Hepatocellular Carcinoma |
 |
Figure 1 Management algorithm for advanced hepatocellular carcinoma. |
Multikinase Inhibitors
Sorafenib
Sorafenib is a small oral molecule that exhibits multikinase inhibitory activity and targets the vascular endothelial growth factor receptor (VEGFR), platelet derived growth factor receptor (PDGFR) and the serine/threonine kinase Raf.25 The landmark SHARP trial (NCT00105443) demonstrated that in patients with unresectable HCC, sorafenib had significantly improved OS (10.7 vs 7.9 months, hazard ratio [HR] 0.69, 95% CI 0.55–0.87, p<0.001) when compared to placebo.26 The majority of the patients had preserved liver function (≥95% of patients classified as Child-Pugh class A) and good performance status (>90% of patients had an Eastern Cooperative Oncology Group [ECOG] performance status of 0 or 1). From 2007 to 2018, sorafenib was the only approved systemic therapy for advanced HCC. Adverse effects demonstrated in multiple studies included diarrhea and dermatological side-effects, which were seen in 30–40% of patients.27–29 Sorafenib is also associated with an increased risk of bleeding, and patients require close monitoring of their international normalized ratio (INR), especially those on anticoagulation.30
Lenvatinib
Almost a decade later, a second systemic agent was approved for first line therapy for advanced HCC. Like sorafenib, lenvatinib is an oral small molecule that inhibits multiple tyrosine kinase receptors, including VEGFR, PDGFR, fibroblast growth factor receptor (FGFR), and other growth-signaling kinases.31 The REFLECT trial (NCT01761266) was an open-label, multinational, non-inferiority Phase III trial which included patients who had not received treatment for advanced HCC.32 Patients were randomized in 1:1 fashion to either receive lenvatinib or sorafenib. The trial demonstrated that lenvatinib was non-inferior to sorafenib in OS (HR, 0.92; 95% CI, 0.79–1.06; p > 0.05). Lenvatinib also had a superior objective response rate (ORR; odds ratio [OR], 3.34; 2.17–5.14; p < 0.0001) and PFS (HR, 0.66; 0.57–0.77; p < 0.0001) to sorafenib. Time to progression was also significantly longer in the lenvatinib group (HR, 0.61; 0.51–0.72; p < 0.0001). The safety and tolerability profile of lenvatinib was found to be manageable. The most commonly reported adverse events were hypertension (42%), diarrhea (39%), decreased appetite (34%), and decreased weight (31%).
Regorafenib
Regorafenib is a multikinase inhibitor targeting oncogenic receptor tyrosine kinases (eg, Raf), angiogenic kinases (eg, VEGFR), and stromal cell protein kinases (eg, FGFR, PDGFR). A 2017 randomized, placebo-controlled, double-blind, Phase 3 trial, RESORCE (NCT01774344) investigated the effectiveness of regorafenib in patients whose HCC had progressed on sorafenib.33 Participants from 152 centers across the world were randomized to each trial arm at a ratio of 2 regorafenib patients to every one control patient, with a median follow-up of 7.0 months. OS, the primary endpoint, was significantly higher in patients receiving regorafenib (HR, 0.63; 95% CI, 0.50–0.79; one-sided p < 0.0001). PFS was also significantly greater in the regorafenib group (HR, 0.46; 95% CI, 0.37–0.56; one-sided p < 0.0001). All (100%) patients receiving regorafenib experienced treatment-emergent adverse events; 93% of these events were possibly related to the study drug. The most frequent grade 3 or 4 events were hypertension (15%), hand-foot skin reaction (13%), fatigue (9%), and diarrhea (3%). Serious adverse events occurred in 44% of patients taking regorafenib, including 50 deaths (13%), 7 (2%) of which were thought to be directly linked to the drug.
Cabozantinib
Cabozantinib is a multikinase inhibitor with both antitumor and immunomodulatory activity. It targets VEGFR, mesenchymal epithelial transition (MET) receptor tyrosine kinase, and members of the Tyro-3, Axl, and Mer (TAM) family of receptor kinases.34 The CELESTIAL trial (NCT01908426) was a Phase 3, double-blind trial in which patients were randomized in 2:1 ratio to receive cabozantinib or placebo. The primary endpoint of the CELESTIAL study was OS, and secondary efficacy endpoints included PFS and ORR. In this trial, cabozantinib showed significantly improved OS (10.2 months vs 8.0 months) and PFS (5.2 months vs 1.9 months) compared to placebo.35 The most common adverse effect reported was palmar–plantar erythrodysesthesia (17% with cabozantinib vs 0% with placebo), followed by hypertension (16% vs 2%), increased aspartate aminotransferase level (12% vs 7%), fatigue (10% vs 4%), and diarrhea (10% vs 2%).35
Combination Therapy (VEGF Inhibitor + Immune Checkpoint Inhibitor)
Atezolizumab and Bevacizumab
Atezolizumab plus bevacizumab emerged as the first successful combination immunotherapy for patients with unresectable HCC. Atezolizumab targets programmed death-ligand 1 (PD-L1)36 and bevacizumab is a monoclonal antibody that targets vascular endothelial growth factor (VEGF).37 The landmark IMbrave150 trial (NCT03434379) was a global, open-label, Phase 3 trial evaluating the effectiveness of combination atezolizumab and bevacizumab.20 In this study, patients with unresectable HCC were randomized 2:1 to receive either atezolizumab plus bevacizumab or sorafenib. The primary endpoint of this intention to treat analysis was OS and PFS. In this study, combination atezolizumab/bevacizumab demonstrated better OS (67.2% vs 54.6% at 6 months) and PFS (6.8 months vs 4.3 months) compared to sorafenib. The most common grade 3 or 4 adverse event with atezolizumab/bevacizumab was hypertension, which occurred in 15.2% of patients in the experimental arm of the trial.20 All patients included in the IMbrave150 trial had to undergo endoscopy and management of varices prior to treatment. Gastrointestinal bleeding is a well-reported side effect of bevacizumab therapy38,39 and was observed in 7% of patients on atezolizumab/bevacizumab therapy in the trial. Given the significant risk of bleeding, adequate endoscopic evaluation and management for esophageal varices ideally within 6 months before initiation of atezolizumab plus bevacizumab regimen is recommended. Despite these side effects, based on the promising results, combination atezolizumab/bevacizumab is now recommended as first-line therapy for advanced HCC.40
Immune Checkpoint Inhibitors
Nivolumab
In 2017, the multicenter, open-label, dose escalation and expansion-based CheckMate 040 trial (NCT01658878) evaluated the safety and efficacy of nivolumab, a PD-1 immune checkpoint inhibitor.41 The trial included 48 patients with advanced HCC in a dose-escalation phase and 214 patients in a dose-expansion phase. A 3 mg/kg dose of nivolumab showed an ORR of 20% for patients in the dose-expansion phase and 15% for patients in the dose-escalation phase. The disease control rates in these phases were 64% and 58%, respectively. Grade 3/4 treatment-related adverse events were seen in 40 (19%) patients and grade 3/4 treatment-related serious adverse events were seen in 9 (4%) patients. Based on durable response rates from this trial, the US Food and Drug Administration (FDA) granted accelerated approval for use of nivolumab in patients with advanced HCC. Additional studies examining combined therapy of nivolumab and the CTLA-4 antibody ipilimumab have shown promising clinical response rates with manageable safety profiles.42
Pembrolizumab
The KEYNOTE-224 trial (NCT02702414) in 2018 evaluated the efficacy and safety of PD-1 inhibitor pembrolizumab. This was a non-randomized, open-label, Phase 2 trial that included 104 patients with HCC who progressed on or were intolerant to sorafenib.43 An ORR of 17% was noted, with one (1%) complete response and 17 (16%) partial responses. Overall, 46 (44%) patients had stable disease and 34 (33%) had progressive disease.43 The safety and tolerability profile of pembrolizumab was similar to other immune checkpoint inhibitors. The results from this trial led to approval of pembrolizumab for patients with advanced HCC previously treated with sorafenib.
Subsequently, a phase III trial KEYNOTE-240 (NCT02702401) was conducted comparing pembrolizumab to a placebo as second-line HCC treatment.44 Interestingly, in this study, the primary endpoints, OS and PFS, were not statistically different between the experimental and placebo arms. Data from the KEYNOTE-240 trial showed that the median OS with pembrolizumab versus placebo was 13.9 vs 10.6 months, respectively (HR, 0.781, 95% CI, 0.611–0.998; p = 0.023) and the median PFS was 3.3 vs 2.8 months, respectively (HR, 0.718; 95% CI, 0.570–0.904; p = 0.0022). In previously treated patients with advanced HCC, pembrolizumab maintained improvements in OS and PFS over time. Hence, pembrolizumab has maintained its accelerated approval for HCC treatment.
Clinical trials have also examined the efficacy of lenvatinib combined with pembrolizumab. A 2020 Phase 1b trial (NCT03006926) examined the combination of lenvatinib and pembrolizumab.45 In 104 patients with unresectable HCC, the ORR was 46.0%. The median PFS and OS were 9.3 and 22 months, respectively. This drug combination was also investigated in the phase 3 LEAP-002 trial (NCT03713593).46 This trial investigated pembrolizumab plus lenvatinib versus lenvatinib with placebo (lenvatinib monotherapy) as a first-line treatment for patients with unresectable HCC. Unfortunately, the primary endpoints of OS and PFS were not met. There were trends toward improvement in OS and PFS for patients who received pembrolizumab plus lenvatinib versus lenvatinib monotherapy; however, these results did not meet statistical significance. Interestingly, the median OS of the lenvatinib monotherapy arm in LEAP-002 was longer than that observed in previously reported clinical trials evaluating lenvatinib monotherapy in unresectable HCC. This data lends further support for the efficacy and use of lenvatinib in patients with unresectable HCC.
Combined lenvatinib and locoregional therapy also shows great promise for treating HCC. The LEAP-012 study (NCT04246177) is an ongoing randomized, double-blind, Phase 3 study examining the effects of lenvatinib and TACE.47 This trial enrolled 450 adults with confirmed HCC localized to the liver without portal vein thrombosis and not amenable to curative treatment, ≥1 measurable tumor per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria, ECOG status 0 or 1, Child-Pugh class A and no previous systemic treatment for HCC. Patients were randomly assigned to lenvatinib once daily plus pembrolizumab every 6 weeks plus TACE or placebo plus TACE. Dual primary endpoints are PFS up to 43 months and OS up to 95 months after randomization. Secondary endpoints are PFS up to 43 months, as well as ORR, disease control rate, duration of response, and time to progression up to 95 months after randomization. LEAP-002 is also measuring the incidence of adverse events, serious adverse events, and hepatic events of clinical interest.
Tremelimumab
Tremelimumab is a CTLA-4-blocking antibody approved in October 2022 in combination with durvalumab as first-line therapy for advanced HCC (Figure 2). Both CTLA-4 and PD-1 blunt T cell priming at the synapse between T cells and antigen presenting cells. The blockade of this interaction plays a vital role in tumorigenesis.18
Tremelimumab and Durvalumab
The first Phase 1/2 trial (NCT02519348) that evaluated this drug combination was Study 22 of the Single Tremelimumab Regular Interval Durvalumab (STRIDE) trial.48,49 This study recruited patients with advanced disease who were not candidates for sorafenib as they either had progression of disease on it or refused/were intolerant to sorafenib. A total of 332 patients were randomly assigned to four cohorts: (1) the T300 + D arm: 300 mg tremelimumab mg plus 1500 mg durvalumab for one dose, followed by durvalumab 1500 mg once every 4 weeks; (2) a durvalumab monotherapy arm (D arm), receiving 1500 mg every 4 weeks; (3) a tremelimumab monotherapy arm (T arm), receiving tremelimumab 750 mg once every 4 weeks; and the T75 + D arm, receiving 75 mg tremelimumab plus 1500 mg durvalumab once every 4 weeks for four doses, followed by 1500 mg durvalumab once every 4 weeks. The primary endpoint of this Phase 1/2 trial was safety, measured by the frequency of adverse events. Treatment-related adverse events and immune-mediated adverse events, as determined by the investigators, were also noted. Secondary end points included ORR by RECIST 1.1 guidelines, duration of response, time to response, PFS, and OS.
Treatment-related adverse events were most common in the T arm of the STRIDE study: 43.5% of participants in the T arm had grade 3 or higher treatment-related adverse events, compared to 37.8% in the T300 + D arm, 20.8% in the D arm, and 24.4% in the T75 + D arm.49 Immune-related adverse events were also more common in the arms that received tremelimumab: 31.1% in the T300 + D arm, 15.8% in the D arm, 24.6% in the T arm, and 26.8% in the T75 + D arm. The T300+D arm produced the most encouraging benefits, with an ORR of 24% (95% CI, 14.9–35.3%), compared to 10.6% (5.4%–18.1%) in the D arm, 7.2% (2.4–6.1%) in the T arm, and 9.5% (4.2–17.9%) in the T75 + D arm. Median PFS was 2.17 (1.91–5.42) months in the T300 + D arm, 2.07 (1.84–2.83) months in the D arm, 2.69 (1.87–5.29) months in the T arm, and 1.87 (1.77–2.53) months in the T75 + D arm. OS rates were also highest in the T300 + D arm, with patients surviving a median of 18.73 (95% CI, 10.78–27.27) months. Median OS was 15.11 (11.33–20.50) months in the T arm, 13.57 (8.74–17.64) months in the D arm, and 11.30 (8.83–14.95) months in the T75 + D arm.
Following the encouraging results from STRIDE, the HIMALAYA Phase 3 open label multicenter study (NCT03298451) was conducted to assess the efficacy and safety of durvalumab/tremelimumab combination therapy, durvalumab monotherapy, and sorafenib to treat patients with unresectable HCC and no prior systemic therapy.50 Patients in this study were not eligible for locoregional therapies. Additionally, unlike other immunotherapy trials, the HIMALAYA study excluded patients with portal vein invasion. They included 1171 patients randomly assigned to one of the three arms: (1) tremelimumab (300 mg, one dose) plus durvalumab (1500 mg every 4 weeks; the “STRIDE” arm), (2) durvalumab (1500 mg every 4 weeks, the “D” arm), or (3) sorafenib (400 mg twice daily, the “S” arm). The primary objective was OS in the STRIDE arm relative to the S arm. Noninferiority of OS for the D arm versus the S arm was a secondary objective.
The study met the primary objective, with significantly improved OS with the STRIDE protocol versus the S arm (HR, 0.78; 95% CI, 0.65–0.92; p = 0.0035).50 The median OS was 16.4 (95% CI, 14.2–19.6) months in the STRIDE arm, 16.6 (14.1–19.1) months in the D arm, and 13.8 (12.3–16.1) months in the S arm. Participants in the STRIDE arm had more grade 3/4 immune-related adverse events (50.5%) compared to durvalumab (37.1%). However, STRIDE and durvalumab were not associated with treatment related gastrointestinal or esophageal varices hemorrhage events or serious liver toxicity.51 The results of HIMALAYA trial led to the latest changes in BCLC system, which now recommends combination tremelimumab/durvalumab treatment as first-line therapy for HCC.40
Tremelimumab/Durvalumab vs Atezolizumab/Bevacizumab
Criteria for choosing between atezolizumab/bevacizumab or tremelimumab/durvalumab as first-line therapy need to be established. The key factor to consider when choosing between the two therapies is the safety and tolerability of the anti-VEGF therapy. Gastrointestinal bleeding from bevacizumab is a well-known adverse event, sometimes resulting in fatalities.52 In patients with known esophageal varices and risk for bleeding, which includes many patients with HCC, tremelimumab/durvalumab may be a better regimen, as it does not contain a VEGF inhibitor. Other important deciding factors include etiology of HCC, renal disease, and the cardiovascular risk factors for patients. Atezolizumab/bevacizumab is not suitable for patients with cardiovascular risk factors or with non-viral etiologies of HCC like metabolic syndrome.53 Bevacizumab can also lead to renal disease with proteinuria54 and is not favored in patients with advanced kidney disease. In these circumstances, the tremelimumab/durvalumab combination should be considered. Although the HIMALYA trial excluded patients with main portal vein thrombus, clinicians may consider using the tremelimumab/durvalumab combination in such patients if they are not good candidates for atezolizumab/bevacizumab combination therapy. Future studies should be conducted to inform evidence-based approaches to managing HCC in these patients.
Tremelimumab and Radiotherapy
The success of tremelimumab combination systemic therapies has led to interest in combining tremelimumab with other types of cancer therapies, such as radiotherapy. The DEPARTURE phase Ib study (jRCT2031210046) is multicenter, open-label, single-arm clinical trial being conducted in Japan.55 The study is evaluating the role of role of durvalumab monotherapy in combination with particle therapy and that of durvalumab plus tremelimumab in combination with particle therapy for patients with advanced hepatocellular carcinoma and macrovascular invasion. Patients in cohort A will receive durvalumab monotherapy (1500 mg every 4 weeks) combined with carbon-ion radiotherapy. In cohort B, patients will receive durvalumab (1500 mg every 4 weeks) and tremelimumab (300 mg on cycle 1, day 1) combined with carbon-ion radiotherapy.
The primary endpoint of the DEPARTURE trial is the frequency of serious adverse events, which includes dose-limiting toxicities.55 Secondary endpoints include OS over the course of the study and at 6 months, 6-month PFS, and time to progression. The results of this trial will provide new insight into the safety and efficacy of combined tremelimumab and radiotherapy, and combination immunotherapy and radiotherapy for HCC in general. If this combination of therapies is effective, it will open new avenues for HCC treatment.
There are also ongoing single-center clinical trials examining the effectiveness of tremelimumab/durvalumab therapy combined with radiotherapy. For example, an ongoing single-center trial at Massachusetts General Hospital is examining the efficacy of this combination in patients with HCC or biliary tract cancer (NCT03482102). A team from the University of Hong Kong is investigating combined TACE, stereotactic body radiotherapy, and tremelimumab/durvalumab (NCT04988945).
Tremelimumab and Locoregional Therapy
Several clinical trials are currently underway to investigate tremelimumab combined with locoregional therapy in patients with HCC. The ROWAN study (NCT05063565) is an ongoing open-label, prospective, multi-center, randomized, Phase 2 trial.56 This study will assess the safety and efficacy of yttrium-90 (Y90) radioembolization administered before initiation of durvalumab with tremelimumab in patients with advanced HCC who are not candidates for resection and liver transplantation. The primary outcome of the study is ORR measured using mRECIST criteria. Immune-mediated adverse events and serious adverse events, interruption of treatment due to an adverse event, change in liver function tests from baseline, change in ECOG score from baseline, duration of response, disease control rate, time to best response, complete response rate, and PFS are among the secondary endpoints being measured. The study should conclude in 2026, with results available in 2027. Other single-center studies examining the effects of tremelimumab combined with Y-90 are also underway (NCT05701488, NCT04605731).
The ongoing EMERALD-3 study (NCT05301842) is also examining the efficacy of tremelimumab and locoregional therapy.57 Specifically, this is a Phase 3, randomized, open-label, multicenter trial examining safety and efficacy of tremelimumab, durvalumab, and TACE, with and without lenvatinib. Recruitment is still underway, as of the time of writing. The study has 3 arms: (1) patients receiving tremelimumab, durvalumab, TACE, and lenvatinib, (2) patients receiving tremelimumab, durvalumab, and TACE (no lenvatinib), and (3) patients receiving TACE only. The primary outcome is PFS (per RECIST 1.1 criteria) of patients receiving all 4 treatments relative to patients receiving TACE alone at approximately 5 years post-randomization. OS and PFS will also be compared between the 3 study arms as secondary outcomes. The study is estimated to be complete in December 2025. Single-center trials looking at tremelimumab/durvalumab therapy and TACE are also ongoing (NCT03638141, NCT03937830).
Clinical trials have also examined combination tremelimumab and ablation therapy in HCC patients. For example, a 2017 clinical trial out of the US National Cancer Institute (NCT01853618) explored the potential benefit of combined effect of tremelimumab and ablative therapy in patients with advanced HCC.58 This study included 32 patients who received tremelimumab at two dose levels (3.5 and 10 mg/kg) every 4 weeks for 6 doses, followed by 3-monthly infusions. On day 36, patients received subtotal radiofrequency ablation or chemoablation. Staging with contrast-enhanced CT or MRI was performed every 8 weeks. This study demonstrated that combining tremelimumab with radiological procedures had a good clinical response. Although only 26.3% (95% CI, 9.1–51.2%) of participants had a confirmed partial response, and overall median time to progression was 7.4 (4.6–19.4) months. Median OS was 12.3 (9.3–15.4) months. Interestingly, patients with hepatitis C experienced a decrease in median viral load from 1275 × 103 IU/mL at baseline to 351 × 103 IU/mL 3 months post-treatment. Patients also experienced an increase in intratumoral CD8+ T cells. No dose limiting toxicity was encountered. This can potentially be a management option for patients with unresectable HCC and needs to be further validated.
Immune Checkpoint Inhibitor Toxicity and Treatment
Although immune checkpoint inhibitors are generally well tolerated, some patients do experience toxicity and side effects. In HCC patients, there is particular concern over potential hepatotoxicity,59 given that most patients already have impaired liver function. Hepatotoxicity is generally diagnosed via liver function tests,60 but some patients may present with more severe symptoms such as jaundice or abdominal pain.59 Hy’s law is frequently used to identify patients with drug-induced liver injury.61,62 According to these guidelines, hepatotoxicity occurs when aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels are more than 3 times the upper limit of normal or total bilirubin levels are more than twice the upper limit of normal.62 Additional tests should be conducted to rule out other possible causes of liver injury, such as progressive liver disease or cholestasis.
Other types of toxicities are common in patients on immune checkpoint inhibitors. For example, around 30–50% of patients may experience skin toxicities.63 Thyroid problems can occur in up to 20% of patients receiving immune checkpoint inhibitor therapy, and other rare endocrine problems may develop as well.63 Colitis is also relatively common, occurring in up to 22% of patients.64 Pulmonary toxicities also occur, which frequently present as pneumonitis.65 Cardiac, immunologic, neurologic, and renal complications may also occur, but they are seen less frequently.63
Treatment of immune checkpoint inhibitor-related toxicity depends on the severity of the patient’s symptoms. According to 2018 guidelines from the American Society of Clinical Oncology,66 treatment should be continued after most grade 1 toxicities. Clinicians should pause therapy in most patients who develop a grade 2 toxicity and administer corticosteroids. Grade 3 toxicities are treated similarly to grade 2, but patients should be given a larger dose of corticosteroids. Immune checkpoint inhibitor therapy should be discontinued in patients who experience most grade 4 toxicities. If the toxicity resolves in a patient with grade 1–3 toxicity, therapy may be continued, but clinicians should utilize caution with these patients.
Patients with HCC who are liver transplant candidates may be at a higher risk of graft rejection or even death if they receive neoadjuvant immune checkpoint inhibitor therapy.67,68 For this reason, most centers incorporate a washout period between the administration of the immunotherapy and the liver transplant surgery.69
Conclusion
The treatment paradigm for unresectable HCC is rapidly changing, and immune checkpoint inhibitors have become an area of great interest and exploration. Combination regimens using immune checkpoint inhibitors have shown promising results. Combined tremelimumab-durvalumab therapy has shown clinical utility in patients with unresectable HCC, which is now recommended as a first-line therapy in the latest BCLC guidelines. Ongoing clinical trials are assessing the safety and efficacy of tremelimumab in combination with radiotherapy, locoregional therapies, and other drugs. The utilization of tremelimumab in management algorithms will be guided by the data on efficacy, tolerability, and safety when combined with other drugs and treatment modalities in these trials.
Funding
There was no financial support for the article.
Disclosure
Dr David Victor 3rd is part of the speaker bureau for Gilead and Intercept, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. El–Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. doi:10.1053/j.gastro.2007.04.061
2. Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788.
3. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi:10.1158/0008-5472.CAN-14-0155
4. El-Serag HB. Current concepts hepatocellular carcinoma. N Eng J Med. 2011;365(12):1118–1127. doi:10.1056/NEJMra1001683
5. Brady CW, Smith AD, Stechuchak KM, et al. Frequency and predictors of de novo hepatocellular carcinoma in patients awaiting orthotopic liver transplantation during the model for end-stage liver disease era. Liver Transplantation. 2008;14(2):228–234. doi:10.1002/lt.21346
6. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the US. Hepatology. 2014;59(6):2188–2195. doi:10.1002/hep.26986
7. Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16(7):411–428.
8. Faria SC, Szklaruk J, Kaseb AO, Hassabo HM, Elsayes KM. TNM/Okuda/Barcelona/UNOS/CLIP International Multidisciplinary Classification of Hepatocellular Carcinoma: concepts, perspectives, and radiologic implications. Abdom Imaging. 2014;39(5):1070–1087. doi:10.1007/s00261-014-0130-0
9. Benson AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Nat Comprehensive Cancer Network. 2021;19(5):541–565. doi:10.6004/jnccn.2021.0022
10. Koh JH, Tan DJH, Ong Y, et al. Liver resection versus liver transplantation for hepatocellular carcinoma within Milan criteria: a meta-analysis of 18,421 patients. Hepatobil Surg Nutr. 2021;21.
11. Galle PR, Dufour J-F, Peck-Radosavljevic M, Trojan J, Vogel A. Systemic therapy of advanced hepatocellular carcinoma. Future Oncol. 2021;17(10):1237–1251. doi:10.2217/fon-2020-0758
12. Giannini EG, Farinati F, Ciccarese F, et al. Prognosis of untreated hepatocellular carcinoma. Hepatology. 2015;61(1):184–190. doi:10.1002/hep.27443
13. Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, Greten TF. The yin and yang of evasion and immune activation in HCC. J Hepatol. 2015;62(6):1420–1429. doi:10.1016/j.jhep.2015.02.038
14. Xu D, Fu J, Jin L, et al. Circulating and liver resident CD4+ CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol. 2006;177(1):739–747. doi:10.4049/jimmunol.177.1.739
15. Knoll P, Schlaak J, Uhrig A, Kempf P, Zum Büschenfelde K-HM, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. 1995;22(2):226–229. doi:10.1016/0168-8278(95)80433-1
16. Matsuzaki K, Murata M, Yoshida K, et al. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor β signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology. 2007;46(1):48–57. doi:10.1002/hep.21672
17. Roth GS, Decaens T. Liver immunotolerance and hepatocellular carcinoma: patho-physiological mechanisms and therapeutic perspectives. Eur J Cancer. 2017;87:101–112. doi:10.1016/j.ejca.2017.10.010
18. El Dika I, Khalil DN, Abou‐Alfa GK. Immune checkpoint inhibitors for hepatocellular carcinoma. Cancer. 2019;125(19):3312–3319. doi:10.1002/cncr.32076
19. Wong KM, King GG, Harris WP. The treatment landscape of advanced hepatocellular carcinoma. Curr Oncol Rep. 2022;24(7):917–927. doi:10.1007/s11912-022-01247-7
20. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Eng J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
21. Kim BK, Cheon J, Kim H, et al. Atezolizumab/Bevacizumab vs. Lenvatinib as First-Line Therapy for Unresectable Hepatocellular Carcinoma: a Real-World, Multi-Center Study. Cancers. 2022;14(7):1747. doi:10.3390/cancers14071747
22. Chao J, Zhu Q, Chen D, et al. Case Report: transarterial Chemoembolization in Combination With Tislelizumab Downstages Unresectable Hepatocellular Carcinoma Followed by Radical Salvage Resection. Front Oncol. 2021;11:667555. doi:10.3389/fonc.2021.667555
23. Takamoto T, Maruki Y, Kondo S. Recent updates in the use of pharmacological therapies for downstaging in patients with hepatocellular carcinoma. Expert Opin Pharmacother. 2023;1–9.
24. Gao Q, Anwar IJ, Abraham N, Barbas AS. Liver Transplantation for Hepatocellular Carcinoma after Downstaging or Bridging Therapy with Immune Checkpoint Inhibitors. Cancers. 2021;13(24):6307. doi:10.3390/cancers13246307
25. Mousa AB. Sorafenib in the treatment of advanced hepatocellular carcinoma. Saudi j Gastroenterol. 2008;14(1):40. doi:10.4103/1319-3767.37808
26. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Eng j Medicine. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
27. Berk V, Kaplan M, Tonyali O, et al. Efficiency and side effects of sorafenib therapy for advanced hepatocellular carcinoma: a retrospective study by the Anatolian Society of Medical Oncology. Asian Pacific J Cancer Prevention. 2013;14(12):7367–7369. doi:10.7314/APJCP.2013.14.12.7367
28. Zhang L, Zhou Q, Ma L, Wu Z, Wang Y. Meta‐analysis of dermatological toxicities associated with sorafenib. Clin Exp Dermatol. 2011;36(4):344–350. doi:10.1111/j.1365-2230.2011.04060.x
29. Brose MS, Frenette CT, Keefe SM, Stein SM. Management of sorafenib-related adverse events: a clinician’s perspective. Semin Oncol. 2014;41:S1–S16. doi:10.1053/j.seminoncol.2014.01.001
30. Dai C, Zhou F, Shao J-H, Wu L-Q, Yu X, Yin X-B. Bleeding risk in cancer patients treated with sorafenib: a meta-analysis of randomized controlled trials. J Cancer Res Ther. 2018;14(Suppl 5):S948–S956. doi:10.4103/0973-1482.188430
31. Al-Salama ZT, Syed YY, Scott LJ. Lenvatinib: a review in hepatocellular carcinoma. Drugs. 2019;79:665–674. doi:10.1007/s40265-019-01116-x
32. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
33. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi:10.1016/S0140-6736(16)32453-9
34. El-Khoueiry AB, Hanna DL, Llovet J, Kelley RK. Cabozantinib: an evolving therapy for hepatocellular carcinoma. Cancer Treat Rev. 2021;98:102221. doi:10.1016/j.ctrv.2021.102221
35. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Eng J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002
36. Chen DS, Hurwitz H. Combinations of bevacizumab with cancer immunotherapy. Cancer J. 2018;24(4):193–204. doi:10.1097/PPO.0000000000000327
37. Morse MA, Sun W, Kim R, et al. The Role of Angiogenesis in Hepatocellular CarcinomaRole of Angiogenesis in HCC. Clin Cancer Res. 2019;25(3):912–920. doi:10.1158/1078-0432.CCR-18-1254
38. Boige V, Malka D, Bourredjem A, et al. Efficacy, Safety, and Biomarkers of Single-Agent Bevacizumab Therapy in Patients with Advanced Hepatocellular Carcinoma. Oncologist. 2012;17(8):1063–1072. doi:10.1634/theoncologist.2011-0465
39. Fang P, Hu J-H, Cheng Z-G, Liu Z-F, Wang J-L, Jiao S-C. Efficacy and Safety of Bevacizumab for the Treatment of Advanced Hepatocellular Carcinoma: a Systematic Review of Phase II Trials. PLoS One. 2012;7(12):e49717. doi:10.1371/journal.pone.0049717
40. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
41. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi:10.1016/S0140-6736(17)31046-2
42. Yau T, Kang Y-K, Kim T-Y, et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: the CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6(11):e204564–e204564. doi:10.1001/jamaoncol.2020.4564
43. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label Phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi:10.1016/S1470-2045(18)30351-6
44. Finn RS, Ryoo B-Y, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. Journal of Clinical Oncology. 2020;38(3):193–202. doi:10.1200/JCO.19.01307
45. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/JCO.20.00808
46. Finn R, Kudo M, Merle P, et al. LBA34 Primary results from the phase III LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2022;33:S1401. doi:10.1016/j.annonc.2022.08.031
47. Llovet JM, Vogel A, Madoff DC, et al. Randomized Phase 3 LEAP-012 Study: transarterial Chemoembolization With or Without Lenvatinib Plus Pembrolizumab for Intermediate-Stage Hepatocellular Carcinoma Not Amenable to Curative Treatment. Cardiovasc Intervent Radiol. 2022;45(4):405–412. doi:10.1007/s00270-021-03031-9
48. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Am Soc Clin Oncol. 2017;35(15_suppl):4073. doi:10.1200/JCO.2017.35.15_suppl.4073
49. Kelley RK, Sangro B, Harris W, et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients With Unresectable Hepatocellular Carcinoma: randomized Expansion of a Phase I/II Study. J Clin Oncol. 2021;39(27):2991–3001. doi:10.1200/JCO.20.03555
50. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(4_suppl):379. doi:10.1200/JCO.2022.40.4_suppl.379
51. Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evidence. 2022;1(8). doi:10.1056/EVIDoa2100070
52. Gu T, Jiang A, Zhou C, et al. Adverse reactions associated with immune checkpoint inhibitors and bevacizumab: a pharmacovigilance analysis. Int J Cancer. 2023;152(3):480–495. doi:10.1002/ijc.34332
53. Hsu C, Rimassa L, Sun HC, Vogel A, Kaseb AO. Immunotherapy in hepatocellular carcinoma: evaluation and management of adverse events associated with atezolizumab plus bevacizumab. Ther Adv Med Oncol. 2021;13:17588359211031141. doi:10.1177/17588359211031141
54. Kanbayashi Y, Ishikawa T, Tabuchi Y, et al. Predictive factors for the development of proteinuria in cancer patients treated with bevacizumab, ramucirumab, and aflibercept: a single-institution retrospective analysis. Sci Rep. 2020;10(1):2011. doi:10.1038/s41598-020-58994-5
55. Ogasawara S, Koroki K, Makishima H, et al. Protocol: durvalumab with or without tremelimumab combined with particle therapy for advanced hepatocellular carcinoma with macrovascular invasion: protocol for the DEPARTURE phase Ib trial. BMJ Open. 2022;12(4):e059779. doi:10.1136/bmjopen-2021-059779
56. Table of Pharmacogenomic Biomarkers in Drug Labeling. US Food & Drug Administration. Updated August 10, 2023. Available from: https://www.fda.gov/drugs/science-and-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling.
57. Abou-Alfa GK, Fan J, Heo J, et al. 727TiP A randomised phase III study of tremelimumab (T) plus durvalumab (D) with or without lenvatinib combined with concurrent transarterial chemoembolisation (TACE) versus TACE alone in patients (pts) with locoregional hepatocellular carcinoma (HCC): EMERALD-3. Ann Oncol. 2022;33:S874.
58. Duffy AG, Ulahannan SV, Makorova-Rusher O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66(3):545–551. doi:10.1016/j.jhep.2016.10.029
59. Remash D, Prince DS, McKenzie C, Strasser SI, Kao S, Liu K. Immune checkpoint inhibitor-related hepatotoxicity: a review. World J Gastroenterol. 2021;27(32):5376–5391. doi:10.3748/wjg.v27.i32.5376
60. Suzman DL, Pelosof L, Rosenberg A, Avigan MI. Hepatotoxicity of immune checkpoint inhibitors: an evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int. 2018;38(6):976–987. doi:10.1111/liv.13746
61. Health UDo, Services H. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v 5.0. 2017; 2023.
62. Food, Administration D. Drug-induced liver injury: premarketing clinical evaluation. Guidance Industry. 2009.
63. Palmieri DJ, Carlino MS. Immune Checkpoint Inhibitor Toxicity. Curr Oncol Rep. 2018;20(9):72. doi:10.1007/s11912-018-0718-6
64. Gupta A, De Felice KM, Loftus EV Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42(4):406–417. doi:10.1111/apt.13281
65. De Velasco G, Je Y, Bossé D, et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol Res. 2017;5(4):312–318. doi:10.1158/2326-6066.CIR-16-0237
66. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–1768. doi:10.1200/JCO.2017.77.6385
67. Nordness MF, Hamel S, Godfrey CM, et al. Fatal hepatic necrosis after nivolumab as a bridge to liver transplant for HCC: are checkpoint inhibitors safe for the pretransplant patient? Am J Transplant. 2020;20(3):879–883. doi:10.1111/ajt.15617
68. Chen GH, Wang GB, Huang F, et al. Pretransplant use of toripalimab for hepatocellular carcinoma resulting in fatal acute hepatic necrosis in the immediate postoperative period. Transpl Immunol. 2021;66:101386. doi:10.1016/j.trim.2021.101386
69. Kuo FC, Chen CY, Lin NC, Liu C, Hsia CY, Loong CC. Optimizing the Safe Washout Period for Liver Transplantation Following Immune Checkpoint Inhibitors with Atezolizumab, Nivolumab, or Pembrolizumab. Transplant Proc. 2023;55(4):878–883. doi:10.1016/j.transproceed.2023.03.064
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.