Back to Journals » Journal of Pain Research » Volume 10
Evidence of both systemic inflammation and neuroinflammation in fibromyalgia patients, as assessed by a multiplex protein panel applied to the cerebrospinal fluid and to plasma
Authors Bäckryd E , Tanum L , Lind AL, Larsson A , Gordh T
Received 23 November 2016
Accepted for publication 14 December 2016
Published 3 March 2017 Volume 2017:10 Pages 515—525
DOI https://doi.org/10.2147/JPR.S128508
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Emmanuel Bäckryd,1,* Lars Tanum,2,* Anne-Li Lind,3 Anders Larsson,4 Torsten Gordh,3
1Pain and Rehabilitation Center, Department of Medical and Health Sciences, Linköping University, Linköping, Sweden; 2Department of R&D in Mental Health, Akershus University Hospital, Lørenskog, Norway; 3Department of Surgical Sciences, 4Department of Medical Sciences, Uppsala University, Uppsala, Sweden
*These authors contributed equally to this work
Abstract: In addition to central hyperexcitability and impaired top–down modulation, chronic inflammation probably plays a role in the pathophysiology of fibromyalgia (FM). Indeed, on the basis of both animal experiments and human studies involving the analysis of cytokines and other inflammation-related proteins in different body fluids, neuroinflammatory mechanisms are considered to be central to the pathophysiology of many chronic pain conditions. However, concerning FM, previous human plasma/serum and/or cerebrospinal fluid (CSF) cytokine studies have looked only at a few predetermined cytokine candidates. Instead of analyzing only a few substances at a time, we used a new multiplex protein panel enabling simultaneous analysis of 92 inflammation-related proteins. Hence, we investigated the CSF and plasma inflammatory profiles of 40 FM patients compared with CSF from healthy controls (n=10) and plasma from blood donor controls (n=46). Using multivariate data analysis by projection, we found evidence of both neuroinflammation (as assessed in CSF) and chronic systemic inflammation (as assessed in plasma). Two groups of proteins (one for CSF and one for plasma) highly discriminating between patients and controls are presented. Notably, we found high levels of CSF chemokine CX3CL1 (also known as fractalkine). In addition, previous findings concerning IL-8 in FM were replicated, in both CSF and plasma. This is the first time that such an extensive inflammatory profile has been described for FM patients. Hence, FM seems to be characterized by objective biochemical alterations, and the lingering characterization of its mechanisms as essentially idiopathic or even psychogenic should be seen as definitively outdated.
Keywords: cerebrospinal fluid, chemokines, chronic pain, cytokines, fibromyalgia, inflammation
Introduction
Fibromyalgia (FM) is a musculoskeletal pain condition characterized by chronic widespread pain and increased pain sensitivity, and it is often accompanied by sleep disturbances, fatigue, memory problems and psychological comorbidities.1 The prevalence of FM is ~2% worldwide,2 and it is more common in women.1 Although pregabalin, duloxetine and milnacipran have been approved by the US Food and Drug Administration (FDA) for use in FM, they provide only partial symptom relief in a minority of patients and have not been approved for this indication by the European Medicines Agency (EMA).3–6 Off-label use of amitriptyline is considered appropriate,3 but most patients on such medication discontinue therapy because of either lack of efficacy or tolerability problems. Drug therapy is not mandatory for the treatment of FM,2 and FM patients are often viewed from a rehabilitative perspective, multidisciplinary pain programs being the state of the art for the management of complex, chronic, nonmalignant pain.7 However, better and safer pharmacological treatment options would of course be a major step forward.
There is a need to better understand the pathophysiological mechanisms of FM, and such knowledge would perhaps enable the development of better therapeutic drugs. Central sensitization, defined as nociception-driven amplification of neural signaling within the central nervous system (CNS) leading to pain hypersensitivity,8 is considered to be an important pathophysiological mechanism in chronic pain conditions, not least in FM.9–11 The top–down modulating systems are impaired in FM,12 and this probably contributes to central hyperexcitability. Hence, it seems probable that central processes and peripheral nociceptive input interact. Indeed, ongoing peripheral input seems important if central hyperexcitability is to be maintained.13
Cytokines are small molecules that are released from immunocompetent cells, and cytokines are classified as either pro- or anti-inflammatory.14,15 Plasma and/or serum levels of proinflammatory cytokines IL-6 and IL-8 are elevated in patients with FM,16,17 as were cerebrospinal fluid (CSF) levels of IL-8 in one study.18 Indeed, the CSF is a relevant body fluid to investigate in pain conditions, as it is in direct contact with the CNS and can be hypothesized to mirror CNS pathology. CSF levels of classical neuropeptides such as substance P, beta-endorphin and other endogenous opioids have therefore been studied in many different pain states.19–24 Neuroinflammatory mechanisms are nowadays considered a very important part of the pathophysiology of chronic pain.25,26
The aim of the present study was to investigate the CSF and plasma inflammatory profiles of FM patients compared with healthy controls. Instead of analyzing only a few substances at a time, we used a multiplex protein panel enabling simultaneous analysis of 92 inflammatory biomarkers. This broader approach increases the chance of both validating previous findings and discovering novel important biomarkers not previously considered in FM. Moreover, in order to analyze data from a systems biology perspective,27 ie, looking at all variables simultaneously and not just one by one, multivariate data analysis (MVDA) by projection was used.28–30
Methods
Subjects and sampling procedures of CSF and blood
Patients
Patients were recruited through study information distributed to members by the Norwegian Fibromyalgia Association, and females 20–60 years of age suffering from FM according to the 1990 criteria of the American College of Rheumatology (ACR) were included. The diagnosis was established or verified by a consultant in rheumatology. Patients were excluded from participation if any verified organic cause of the pain condition was found or if there was an organic condition probably influencing the symptoms of FM. Moreover, any history of any serious medical illness or current or previous Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnosis of mood disorders (last 12 months), anxiety disorders, psychotic disorders, dementia, epilepsy, seizure disorders, alcohol or drug abuse led to exclusion. All patients underwent a structured psychiatric interview for DSM-IV and Montgomery–Åsberg Depression Rating Scale.
Lumbar puncture was performed fasting between eight and nine in the morning. A total of 10 mL of CSF was obtained as 10 separate aliquots and immediately put on dry ice for cooling and freezing and afterwards stored in an ultra freezer until analysis. None of the patients received any psychotropic medication at the time of the lumbar puncture.
CSF from healthy controls
Healthy controls were recruited by local advertisement at Linköping University and by contacting them from earlier studies. Informed consent was obtained. We have elsewhere published a detailed description of how the absence of any significant disease (including chronic pain) was ensured, and this will not be repeated here.29 Intrathecal access was obtained by lumbar puncture, and a 10 mL sample of CSF was taken. The sample was immediately cooled on ice and transported to the Painomics® Laboratory, Linköping University Hospital, where it was checked for blood contamination, centrifuged and divided into aliquots and stored at −70°C until analysis.
Control plasma samples from blood donors
In addition to analyzing CSF from patients and healthy controls, we also compared the plasma inflammatory profile of the same cohort of FM patients with healthy blood donors as controls. For the FM patients, the blood samples were obtained at the same occasion as the lumbar puncture and immediately centrifuged before storage in an ultra freezer.
Analytical method
In the present study, we applied the multiplex proximity extension assay (PEA) in which 92 proteins (Table S1) were simultaneously analyzed.31,32 The multiplex proximity extension assay (PEA) was performed using Proseek® Multiplex Inflammation I (Olink Bioscience, Uppsala, Sweden) according to the manufacturer’s instructions. Briefly, 1 μL sample was mixed with 3 μL incubation mix. The incubation mix contained 94 probe pairs (each pair comprising two target-specific antibodies with unique barcoded DNA oligonucleotides attached). The mixture was incubated at 8°C overnight. Then, 96 μL extension mix including PEA enzyme and polymerase chain reaction (PCR) reagents was added and incubated for 5 min at room temperature, after which the plate was transferred to a thermal cycler for an extension reaction followed by 17 cycles of DNA amplification. A 96.96 Dynamic Array IFC (Fluidigm, South San Francisco, CA, USA) was prepared and primed according to the manufacturer’s instructions. After that, in a new plate, 2.8 μL of sample mixture was mixed with 7.2 μL of detection mix from which 5 μL was loaded into the right side of the primed 96.96 Dynamic Array IFC. In all, 5 μL of the primer pairs, unique for each assay, were loaded into the left side of the 96.96 Dynamic Array IFC, and the protein expression program was run in Fluidigm Biomark Reader according to the instructions for Proseek® Multiplex. Data were expressed as normalized protein expression (NPX). NPX values were acquired by normalizing cq-values against extension control as well as interplate control and a correction factor. NPX values were on log2 scale. A high NPX value corresponds to a high protein concentration and can be linearized by using the formula 2NPX. NPX can be used for statistical multivariate analysis and express relative quantification between samples but is not an absolute quantification.
Statistics
This study generated data on 92 potentially interacting proteins. Traditional univariate statistical analysis can quantify level changes of individual proteins but disregard interrelationships between them and thereby ignore system-wide aspects. Therefore, we used SIMCA-P+ version 13.0 (Umetrics AB, Umeå, Sweden) for MVDA computations, looking at all the variables simultaneously and thereby taking the overall correlation structure of the material into consideration.30 The MVDA workflow and reported parameters were in accordance with Wheelock and Wheelock,28 and we have elsewhere extensively described the detailed rationale for using MVDA as opposed to classical regression in an open access paper.29 Briefly, we first used principal component analysis (PCA) for data overview and detection of outliers. Then, orthogonal partial least squares – discriminant analysis (OPLS-DA) was used to regress (predict) group membership, ie, the purpose was to find which proteins were responsible for group discrimination (patient or healthy control).
Data were log transformed when needed (using the appropriate in-built SIMCA-P+ function) and scaled to unit variance. The R2 value indicates how well the model explains the dataset, whereas cross-validated Q2 indicates the predictive power. Analysis of variance of cross-validated predictive residuals (CV-ANOVA) measures the significance of the observed group separation in OPLS-DA and provides a familiar p-value. Group separation can be visualized in a score plot, using the latent variables of the model as axes; hence, the score plot illustrates how the study subjects relate to each other. The importance of the variables for the OPLS-DA model could be quantified as a variable influence on projection (VIP) value. VIP indicates the relevance of each X-variable pooled over all dimensions and Y-variables – ie, VIP values indicate the group of variables that best explain Y. Variables with a VIP >1.0 and having a 95% confidence interval not including zero are usually considered significant.28,30,33
For traditional univariate statistics, which were used only for MVDA-selected variables (with one exception, refer the “Discussion” section), all computations were made using IBM® SPSS® Statistics version 23, and the results in the text and tables are generally given as median values (range) or proportions. Mann–Whitney U test was used to compare groups, and p<0.05 (two-sided test) was considered significant.
Ethics
Concerning the FM patients, the study protocol was approved by the Regional Committee for Medical Research Ethics, South-East Health Region, Norway, and the patients received both written and oral information about the study and gave their written consent. Protocol for the healthy controls was approved by the Regional Ethics Committee in Linköping, Sweden (Dnr M136-06 and Dnr 2012/94-32). The use of blood donor samples was approved by the local ethical board at Uppsala University (01-167) with the limitation that only information about age and sex was to be stored. The study was performed in full accordance with the Declaration of Helsinki (1965 and later revisions).
Results
CSF analyses
Overview of CSF cohorts
All 40 FM patients were females, whereas seven out of 11 healthy controls (64%) were females. The age of the patients did not significantly differ from healthy controls: 47 years (24–60 years) vs. 54 years (44–57 years), respectively, p=0.092. We excluded proteins with >20% below-limit of detection (LoD) values in both patients and healthy controls (ie, >20% in patients and >20% in controls).32 Hence, the CSF results of the present study are based on 53 proteins.
The CSF protein data of the 51 subjects were overviewed by PCA to check for multivariate outliers. The model had three principal components, R2=0.61 and Q2=0.48. One strong outlier was detected by Hotelling’s T2 (T2Crit 99%), and this subject was excluded from further analyses. Distance to model in the X-space showed four nonserious moderate outliers, and hence (because nonserious), these subjects were not excluded from the analysis. As a consequence, 40 patients and 10 controls were kept for OPLS-DA analysis.
Regression of class-discriminating CSF proteins
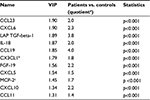
In order to identify important class-discriminating proteins, an OPLS-DA model was computed. The model had two latent variables (one predictive interclass and one orthogonal intraclass), R2=0.80 and Q2=0.75. The model was highly significant by CV-ANOVA (p<0.001), and hence, clear group separation was achieved (Figure 1). In all, 11 substances had VIP >1.3, implying high significance for group separation, and these 11 substances were therefore scrutinized using traditional statistics (Table 1 and Figure 2). To investigate any putative effect of sex, a new OPLS-DA model was computed with the four men excluded, ie, the model compared 40 patients and seven healthy controls. The list of the 11 most discriminating proteins was exactly the same as in Table 1, although the order varied somewhat (data not shown).
Plasma analyses
Overview of plasma cohorts
Plasma data were available for 35 FM patients who were all females, whereas 32 out of 47 blood donor controls were females (68%). The age of the patients did not significantly differ from blood donor controls: 47 years (24–60 years) vs. 48 years (27–67 years), respectively, p=0.599. We excluded proteins with >20% below-LoD values in both patients and blood donors (ie, >20% in patients and >20% in controls).32 Hence, the plasma results of the present study are based on 75 proteins.
The plasma protein data of the 82 subjects were overviewed by PCA to check for multivariate outliers. The model had three PCs, R2=0.50 and Q2=0.34. One strong outlier (a blood donor) was detected by Hotelling’s T2 (T2Crit 99%), and this subject was excluded from further analyses. Distance to model in the X-space showed six nonserious moderate outliers, and hence (because nonserious), these subjects were not excluded from analysis. As a consequence, 35 patients and 46 controls were kept for OPLS-DA analysis.
Regression of class-discriminating plasma proteins
In order to identify important class-discriminating proteins, an OPLS-DA model was computed. The model had three latent variables (one predictive interclass and two orthogonal intraclass), R2=0.97 and Q2=0.92. The model was highly significant by CV-ANOVA (p<0.001), and hence, clear group separation was achieved. In all, 21 substances had VIP >1.3, implying very high significance for group separation, and these 21 substances were therefore scrutinized using traditional statistics (Table 2). To investigate a putative effect of sex, a new OPLS-DA model was computed with men excluded, ie, the model compared 35 patients and 32 healthy controls. The list of the 21 most discriminating proteins was exactly the same as in Table 2, although the order varied somewhat (data not shown).
Of the 11 proteins listed in Table 1, four are also present in Table 2 and were therefore important for group discrimination in both plasma and CSF; these four proteins were CXCL6, LAPTGF-beta-1, CXCL5, and MCP-2. The remaining seven proteins of Table 1 had very low VIPs in the plasma model, VIPs ranging from 0.11 to 0.82. Hence, these seven proteins were very important for group discrimination in CSF but not in plasma. Finally, the top five proteins of Table 2 were not present in Table 1, indicating that these five proteins (STAMBP, SIRT2, CD40, AXIN1, and IL-7) strongly discriminate patients from controls in plasma but not in CSF (Figure 3).
Summary of main results
Instead of looking at a limited number of predetermined proteins, we have analyzed 92 inflammation-related proteins in CSF and plasma from patients with FM, compared to controls. Group separation was achieved for both body fluids using MVDA, and the proteins responsible for group discrimination are presented in Table 1 and Table 2. We found evidence of both neuroinflammation (as assessed in CSF) and chronic systemic inflammation (as assessed in plasma). For detailed descriptive statistics (10th, 25th, 50th, 75th, and 90th percentiles) for all analyzed proteins, see Tables S2 and S3.
Discussion
We have determined the CSF and plasma inflammatory profiles of 40 FM patients compared to healthy controls (CSF) and blood donors (plasma).
CSF vs. systemic levels
The CSF is an important potential “mirror” for pathophysiological processes in the spinal cord.29,34 Neuroinflammation and gliosis are important concepts in modern pain medicine.25,35 Are we perhaps “seeing” some aspects of central neuroinflammation in the protein list of Table 1? This would be a major step forward for pain medicine, as evidence of central neuroinflammation has hitherto been mostly gained through animal experiments.25,36
High systemic levels of proinflammatory cytokines IL-6 and IL-8 have been previously been found in FM.16,17 Here, we investigated a large number of plasma cytokines at the same time. Notably, there is some overlap between the list of discriminating plasma proteins (Table 2) and the CSF protein list (Table 1), reflecting that although plasma and CSF partly mirror different compartments, they are also interlinked. We have also confirmed previous findings, namely, that systemic IL-8 levels are elevated in these patients (Table 2). Although IL-6 does not appear in Table 2, ie, IL-6 is not one of the major group discriminating proteins when taking the whole correlation structure of the material into consideration, when looking at IL-6 with classical univariate statistics, we still found a significant difference between groups, IL-6 being elevated in FM patients (p<0.001).
Neuroimmunity and chronic pain
A large proportion of the chemokines listed in Table 1 belong to the CC or the CXL subfamilies of chemokines.37 Chemokines are expressed by neurons, glia and neural progenitor cells, and the synthesis is increased in response to injury. These chemokines initiate cytokine activations, leading to neuroinflammation.38 Hence, our results are in line with several lines of evidence concerning the role of neuroimmunity in chronic pain.25,26,39 For instance, in animal models of pathological pain conditions, neuron-to-glia communication in the spinal cord has been shown to be mediated by, among other substances, the chemokine CX3CL1 (also known as fractalkine) released from damaged or activated first-order neurons.25 Indeed, CX3CL1/fractalkine has been proposed as one of the most prominent signaling pathways in preclinical models of neuropathic pain.40–42 Hence, the presence of CX3CL1/fractalkine among our principal findings is notable (Table 1 and Figure 2F). Together with its signaling pair cathepsin S, fractalkine is a novel therapeutic approach for the treatment of chronic pain,43 for instance by cathepsin S inhibition.44 Among the substances listed in Table 1, we also want to highlight IL-18. Animal models (albeit for neuropathic pain) suggest that IL-18 is an important mediator for the development of pathological pain.45,46
The process of gliosis is characterized by activated microglia releasing key multifunctional cytokines (TNF-α, IL-1β, IL-6) that orchestrate the subsequent production of downstream algesic mediators.36,47,48 In this context, it is also important to report negative results. Notably, in the present study, IL-6, MCP-1 (also known as CCL-2), and beta-NGF were not important for class discrimination in CSF. It is possible that these “classical” mediators are animal model specific (translation is a well-known problem49) or that they are specific for neuropathic pain as opposed to FM.35 Also, it is important to acknowledge that almost all values of TNF-α, BDNF and GDNF were under the LoD, and these three proteins were hence not part of the OPLS-DA model. Given previous findings,18,50 it is also notable that IL-8 is not listed in our top 11 list in Table 1. However, a retrospective look at this particular cytokine showed a VIP=1.17, ie, higher than the usual cut-off value for “significant” VIP, indicating that IL-8 does contribute to the model, albeit not very strongly. Moreover, the levels of IL-8 were significantly higher in patients by univariate statistics (p=0.001). Hence, the present study confirmed that CSF IL-8 levels are high in FM.18
It is interesting to compare the present findings in Table 2 to the findings of Moen et al,32 who recently used the same multiplex panel in patients with chronic lumbar radicular pain. In all, 16 out of the 21 proteins (76%) listed in Table 2 are described by Moen et al as being significantly upregulated in patients with a high-level pain. This high degree of overlap may perhaps point to a common inflammatory pattern in chronic pain, regardless of which chronic pain condition is studied. However, despite a significant overlap, different chronic pain conditions may exhibit partly different systemic inflammatory profiles. In the present study, considering Table 2, the following five proteins were not described by Moen et al: IL-7, CD244, ADA, MMP-1, and EN-RAGE.
Although many FM patients use nonsteroidal anti-inflammatory drugs (NSAIDs),2 the current scientific evidence is usually not considered strong enough to warrant a general recommendation for the use of NSAIDs.3 Hence, although the results of the present study point to the importance of chronic inflammation in FM, it is important not to jump to conclusions concerning the use of NSAIDs in this pain condition. FM being a chronic condition, it is important to ponder the potential side effects of long-term NSAID use.51 Most FM patients being women, the possible relationship between inflammation and levels of ovarian hormones in FM patients is also a possible area for future investigations.52–54
Study limitations
There are a number of limitations to the present study. First, although the CSF control group was reasonably well matched by age, it was much smaller than the patient group. Second, as the plasma control group consisted of blood donors, limited information was available about them, although blood donors can be expected to be fairly healthy. Indeed, blood donors are often used to determine reference values for new biomarkers. To summarize these two points about the control groups used in this study, the plasma control group was more adequate size-wise than the CSF control group, but on the other hand, it was less well characterized. Ideally, of course, it would have been best to have the same control group for both body fluids, but this was not possible for practical reasons.
Third, the FM patients were all females, whereas the two control groups were mixed. Although neither our CSF nor our plasma main results seemed to be influenced by sex, our findings should therefore not unreflectively be generalized to men. Fourth, body mass index (BMI), which was not registered in this study, can influence the inflammatory profile. Notably, for the interpretation of the results of the present study, it must be acknowledged that this has been shown for CXCL10, CXCL6, CX3CL1 and CCL19; however, Spearman’s rho did not range higher than 0.20–0.26 for these substances.55 In another study, it was shown that 15 out of 63 cytokines in plasma were associated with age;56 hence, having age-matched controls is probably important.
Fifth, the most important limitation of the present study is perhaps that the controls came from other centers than the patients. Hence, the question arises if our results can be explained by different pre-analysis handlings of CSF and/or plasma samples. However, using the same multiplex inflammatory panel in patients with high and low levels of pain, Moen et al32 found a clear inflammatory pattern in patients with high levels of chronic radicular pain. Therefore, even though the handling of samples by different centers remains a major limitation of the present study (making the results somewhat uncertain), the results of Moen et al show that it is possible to find clear inflammatory differences between groups of chronic pain patients even when the controls are recruited from the same center. Hence, dismissing our results outright as an error of measurement due to different sample handling does not seem to be well founded, although it of course remains a possibility. The findings in the present study need to be confirmed in other cohorts where patient and control samples were handled by the same study personnel prior to analysis.
Question of causality
Granted that our results are valid, is the present inflammatory fingerprint directly related to the pathophysiology of FM (eg, central sensitization due to neuroinflammation?) or is it an inflammatory risk factor that was present prior to the development of chronic pain (eg, a genetic susceptibility57)? A third possibility could be that the fingerprint is a consequence of the chronic pain condition, eg, mirroring pain-related stress, inactivity,58 depression59 or bad sleep.60 Whether our findings are a risk factor for, a direct mirror of or a consequence of the pathophysiological processes involved in these patients is hence an important area for further investigations. Of course, all three of these categories may play a role. One could for instance hypothesize that some individuals are more inflammation prone at the outset (a risk factor) and that they therefore develop a strong neuroimmune and/or systemic reaction leading both to the experience of pain and to other symptoms of the “sickness syndrome”.14 All of this is of course highly speculative but makes good physiological sense. Disentangling the contribution of these potentially mutually interacting factors will be very difficult. For instance, levels of peripheral IL-6 are known to be influenced by regular exercise, individuals who are inactive having higher baseline levels of this particular cytokine.58
Statistical considerations
When analyzing many variables, the so-called multiple testing problem is an ever-present concern.61 Theoretically, using a traditional significance level of 0.05, if “k” is the number of comparisons, the risk of a least one false positive is 1-0.95k.30 In this study, the risk of at least one false-positive CSF finding would hence be 93% (see the Results section), and for our plasma results (given the higher number of variables), it would be even higher. However, the formula assumes that the variables are independent of each other, and when looking at cytokines and chemokines, this assumption is not well founded. Moreover, MVDA looks at all the variables together at the same time, taking the correlation structure of the dataset into consideration, thereby favoring structure and information over “noise”.30 Hence, although the multiple testing issue should not be dismissed too lightly, it should not on the other hand be exaggerated. There may of course be some false positives among our findings, but all in all it does not seem sensible to dismiss all our results as a gigantic type I error. The MVDA methodology used in the present study is the same as used by the Linköping group in a number of recent peer-reviewed publications in different journals,22,29,62–66 and it is congruent with the principles argued for by Wheelock and Wheelock.28 Finally, in order to ensure the robustness of our statistical methodology, the CSF data of the present study were recomputed using the statistical methodology described by Moen et al32 (with use of false discovery rate); the result of this recomputation was exactly the same as the list presented in Table 1 (TG being the last author of both articles).
Conclusion
Instead of looking at a limited number of predetermined cytokines, we have used an inflammatory panel on patients with FM, analyzing both CSF and plasma. Group separation was achieved for both body fluids, and the present study is the most extensive, “holistic” inflammatory profiling study of FM patients to date. We found evidence of both neuroinflammation (as assessed in CSF) and chronic systemic inflammation (as assessed in plasma).
Acknowledgment
This research project was supported by Uppsala Berzelii Technology Centre for Neurodiagnostics, with financing from the Swedish Governmental Agency for Innovation Systems (Vinnova) and the Swedish Research Council (grant no. P29797-1).
Disclosure
The authors report no conflicts of interest in this work.
References
Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311(15):1547–1555. | ||
Hauser W, Walitt B, Fitzcharles MA, Sommer C. Review of pharmacological therapies in fibromyalgia syndrome. Arthritis Res Ther. 2014;16(1):201. | ||
Calandre EP, Rico-Villademoros F, Slim M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin Pharmacother. 2015;16(9):1347–1368. | ||
Cording M, Derry S, Phillips T, Moore RA, Wiffen PJ. Milnacipran for pain in fibromyalgia in adults. Cochrane Database Syst Rev. 2015;(10):CD008244. | ||
Derry S, Cording M, Wiffen PJ, Law S, Phillips T, Moore RA. Pregabalin for pain in fibromyalgia in adults. Cochrane Database Syst Rev. 2016;9:CD011790. | ||
Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115. | ||
Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology (Oxford). 2008;47(5):670–678. | ||
Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15. | ||
Desmeules JA, Cedraschi C, Rapiti E, et al. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48(5):1420–1429. | ||
DeSantana JM, Sluka KA. Central mechanisms in the maintenance of chronic widespread noninflammatory muscle pain. Curr Pain Headache Rep. 2008;12(5):338–343. | ||
Boomershine CS. Fibromyalgia: the prototypical central sensitivity syndrome. Curr Rheumatol Rev. 2015;11(2):131–145. | ||
Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70(1):41–51. | ||
Staud R, Nagel S, Robinson ME, Price DD. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. Pain. 2009;145(1–2):96–104. | ||
Ahrens C, Schiltenwolf M, Wang H. Zytokine im psychoneuroendokrin-immunologischen Kontext unspezifischer muskuloskeletaler Schmerzen. [Cytokines in psychoneuroendocrine immunological context of nonspecific musculoskeletal pain]. Schmerz. 2012;26(4):383–388. German. | ||
Kiguchi N, Kobayashi Y, Kishioka S. Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr Opin Pharmacol. 2012;12(1):55–61. | ||
Uceyler N, Hauser W, Sommer C. Systematic review with meta-analysis: cytokines in fibromyalgia syndrome. BMC Musculoskelet Disord. 2011;12:245. | ||
Rodriguez-Pinto I, Agmon-Levin N, Howard A, Shoenfeld Y. Fibromyalgia and cytokines. Immunol Lett. 2014;161(2):200–203. | ||
Kadetoff D, Lampa J, Westman M, Andersson M, Kosek E. Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J Neuroimmunol. 2012;242(1–2):33–38. | ||
Almay BG, Johansson F, Von Knorring L, Le Greves P, Terenius L. Substance P in CSF of patients with chronic pain syndromes. Pain. 1988;33(1):3–9. | ||
Vaeroy H, Helle R, Forre O, Kass E, Terenius L. Elevated CSF levels of substance P and high incidence of Raynaud phenomenon in patients with fibromyalgia: new features for diagnosis. Pain. 1988;32(1):21–26. | ||
Russell IJ, Orr MD, Littman B, et al. Elevated cerebrospinal fluid levels of substance P in patients with the fibromyalgia syndrome. Arthritis Rheum. 1994;37(11):1593–1601. | ||
Bäckryd E, Ghafouri B, Larsson B, Gerdle B. Do low levels of beta-endorphin in the cerebrospinal fluid indicate defective top-down inhibition in patients with chronic neuropathic pain? A cross-sectional, comparative study. Pain Med. 2014;15(1):111–119. | ||
Vaeroy H, Helle R, Forre O, Kass E, Terenius L. Cerebrospinal fluid levels of beta-endorphin in patients with fibromyalgia (fibrositis syndrome). J Rheumatol. 1988;15(12):1804–1806. | ||
Baraniuk JN, Whalen G, Cunningham J, Clauw DJ. Cerebrospinal fluid levels of opioid peptides in fibromyalgia and chronic low back pain. BMC Musculoskelet Disord. 2004;5:48. | ||
Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14(4):217–231. | ||
Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16(11):1267–1276. | ||
Antunes-Martins A, Perkins JR, Lees J, Hildebrandt T, Orengo C, Bennett DL. Systems biology approaches to finding novel pain mediators. Wiley Interdiscip Rev Syst Biol Med. 2013;5(1):11–35. | ||
Wheelock AM, Wheelock CE. Trials and tribulations of ’omics data analysis: assessing quality of SIMCA-based multivariate models using examples from pulmonary medicine. Mol Biosyst. 2013;9(11):2589–2596. | ||
Bäckryd E, Ghafouri B, Carlsson AK, Olausson P, Gerdle B. Multivariate proteomic analysis of the cerebrospinal fluid of patients with peripheral neuropathic pain and healthy controls – a hypothesis-generating pilot study. J Pain Res. 2015;8:321–333. | ||
Eriksson L, Byrne T, Johansson E, Trygg J, Vikström C. Multi- and Megavariate Data Analysis: Basic Principles and Applications. 3rd ed. Malmö: MKS Umetrics AB; 2013. | ||
Assarsson E, Lundberg M, Holmquist G, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9(4):e95192. | ||
Moen A, Lind AL, Thulin M, et al. Inflammatory serum protein profiling of patients with lumbar radicular pain one year after disc herniation. Int J Inflam. 2016;2016:3874964. | ||
Wold S, Sjöström M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemometr Intell Lab Syst. 2001;58(2):109–130. | ||
Roche S, Gabelle A, Lehmann S. Clinical proteomics of the cerebrospinal fluid: towards the discovery of new biomarkers. Proteomics Clin Appl. 2008;2(3):428–436. | ||
Ellis A, Bennett DL. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth. 2013;111(1):26–37. | ||
Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010;10(3):167–184. | ||
Palomino DC, Marti LC. Chemokines and immunity. Einstein (Sao Paulo). 2015;13(3):469–473. | ||
White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov. 2005;4(10):834–844. | ||
Liou JT, Lee CM, Day YJ. The immune aspect in neuropathic pain: role of chemokines. Acta Anaesthesiol Taiwan. 2013;51(3):127–132. | ||
Old EA, Clark AK, Malcangio M. The role of glia in the spinal cord in neuropathic and inflammatory pain. Handb Exp Pharmacol. 2015;227:145–170. | ||
Clark AK, Malcangio M. Fractalkine/CX3CR1 signaling during neuropathic pain. Front Cell Neurosci. 2014;8:121. | ||
Clark AK, Yip PK, Malcangio M. The liberation of fractalkine in the dorsal horn requires microglial cathepsin S. J Neurosci. 2009;29(21):6945–6954. | ||
Clark AK, Malcangio M. Microglial signalling mechanisms: cathepsin S and Fractalkine. Exp Neurol. 2012;234(2):283–292. | ||
Clark AK, Yip PK, Grist J, et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc Natl Acad Sci U S A. 2007;104(25):10655–10660. | ||
Pilat D, Piotrowska A, Rojewska E, et al. Blockade of IL-18 signaling diminished neuropathic pain and enhanced the efficacy of morphine and buprenorphine. Mol Cell Neurosci. 2016;71:114–124. | ||
Xu J, Chen XM, Zheng BJ, Wang XR. Electroacupuncture relieves nerve injury-induced pain hypersensitivity via the inhibition of spinal P2´7 receptor-positive microglia. Anesth Analg. 2016;122(3):882–892. | ||
Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial cells and chronic pain. Neuroscientist. 2010;16(5):519–531. | ||
Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009;(194):417–449. | ||
Mao J. Translational pain research: achievements and challenges. J Pain. 2009;10(10):1001–1011. | ||
Lundborg C, Hahn-Zoric M, Biber B, Hansson E. Glial cell line-derived neurotrophic factor is increased in cerebrospinal fluid but decreased in blood during long-term pain. J Neuroimmunol. 2010;220(1–2):108–113. | ||
Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 2013;16(5):821–847. | ||
Hassan S, Muere A, Einstein G. Ovarian hormones and chronic pain: a comprehensive review. Pain. 2014;155(12):2448–2460. | ||
Shivers KY, Amador N, Abrams L, Hunter D, Jenab S, Quinones-Jenab V. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic-pituitary-adrenal axis activity. Cytokine. 2015;72(2):121–129. | ||
Chaireti R, Lindahl TL, Bystrom B, Bremme K, Larsson A. Inflammatory and endothelial markers during the menstrual cycle. Scand J Clin Lab Invest. 2016;76(3):190–194. | ||
Larsson A, Carlsson L, Lind AL, et al. The body mass index (BMI) is significantly correlated with levels of cytokines and chemokines in cerebrospinal fluid. Cytokine. 2015;76(2):514–518. | ||
Larsson A, Carlsson L, Gordh T, Lind AL, Thulin M, Kamali-Moghaddam M. The effects of age and gender on plasma levels of 63 cytokines. J Immunol Methods. 2015;425:58–61. | ||
Dominguez CA, Kalliomaki M, Gunnarsson U, et al. The DQB1 *03:02 HLA haplotype is associated with increased risk of chronic pain after inguinal hernia surgery and lumbar disc herniation. Pain. 2013;154(3):427–433. | ||
Pedersen BK. Muscles and their myokines. J Exp Biol. 2011;214(pt 2):337–346. | ||
Walker AK, Kavelaars A, Heijnen CJ, Dantzer R. Neuroinflammation and comorbidity of pain and depression. Pharmacol Rev. 2014;66(1):80–101. | ||
Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010;24(5):775–784. | ||
Streiner DL, Norman GR. Correction for multiple testing: is there a resolution? Chest. 2011;140(1):16–18. | ||
Olausson P, Gerdle B, Ghafouri N, Sjostrom D, Blixt E, Ghafouri B. Protein alterations in women with chronic widespread pain – an explorative proteomic study of the trapezius muscle. Sci Rep. 2015;5:11894. | ||
Olausson P, Ghafouri B, Ghafouri N, Gerdle B. Specific proteins of the trapezius muscle correlate with pain intensity and sensitivity – an explorative multivariate proteomic study of the trapezius muscle in women with chronic widespread pain. J Pain Res. 2016;9:345–356. | ||
Bäckryd E, Ghafouri B, Larsson B, Gerdle B. Plasma pro-inflammatory markers in chronic neuropathic pain: a multivariate, comparative, cross-sectional pilot study. Scand J Pain. 2016;10:1–5. | ||
Hadrevi J, Ghafouri B, Larsson B, Gerdle B, Hellstrom F. Multivariate modeling of proteins related to trapezius myalgia, a comparative study of female cleaners with or without pain. PLoS One. 2013;8(9):e73285. | ||
Gerdle B, Kristiansen J, Larsson B, Saltin B, Sogaard K, Sjogaard G. Algogenic substances and metabolic status in work-related Trapezius Myalgia: a multivariate explorative study. BMC Musculoskelet Disord. 2014;15:357. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.