Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Evaluation of the Effect of Fish Oil Alone and in Combination with a Proprietary Chromium Complex on Endothelial Dysfunction, Systemic Inflammation and Lipid Profile in Type 2 Diabetes Mellitus – A Randomized, Double-Blind, Placebo-Controlled Clinical Study
Authors Pingali U , Nutalapati C , Illendulla VS
Received 19 June 2019
Accepted for publication 7 December 2019
Published 7 January 2020 Volume 2020:13 Pages 31—42
DOI https://doi.org/10.2147/DMSO.S220046
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Usharani Pingali, 1 Chandrasekhar Nutalapati, 1 Vijay Sravanthi Illendulla 2
1Department of Clinical Pharmacology & Therapeutics, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, 500082, India; 2Govt. Ayurvedic Dispensary, Primary Health Center Gummadidala, Sangareddy, Telangana, 502313, India
Correspondence: Usharani Pingali
Department of Clinical Pharmacology & Therapeutics, Nizam’s Institute of Medical Sciences, Hyderabad 500082, Telangana, India
Tel +91 9849574143
Email [email protected]
Purpose: This study was conducted to evaluate the effectiveness of fish oil alone and with an adjunct, a proprietary chromium complex (PCC), on cardiovascular parameters – endothelial dysfunction, lipid profile, systemic inflammation and glycosylated hemoglobin – in a 12-week randomized, double-blind, placebo-controlled clinical study in type 2 diabetes mellitus subjects.
Patients and Methods: In this randomized, double-blind, parallel group study, 59 subjects in three groups completed the study: Group A, fish oil 2000 mg; Group B, fish oil 2000 mg + PCC 10 mg (200 μg of Cr 3+); and Group C, fish oil 2000 mg + PCC 20 mg (400 μg of Cr 3+) daily for 12 weeks (2000 mg of fish oil contained 600 mg of eicosapentaenoic acid [EPA] and 400 mg of docosahexaenoic acid [DHA], the omega-3 fatty acids). Endothelial function, by estimating reflection index (RI), biomarkers of oxidative stress (nitric oxide [NO], malondialdehyde [MDA], glutathione [GSH]) and inflammatory biomarkers (high-sensitivity C-reactive protein [hsCRP], intercellular adhesion molecule-1 [ICAM-1], vascular cell adhesion molecule-1 [VCAM-1], endothelin-1) were evaluated at baseline, and 4 and 12 weeks. Lipid profile, platelet aggregation and glycosylated hemoglobin [HbA1c) were tested at baseline and 12 weeks. Any reported adverse drug reactions were recorded. Statistical analysis was performed using GraphPad Prism 8.
Results: The present study shows that fish oil by itself, at a dose of 2000 mg (600 mg of EPA + 400 mg of DHA) per day, led to significant, but only modest, improvement in cardiovascular parameters (RI from − 2.38± 0.75 to − 3.92± 0.60, MDA from 3.77± 0.16 to 3.74± 0.16 nM/mL, NO from 30.60± 3.18 to 32.12± 3.40 μM/L, GSH from 568.93± 5.91 to 583.95± 6.53 μM/L; p≤ 0.0001), including triglyceride levels. However, when PCC was added to fish oil, especially at the 20 mg dose, there were highly significant improvements in all the parameters tested (RI from − 2.04± 0.79 to − 8.73± 1.36, MDA from 3.67± 0.39 to 2.89± 0.34 nM/mL, NO from 28.98± 2.93 to 40.01± 2.53 μM/L, GSH from 553.82± 8.18 to 677.99± 10.19 μM/L; p≤ 0.0001), including the lipid profile. It is noteworthy that the triglycerides were decreased significantly by addition of 20 mg of PCC although the dose of fish oil was only 2 g/day and the baseline triglyceride levels were only about 200 mg/dL. Fish oil alone did not significantly decrease the HbA1c, whereas the addition of 20 mg of PCC did.
Conclusion: Addition of PCC, especially at 20 mg dose, significantly improves the efficacy of fish oil in addressing cardiovascular risk factors compared to fish oil given alone.
Keywords: fish oil, Crominex®, diabetes mellitus, omega-3 fatty acids, trivalent chromium, Cr3+
Introduction
Cardiovascular diseases (CVDs), which include coronary heart disease (CHD) and cerebrovascular diseases, account for 21.9% of total mortality and are predicted to increase to 26.3% by 2030. They are said to be the dominant cause of death globally.
CVD is a major cause of death and disability among people with type 2 diabetes mellitus (T2DM). Adults with diabetes historically have a higher prevalence rate of CVD than adults without diabetes, and the risk of CVD increases continuously with rising fasting plasma glucose levels, even before reaching levels sufficient for a diabetes diagnosis. Among the specific diseases within that term, coronary artery disease is supposed to be the most lethal. An estimated 17.9 million people died from CVDs in 2016, representing 31% of all global deaths. Of these deaths, 85% are due to heart attack and stroke.1–3
Endothelial dysfunction (EnD) is presumed to play a significant role in the development of microvascular and macrovascular disease, causing a considerable increase in atherosclerotic vascular disease.3 Diminished bioavailability of the vasodilator nitric oxide (NO), chiefly due to increase in the deterioration of NO levels by reactive oxygen species (ROS), results in EnD.4 Oxidative stress, through a single unifying mechanism of superoxide production, is the common pathogenic factor leading to insulin resistance, β-cell dysfunction, impaired glucose tolerance and ultimately to T2DM. It is suspected to be the underlying cause of both the macrovascular and microvascular complications associated with T2DM. So, therapies aimed at decreasing oxidative stress would be of use in patients with T2DM, and those at risk for developing diabetes.5 T2DM is managed with oral anti-diabetic drugs. However, lifestyle modifications, such as daily exercise and diet control, are essential components in addressing the problem.
Dyslipidemia is a common feature of diabetes.6 It seems that dyslipidemia substantially alters endothelial function, causing a decrease in relaxation of the arterial vessels.7,8 This irregularity has been observed mainly in atherosclerotic patients as well as in asymptomatic subjects with hypercholesterolemia.9,10
Diabetes mellitus and hypertension are frequently present together. Among many microvascular complications of diabetes, hypertension has a predominant role in the progression of diabetic nephropathy by glomerular hyperfiltration. Hypertension also induces atherosclerosis in diabetes.11 Data from the MRFIT study12 show that the association between cholesterol and coronary artery disease is strong and graded across all levels of blood pressure; similarly, the association between blood pressure and coronary artery disease is strong and graded across all levels of cholesterol.
Dietary supplementation with omega-3 fatty acids (ω-3FAs) from fish and krill oils has been a popular way to improve the lipid profile, especially the triglyceride (TG) levels, in the blood.13–22 Despite the controversy regarding the benefits of ω-3FAs, as per the latest Science Advisory from the American Heart Association, treatment with ω-3FA supplements in patients with CHD, such as myocardial infarction, is considered reasonable, and ω-3FA treatment is also suggested for patients with prevalent heart failure without conserved left ventricular function to reduce mortality and hospitalizations.23
Proprietary chromium complex (PCC; Crominex® 3+) is prepared by complexation of trivalent chromium chloride with an aqueous extract of Phyllanthus emblica fruit, which contains polyphenolic compounds, and purified Shilajit, which, by virtue of its liposomal fulvic acid content, increases the bioavailability of chromium. Microcrystalline cellulose is used as a placebo carrier in the complex.24 Trivalent chromium is an essential mineral that appears to play a favorable role in the regulation of insulin action, metabolic syndrome and CVD.25,26 Phyllanthus emblica, generally recognized as Indian gooseberry or “Amla”, has been utilized as a well-being product for centuries in India and other Asian nations. Clinical studies in humans have proven the hypolipidemic, antioxidant and cardioprotective activities of P. emblica.27–33 Shilajit, a rock exudate containing free and chromoprotein-conjugated dibenzo-α-pyrones (urolithins) and fulvic acids as bioactives, finds broad use in Ayurveda for different clinical applications, such as improving cardiovascular health, upregulating collagen and related extracellular matrix protein genes34–38 Shilajit seems to induce the growth of blood vessels39 and has a prominent cardioprotective effect.40
A clinical study with ω-3FA, in combination with another product, to broaden the spectrum of cardiovascular benefits of ω-3FA in T2DM, without increasing the size or cost of the dosage form significantly, is warranted. PCC, which has been shown previously in T2DM patients to improve endothelial function and the lipid profile,41–43 may prove to be an ideal candidate to be added to ω-3FA, as the dose is small (10–20 mg). So, this study was planned to evaluate the effect of fish oil alone and in combination with a PCC on endothelial dysfunction, systemic inflammation and the lipid profile in T2DM subjects.
Materials and Methods
This prospective, randomized, double-blind, parallel-group study was conducted in the Department of Clinical Pharmacology and Therapeutics, Nizam’s Institute of Medical Sciences, Hyderabad, India. The study was initiated after prior approval from the Institutional Ethics Committee. This trial was conducted in accordance with the Declaration of Helsinki. The CONSORT flow diagram is depicted in Figure 1. Written informed consent was obtained from all subjects prior to their participation in the study.
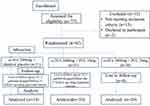 |
Figure 1 CONSORT flow diagram. |
Subjects
Inclusion Criteria
The study included male and female subjects aged 30–65 years, willing to give informed consent and to adhere to protocol-related study procedures, with fasting plasma glucose of 110–126 mg/dL and glycosylated hemoglobin (HbA1c) between 6.5% and 8%; patients on a steady dose of anti-diabetic treatment (metformin 1500–2500 mg/day) for the past 8 weeks preceding the screening; and with EnD (salbutamol challenge test), reported as ≤6% change in reflection index (RI), and not on any investigational products in the previous 6 months.
Exclusion Criteria
Subjects were excluded if they had abnormal hematological or biochemical parameters considered significant by the investigators, uncontrolled hypertension (SBP >180 mmHg and DBP >100 mmHg), aspartate transaminase (AST) and alanine transaminase (ALT) elevation greater than three times the upper limit of normal, serum creatinine >1.5 mg/dL, serum triglycerides (TGs) >500 mg/dL, were on any other dietary or herbal supplements, and/or had any medical condition where the physician felt that participation in the study could be detrimental to their well-being.
Outcome Measures
Primary
Primary outcome measures were:
- Improvement in endothelial function as estimated by >6% change in RI and endothelial function biomarkers (nitric oxide [NO], glutathione [GSH] and malondialdehyde [MDA]), at 12 weeks in all treatment groups.
- Improvement in vascular inflammation biomarkers (intercellular adhesion molecule-1 [ICAM-1], human vascular cell adhesion molecule-1 [VCAM-1] and endothelin-1 [ET-1]), systemic inflammation biomarker (high-sensitivity C-reactive protein [hsCRP]), glycosylated hemoglobin (HbA1c), platelet aggregation and lipid profile.
Secondary
The secondary outcome measure was to assess the safety and tolerability of the test medications.
Randomization and Blinding
Out of 77 subjects who were screened, 62 subjects complying with the inclusion criteria were randomized by computer-generated block randomization to one of the three treatment groups in a double-blinded fashion. Three subjects dropped out of the study before the first follow-up; 59 subjects completed 12 weeks of treatment.
All the study medications (capsules) were similar in size, color, shape and texture. Each subject was given a sealed packet containing two sealed bottles, each bottle containing 33 capsules. They were instructed to take one capsule from each bottle daily each morning with water, after breakfast, for a period of 4 weeks. Thereafter, at each visit the subject was given a new sealed packet containing two sealed bottles and asked to consume the contents in the same pattern as instructed earlier.
- Group A: one capsule from each bottle once a day, each soft gelatin capsule containing 1000 mg of a refined fish oil concentrate having 300 mg of eicosapentaenoic acid (EPA) (TG) and 200 mg of docosahexaenoic acid (DHA) (TG), to provide a total dose of 2000 mg of fish oil concentrate per day.
- Group B: one capsule from each bottle once a day, one bottle containing a soft gelatin capsule of 1000 mg of a refined fish oil concentrate having 300 mg of EPA (TG) and 200 mg of DHA (TG), and the other bottle containing a soft gelatin capsule of 1000 mg of a refined fish oil concentrate, containing 300 mg of EPA (TG) and 200 mg of DHA (TG), and 10 mg of PCC (equivalent to 200 µg of trivalent chromium) to provide a total dose of 2000 mg of fish oil and 10 mg of PCC (equivalent to 200 µg of trivalent chromium) per day.
- Group C: one capsule from each bottle once a day, each soft gelatin capsule containing 1000 mg of a refined fish oil concentrate having 300 mg of EPA (TG) and 200 mg of DHA (TG), and 10 mg of PCC (equivalent to 200 µg of trivalent chromium) to provide a total dose of 2000 mg of fish oil and 20 mg of PCC (equivalent to 400 µg of trivalent chromium) per day.
The bottles containing the test products were sequentially designated numbers and were dispensed by the pharmacist to the subjects as per the randomly allocated sequence. The principal investigator and the subjects were blinded. After the completion of the study period the allocations were unblinded to tabulate the data and perform the statistical analyses.
All subjects were on a steady dose of concomitant medication (metformin 1500–2500 mg/day) over previous 8 weeks as prescribed by the physician, and the same was continued throughout the study.
Follow-Up Visits
Subjects were recalled for follow-up visits at 4, 8 and 12 weeks of therapy; they were evaluated for efficacy and safety at these visits. Endothelial function as estimated by RI was performed at every visit (baseline, 4, 8 and 12 weeks). Blood samples were collected for evaluation of oxidative markers (MDA, NO, GSH) and inflammatory markers (hsCRP, ICAM-1, VCAM-1, ET-1) at baseline and 4 and 12weeks. Lipid profile, HbA1c and platelet aggregation were evaluated at baseline and at the end of 12 weeks. A complete physical examination and laboratory investigations for safety parameters including hematological, hepatic and renal biochemical parameters were conducted at baseline and at the end of 12 weeks, and, if deemed necessary, during the study. At each visit subjects were asked to report the presence of adverse drug reaction, and these were recorded in the case report form, if any. The pill-count method was adapted to check compliance with study medications. Compliance was considered good if >80% of the time >80% of medication was taken, fair if it was between 60% and 80%, and poor if it was <60%. In the present study the average compliance in all three groups was 90%.
Methods of Assessment
Endothelial function was evaluated by a salbutamol challenge test using digital volume plethysmography, as reported by Chowienczyk et al44 and Naidu et al.45 Subjects were examined in the supine position after 5 min of rest. A digital volume pulse (DVP) was obtained using a photoplethysmograph (Pulse Trace PCA2, PT200; Micro Medical, Kent, UK) transmitting infrared light at 940 nm, placed on the index finger of the right hand. The signal from the plethysmograph was digitized using a 12-bit analogue-to-digital converter with a sampling frequency of 100 Hz. DVP waveforms were recorded over a 20-s period and the height of the late systolic/early diastolic portion of the DVP was expressed as a percentage of the amplitude of the DVP to yield the RI, as per the procedure described in detail by Millasseau et al.46 After DVP recordings had been taken, three measurements of RI were calculated and the mean value was determined. Subjects were then administered 400 µg of salbutamol by inhalation. After 15 min, three measurements of RI were obtained again and the difference in mean RI before and after administration of salbutamol was used for assessing endothelial function. A decrease of ≤6% in RI post-salbutamol was considered as EnD.
NO47 (by colorimetric detection with Griess reagents), MDA48 (by thiobarbituric acid reactive substance test) and GSH49 (by Ellman’s method) levels were estimated spectrophotometrically. The ELISA method was employed to assess hsCRP, ET-1, ICAM-1 and VCAM-1 levels. Subjects were asked to come to the department after an overnight fast, for collection of samples for determination of hemoglobin, blood urea and serum creatinine, liver function tests, and lipid profile, ie, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C) and TGs, using appropriate standard techniques. A platelet aggregation test with 10 µM/mL ADP and 2 µg/mL collagen was performed by employing a platelet aggregometry test (Chrono-log light transmittance aggregometry).
Statistical Analysis
A total sample size of 78 was predicted, to enroll 66 subjects, assuming a pooled standard deviation of 2 units to achieve a power of 80%, 5% significance, screening failure of 10% and dropout rate of 10%. Study data are shown as mean ± SD. The paired t-test was applied to compare the mean change from baseline to post-treatment (4, 8 and 12 weeks of treatment) within groups and ANOVA for between-group comparisons. Post-hoc analysis between the groups was conducted using Tukey’s test. A p-value of ≤0.05 was taken to be statistically significant. GraphPad Prism 8 software was used to conduct statistical analyses.
Results
In this randomized study 59 subjects completed 12 weeks of treatment. A total of 19 subjects in Group A (fish oil 2000 mg), 20 subjects in Group B (fish oil 2000 mg + PCC 10 mg, equivalent to 200 µg of Cr3+) and 20 subjects in Group C (fish oil 2000 mg + PCC 20 mg, equivalent to 400 µg of Cr3+) completed the 12 weeks of treatment.
Table 1 depicts the demographic data of the study population.
 |
Table 1 Baseline Characteristics of Subjects |
As seen from Table 2, all groups showed significant improvement in RI, illustrating an improvement in endothelial function at 12 (p≤0.0001), 8 (p≤0.0001) and 4 (p≤0.001) weeks. On further analysis, treatment in Group C gave significantly better results than in Groups B and Group A at 12 weeks (p≤0.0001).
 |
Table 2 Effect of Treatment on Reflection Index % (RI, Measure of Endothelial Function) |
The biomarkers of oxidative stress showed improvement at 12 weeks in all the groups. As depicted in Table 3, statistically significant differences in mean percentage change were seen for MDA, NO and GSH. Similar results were obtained for the markers of inflammation at 12 weeks (hsCRP, ICAM-1, VCAM-1 and ET-1). Group B treatment was better than Group A (p<0.0001), while Group C treatment was more efficacious than the other groups (p<0.0001). The level of significance between Groups C and B in improving the ET-1 levels was p<0.05.
 |
Table 3 Effect of Treatment on Biomarkers of Oxidative Stress, Systemic Inflammation and Vascular Inflammation at 12 Weeks |
As shown in Table 4, at 12 weeks, statistical significance was seen with all three groups in mean percentage change in TC (−7.53%, −14.62%, −23.68 in Groups A, B, C, respectively), HDL (4.74%, 14.14%, 25.11%), LDL-C (−11.06%, −26.14%, −37.20%), VLDL (−10.23%, −15.98%, −28.06%) and TGs (−10.10%, −15.00%, −28.11%). The significance at the end of 12 weeks for Groups A, B and C was p<0.0001 compared to baseline. Between-group analysis showed Group C to be better than Group A (p<0.0001) and Group B (p<0.0001) in improving all the parameters except LDL-C, where there was no significant difference between Groups C and B. No effect was recorded on platelet aggregation in any of the groups.
 |
Table 4 Effect of Treatment on Lipid Profile at 12 Weeks |
 |
Table 5 Effect of Treatment on HbA1c at 12 Weeks |
A statistically significant (p<0.0001) decrease in HbA1c after 12 weeks of treatment was seen in Groups C (from 7.3% to 6.8%) and B (from 7.1% to 6.8%) (Table 5). Group A tended to show an increase in HbA1c, while both Groups B and C had decreased HbA1c, by a modest 3.2% and 6.4%, respectively. Between-group analysis showed Group C to be better than both Group A (p<0.0001) and Group B (p<0.05).
All safety hematological and biochemical parameters were found to be within normal limits in all groups at the end of 12 weeks. Only one subject in Group C complained of nausea, which subsided with symptomatic treatment. However, no subject in any of the groups dropped out of the study owing to adverse events.
Discussion
Significant, though modest, improvement in endothelial function (RI), biomarkers of oxidative stress, systemic and vascular inflammation, and lipid profile was seen with fish oil alone. Addition of PCC, especially at 20 mg dose (400 µg of Cr3+), significantly increased the efficacy of fish oil.
In a 12-week study by Wong et al50 on T2DM patients, no improvement in EnD was seen with ω-3FA (4 g/day). Similarly, Siniarski et al51 reported no improvement in EnD with 1000mg EPA plus 1000mg DHA taken per day. In a previous study52 in our department, we showed the beneficial effect of PCC on endothelial function in T2DM patients. In the present study, we observed that addition of PCC, especially at 20 mg (400 µg of Cr3+), significantly complemented the efficacy of ω-3FA in improving EnD in T2DM subjects. We presume that the highly significant improvement in endothelial function in the present study is associated with the combining of fish oil with PCC.
MDA is a by-product formed by lipid peroxidation due to free radical generation, whereas GSH is an endogenous antioxidant and is known to minimize lipid peroxidation resulting from excessive ROS.53 In our study we have shown statistically significant improvement in the levels of MDA and GSH in all three treatment groups. Although the effect of Group A treatment on MDA and GSH was modest, the endogenous antioxidant power increased significantly with the addition of PCC to fish oil. Group C showed significantly better results than Groups A and B. The results in the present study are similar to those of Shidfar et al,54 who related the potential mechanism for the decrease in MDA to the inhibitory effect of ω-3FAs on the pro-oxidant enzyme phospholipase A2 and stimulation of antioxidant enzymes. The improvement in GSH levels could be due to the positive effect of ω-3FAs in improving the levels of glutathione reductase.55 So, in our study we assume that the combination of fish oil with PCC is the reason for the better results compared to fish oil alone.
NO has an important role in the determination of vascular health. Impaired bioavailability of NO represents an essential feature of EnD.56 In a 12-week study by Asemi et al,57 ω-3FA alone and its combination with alpha-tocopherol significantly increased NO levels. This was attributed to direct and indirect effects of ω-3FA on the arterial wall. In the present study, after 12 weeks of treatment, levels of NO increased significantly in all the groups. Group C showed significantly better results than both Groups A and B, substantiating that endothelial function had indeed improved very significantly with the addition of PCC to fish oil. The probable mechanism in improved NO levels could be as reported by Asemi et al.57 We assume that the combination of fish oil with PCC is more beneficial than fish oil alone.
Yamada et al,58 in their study on metabolic syndrome subjects, reported that EPA improved the levels of the adhesion molecules ICAM-1 and VCAM-1. Their results are in concurrence with the highly significant reductions in ICAM-1, VCAM-1 and ET-1 in all three treatment groups in our study, with the best improvement seen in Group C. NO, a vasodilator, seems to have anti-inflammatory properties and thus decreases ICAM-1 and VCAM-1 expression.59 In our study, as mentioned in the previous paragraph, a significant improvement in NO levels was seen. The positive effect of ω-3FA on NO levels, along with the inhibitory effect on endothelial expression of adhesion molecules, is the possible mechanism of action of ω-3FAs.60 The addition of PCC to fish oil in our study seems to have provided a highly significant response on biomarkers of vascular inflammation, compared to fish oil given alone.
C-reactive protein is a robust biomarker of chronic systemic inflammation. Higher values of hsCRP are associated with metabolic disorders and its components.61 Schiano et al62 and Mackay et al63 reported no change in hsCRP and platelet aggregation with ω-3FAs. In previous studies27,64 carried out in our department, we reported the beneficial effects of PCC and its constituent materials on hsCRP. In the present study, we observed a significant change in hsCRP values at 12 weeks in all three treatment groups. Group C showed more robust improvement compared to the other two groups. We presume that this was due to the addition of PCC. No change in platelet aggregation, akin to the study by Mackay et al,63 was seen in this study.
In a study by Harris et al65 and Koski RR66 in hypertriglyceridemia subjects, fish oil (OMACOR – a prescription product) improved TG, TC, VLDL-C and HDL-C levels significantly, but increased the LDL-C levels. In our study, improvement in LDL-C was observed along with other parameters mentioned in the above studies.65,66 In a similar study by Bays et al,67 4g/day of ω-3FA (VASCEPA containing purified EPA and no DHA) significantly improved TGs without significantly increasing the LDL-C levels. Katoka Y et al,68 also reported the same. We observed a decrease in the LDL-C levels in our study. A study by Cicero et al69 in hypertriglyceridemia subjects reported improvements in TG and HDL-C levels while no significant difference was seen in TC and LDL-C levels. Sawada et al,70 in subjects with diminished glucose metabolism and coronary artery disease, reported improvement in fasting TG levels and TG/HDL-C ratio. They related this to improved endothelial function. In our study we observed significant improvements in all the parameters of the lipid profile in the three treatment groups. The most significant improvement, seen in Group C, can be attributed to the addition of PCC.
A meta-analysis of 83 randomized trials by Brown et al71 showed that increasing omega-3, omega-6 or total polyunsaturated fatty acids has little or no effect on the prevention and treatment of T2DM. In our study there was a significant improvement in HbA1c levels in Groups B and C, while Group A showed no improvement. The improvement in Groups B and C can be attributed to PCC.
Conclusion
It can be concluded from this study in T2DM subjects that fish oil by itself, at a dose of 2000 mg/day, containing 600 mg of EPA plus 400 mg of DHA, led to a modest but statistically significant improvement in the lipid profile, including the TG levels and the adhesion molecules, but only small effects on EnD, oxidative stress biomarkers and the systemic inflammation biomarker, and no effect on HbA1c. However, addition of PCC to fish oil, especially at 20 mg dose, significantly and robustly improved EnD, the biomarkers of oxidative stress, systemic inflammation and the lipid profile, but HbA1c only modestly. It is noteworthy that all three groups showed significant changes in lipid profile, although the most significant improvement in lipid profile was obtained with the group receiving fish oil plus 20 mg PCC. Furthermore, these results were obtained with only 2 g/day of fish oil. This study provides initial evidence that the addition of PCC (Crominex 3+) to fish oil significantly improved the cardiovascular health benefits of fish oil. The strengths of the study include that we evaluated the improvement in EnD by estimating the RI and biomarkers MDA, NO and GSH. We also evaluated the effect on the vascular inflammation biomarkers ICAM-1, VCAM-1 and ET-1, and the systemic inflammation biomarker hsCRP. A limitation of our study is that we did not include a placebo group.
Abbreviations
ALT, alanine transaminase; AST, aspartate transaminase; CHD, coronary heart disease; CRP, C-reactive protein; CVD, cardiovascular disease; DBP, diastolic blood pressure; DHA, docosahexaenoic acid; EnD, endothelial dysfunction; EPA, eicosapentaenoic acid; ET-1, endothelin-1; GSH, glutathione; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; ICAM-1, intercellular adhesion molecule-1; LDL-C, low-density lipoprotein cholesterol; MDA, malondialdehyde; MRIFT, Multiple Risk Factor Intervention Trial; NO, nitric oxide; PCC, proprietary chromium complex; ROS, reactive oxygen species; RI, reflection index; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; T2DM, type 2 diabetes mellitus; VCAM-1, vascular cell adhesion molecule-1; VLDL-C, very low-density lipoprotein cholesterol; ω-3FA, omega-3 fatty acid.
Data Sharing Statement
The data will be handled on a case-by-case basis as per institutional policy.
Acknowledgments
The authors are indebted to Natreon, Inc, USA, for arranging the study medications and kits for biomarkers, and study coordinator Mr Muralidhar. We express gratitude to Dr Y.S.N. Raju, Professor of General Medicine, NIMS, for his clinical support.
Ethics and Consent
The present study was started after obtaining approval from NIMS – Institutional Ethics Committee, review letter no. EC/NIMS/1978/2017; ESGS no. 520/2017. Prior to participation in the study, all subjects had provided written informed consent.
Registration of the trial: The study has been registered with Clinical Trials Registry – India (CTRI). Registration number: CTRI/2018/05/013547 [Registered on: 01/05/2018] trial registered retrospectively.
Disclosure
The authors declare no competing interests.
References
1. World Health Organization. World Health Statistics. Department of Measurement & Health Information Systems of the Information, Evidence and Research Cluster. Geneva: WHO Press; 2018:7.
2. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83. doi:10.1186/s12933-018-0728-6
3. Strain WD, Paldánius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018;17(1):57. doi:10.1186/s12933-018-0703-2
4. Stehouwer CD, Lambert J, Donker AJ, van Hinsbergh VW. Endothelial dysfunction and pathogenesis of diabetic angiopathy. Cardiovasc Res. 1997;34(1):55–68. doi:10.1016/S0008-6363(96)00272-6
5. Pandolfi A, De Filippis EA. Chronic hyperglicemia and nitric oxide bioavailability play a pivotal role in pro-atherogenic vascular modifications. Genes Nutr. 2007;2(2):195–208. doi:10.1007/s12263-007-0050-5
6. Schofield JD, Liu Y, Balakrishna PR, Malik RA, Soran H. Diabetes dyslipidemia. Diabetes Ther. 2016;7(2):203–219. doi:10.1007/s13300-016-0167-x
7. Kolluru GK, Bir SC, Kevil CG.Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med.2012;2012:1–30. doi:10.1155/2012/918267
8. Bretón-Romero R, Feng B, Holbrook M, et al. Endothelial dysfunction in human diabetes is mediated by Wnt5a-JNK signaling. Arterioscler Thromb Vasc Biol. 2016;36(3):561–569. doi:10.1161/ATVBAHA.115.306578
9. Park KH, Park WJ. Endothelial dysfunction: clinical implications in cardiovascular disease and therapeutic approaches. J Korean Med Sci. 2015;30(9):1213–1225. doi:10.3346/jkms.2015.30.9.1213
10. Daiber A, Steven S, Weber A, et al. Targeting vascular (endothelial) dysfunction. Br J Pharmacol. 2017;174(12):1591–1619.
11. Yamazaki D, Hitomi H, Nishiyama A. Hypertension with diabetes mellitus complications. Hypertens Res. 2018;41:147–156. doi:10.1038/s41440-017-0008-y
12. Neaton JD, Blackburn H, Jacobs D, Kuller L, Lee DJ, Sherwin R. Serum cholesterol level and mortality findings for men screened in the multiple risk factor intervention trial. Multiple risk factor intervention trial research group. Arch Intern Med. 1992;152(7):1490–1500.
13. Aung T, Halsey J, Kromhout D, et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77917 Individuals. JAMA Cardiol. 2018;3(3):225–233. doi:10.1001/jamacardio.2017.5205
14. Zivkovic AM, Telis N, German JB, Hammock BD. Dietary omega-3 fatty acids aid in the modulation of inflammation and metabolic health. Calif Agric (Berkeley). 2011;65(3):106–111. doi:10.3733/ca.v065n03p106
15. Zhang YF, Gao HF, Hou AJ, Zhou YH. Effect of omega-3 fatty acid supplementation on cancer incidence, non-vascular death and total mortality: a meta-analysis of randomized controlled trials. BMC Public Health. 2014;14:204. doi:10.1186/1471-2458-14-204
16. Din JN, Sarma J, Harding SA, Lyall K, Newby DE, Flapan AD. Effect of ω-3 fatty acid supplementation on endothelial function, endogenous fibrinolysis and platelet activation in patients with a previous myocardial infarction: a randomised controlled trial. BMJ Open. 2013;3:e003054. doi:10.1136/bmjopen-2013-003054
17. Chan DC, Watts GF, Barrett PHR, Beilin LJ, Mori TA. Effect of atorvastatin and fish oil on plasma high-sensitivity C-reactive protein concentrations in individuals with visceral obesity. Clin Chem. 2002;48(6):877–883.
18. Kromhout D, Yasuda S, Geleijnse JM, Shimokawa H. Fish oil and omega-3 fatty acids in cardiovascular disease: do they really work? Eur Heart J. 2012;33(4):436–443. doi:10.1093/eurheartj/ehr362
19. Aucoin M, Cooley K, Knee C, et al. Fish-derived omega-3 fatty acids and prostate cancer: a systematic review. Integr Cancer Ther. 2017;16:32–62. doi:10.1177/1534735416656052
20. Kwak SM, Myung SK, Lee YJ, Seo HG; Korean Meta-analysis Study Group. Efficacy of omega-3 Fatty acid supplements (Eicosapentaenoic acid and Docosahexaenoic acid) in the secondary prevention of cardiovascular disease. Arch Intern Med. 2012;172(9):686–694. doi:10.1001/archinternmed.2012.262
21. Grosso G, Galvano F, Marventano S, et al. Omega-3 fatty acids and depression: scientific evidence and biological mechanisms. Oxid Med Cell Longev. 2014;313570.
22. Weylandt KH, Serini D, Chen YQ, et al. Omega-3 polyunsaturated fatty acids: the way forward in times of mixed evidence. Biomed Res Int. 2015;143109.
23. Siscovick DS, Barringer TA, Fretts AM, et al. Omega-3 polyunsaturated fatty acid (Fish oil) supplementation and the prevention of clinical cardiovascular disease. A science advisory from the American Heart Association. Circulation. 2017;135(15):e867–84. doi:10.1161/CIR.0000000000000482
24. Nutalapati C, Chiranjeevi UK, Kishan PV, Kiran KK, Pingali U. A randomized, double-blind, placebo-controlled, parallel group clinical study to evaluate the analgesic effect of aqueous extract of terminalia chebula, a proprietary chromium complex, and their combination in subjects with joint discomfort. Asian J Pharm Clin Res. 2016;9(3):264–269.
25. Hua Y, Clark S, Ren J, Nair S. Molecular mechanisms of chromium in alleviating insulin resistance. J Nutr Biochem. 2012;23(4):313–319. doi:10.1016/j.jnutbio.2011.11.001
26. Bai J, Xun P, Morris S, Jacobs DR, Liu K, He K. Chromium exposure and incidence of metabolic syndrome among American young adults over a 23-year follow-up: the CARDIA trace element study. Sci Rep. 2015;5:15606. doi:10.1038/srep15606
27. Usharani P, Fatima N, Muralidhar N. Effects of Phyllanthus emblica extract on endothelial dysfunction and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: a randomized, double-blind, controlled study. Diabetes Metab Syndr Obes. 2013;6:275–284. doi:10.2147/DMSO.S46341
28. Biswas TK, Chakrabarty S, Pandit S, Jana U, Dey SK. Pilot study evaluating the use of Emblica officinalis standardized fruit extract in cardio-respiratory improvement and antioxidant status of volunteers with smoking history. J Herb Med. 2014;4(4):188–194. doi:10.1016/j.hermed.2014.09.002
29. Khanna S, Das A, Spieldenner J, Rink C, Roy S. Supplementation of a standardized extract from Phyllanthus emblica Improves cardiovascular risk factors and platelet aggregation in overweight/class-1 obese adults. J Med Food. 2015;18(4):415–420. doi:10.1089/jmf.2014.0178
30. Fatima N, Pingali U, Muralidhar N. Study of pharmacodynamic interaction of Phyllanthus emblica extract with clopidogrel and ecosprin in patients with type II diabetes mellitus. Phytomedicine. 2014;21(5):579–585. doi:10.1016/j.phymed.2013.10.024
31. Thirunavukkarasu M, Selvaraju V, Tapias L, Sanchez JA, Palesty JA, Maulik N. Protective effects of Phyllanthus emblica against myocardial ischemia-reperfusion injury: the role of PI3-kinase/glycogen synthase kinase 3β/β-catenin pathway. J Physiol Biochem. 2015;71(4):623–626. doi:10.1007/s13105-015-0426-8
32. Fatima N, Pingali U, Pilli R. Evaluation of Phyllanthus emblica extract on cold pressor induced cardiovascular changes in healthy human subjects. Pharmacognosy Res. 2014;6(1):29–35. doi:10.4103/0974-8490.122914
33. Usharani P, SudhaRani E, KiranKishore K, Raveendranath P. Evaluation of the effect of a standardized aqueous extract of the fruits of Emblica officinalis on mental stress induced cardiovascular changes in healthy human subjects. IJPSR. 2017;8(10):4138–4146.
34. Biswas TK, Pandit S, Mondal S, et al. Clinical evaluation of spermatogenic activity of processed Shilajit in oligospermia. Andrologia. 2010;42(1):48–56. doi:10.1111/and.2009.42.issue-1
35. Pandit S, Biswas S, Jana U, De RK, Mukhopadhyay SC, Biswas TK. Clinical evaluation of purified Shilajit on testosterone levels in healthy volunteers. Andrologia. 2015;xx:1–6.
36. Das A, Datta S, Rhea B, et al. The human skeletal muscle transcriptome in response to oral shilajit supplementation. J Med Food. 2016;19(7):701–709. doi:10.1089/jmf.2016.0010
37. Niranjan K, Ramakanth GSH, Fatima N, Usharani P. Evaluation of the effect of purified aqueous extract of shilajit in modifying cardiovascular risk with special reference to endothelial dysfunction in patients with type 2 diabetes mellitus. Int J Ayur Pharma Res. 2016;4(4):1–7.
38. Lawley S, Gupta RC, Goad JT, Canerdy TD, Kalidindi SR. Anti-inflammatory and antiarthritic efficacy and safety of purified shilajit in moderately arthritic dogs. J Vet Sci Anim Husb. 2013;1(3):302.
39. Das A, El Masry SM, Gnyawali SC, et al. Skin transcriptome of middle-aged women supplemented with natural herbo-mineral shilajit shows induction of microvascular and extracellular matrix mechanisms. J Am Coll Nutr. 2019;38(6):526–536. doi:10.1080/07315724.2018.1564088
40. Joukar S, Najafipour H, Dabiri S, Sheibani M, Sharokhi N. Cardioprotective effect of mumie (shilajit) on experimentally induced myocardial injury. Cardiovasc Toxicol. 2014;14(3):214–221. doi:10.1007/s12012-014-9245-3
41. Biswas TK, Polley G, Pandit S, et al. Effects of adjunct therapy of a proprietary herbo-chromium supplement in type 2 diabetes: a randomized clinical trial. Int J Diabetes Dev Ctries. 2010;30(3):153–161. doi:10.4103/0973-3930.66512
42. Peng M, Yang X. Controlling diabetes by chromium complexes: the role of the ligands. J Inorg Biochem. 2015;146:97–103. doi:10.1016/j.jinorgbio.2015.01.002
43. Saiyed ZM, Lugo JP. Impact of chromium dinicocysteinate supplementation on inflammation, oxidative stress, and insulin resistance in type 2 diabetic subjects: an exploratory analysis of a randomized, double-blind, placebo-controlled study. Food Nutr Res. 2016;60:31762. doi:10.3402/fnr.v60.31762
44. Chowienczyk PJ, Kelly RP, MacCallum H, et al. Photoplethysmographic assessment of pulse wave reflection: blunted response to endothelium-dependent beta2-adrenergic vasodilation in type II diabetes mellitus. J Am Coll Cardiol. 1999;34(7):2007–2014. doi:10.1016/S0735-1097(99)00441-6
45. Naidu MUR, Sridhar Y, Usharani P, Mateen AA. Comparison of two β2 adrenoceptor agonists by different routes of administration to assess human endothelial function. Indian J Pharmacol. 2007;39(3):168–169. doi:10.4103/0253-7613.33439
46. Millasseau SC, Kelly RP, Ritter JM, Chowienczyk PJ. Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin Sci. 2002;103:371–377. doi:10.1042/cs1030371
47. Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. doi:10.1006/niox.2000.0319
48. Vidyasagar J, Karunakar N, Reddy MS, Rajnarayana K, Surender T, Krishna DR. Oxidative stress and antioxidant status in acute organophosphorous insecticide poisoning. Indian J Pharmacol. 2004;36(2):76–79.
49. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi:10.1016/0003-9861(59)90090-6
50. Wong CY, Yiu KH, Li SW, et al. Fish-oil supplement has neutral effects on vascular and metabolic function but improves renal function in subjects with Type 2 diabetes mellitus. Diabet Med. 2010;27(1):54–60. doi:10.1111/j.1464-5491.2009.02869.x
51. Siniarski A, Haberka M, Mostowik M, et al. Treatment with omega-3 polyunsaturated fatty acids does not improve endothelial function in patients with type 2 diabetes and very high cardiovascular risk: a randomized, double-blind, placebo-controlled study (Omega FMD). Atherosclerosis. 2018;271:148–155. doi:10.1016/j.atherosclerosis.2018.02.030
52. Usharani P, Devi CG, Kishore KK, Kumar CU. Effect of proprietary chromium complex and its individual components versus chromium picolinate, chromium polynicotinate and chromium dinicocysteinate on endothelial function, biomarkers and lipid profile in type 2 diabetics - a randomized, double-blind, placebo-controlled study. Int J Pharm Sci Res. 2017;8(5):2267–2276.
53. Kerksick C, Willoughby D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J Int Soc Sports Nutr. 2005;2:38–44. doi:10.1186/1550-2783-2-2-38
54. Shidfar F, Keshavarz A, Hosseyni S, Ameri A, Yarahmadi S. Effects of omega-3 fatty acid supplements on serum lipids, apolipoproteins and malondialdehyde in type 2 diabetes subjects. East Mediterr Health J. 2008;14(2):305–313.
55. Sorto-Gomez TE, Ortiz GG, Pacheco-Moises FP, et al. Effect of fish oil on glutathione redox system in multiple sclerosis. Am J Neurodegener Dis. 2016;5(2):145–151.
56. Jin RC, Loscalzo J. Vascular nitric oxide: formation and function. J Blood Med. 2010;1:147–162.
57. Asemi Z, Soleimani A, Shakeri H, Mazroii N, Esmaillzadeh A. Effects of omega-3 fatty acid plus alpha-tocopherol supplementation on malnutrition-inflammation score, biomarkers of inflammation and oxidative stress in chronic hemodialysis subjects. Int Urol Nephrol. 2016;48(11):1887–1895. doi:10.1007/s11255-016-1399-4
58. Yamada H, Yoshida M, Nakano Y, et al. In vivo and in vitro inhibition of monocyte adhesion to endothelial cells and endothelial adhesion molecules by eicosapentaenoic acid. Arterioscler Thromb Vasc Biol. 2008;28(12):2173–2179. doi:10.1161/ATVBAHA.108.171736
59. Hocaoglu-Emre FS, Saribal D, Yenmis G, Guvenen G. Vascular cell adhesion molecule 1, intercellular adhesion molecule 1, and cluster of differentiation 146 levels in patients with Type 2 diabetes with complications. Endocrinol Metab (Seoul). 2017;32(1):99–105. doi:10.3803/EnM.2017.32.1.99
60. Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75(3):645–662. doi:10.1111/j.1365-2125.2012.04374.x
61. Kamath DY, Xavier D, Sigamani A, Pais P. High sensitivity C-reactive protein (hsCRP) & cardiovascular disease: an Indian perspective. Indian J Med Res. 2015;142(3):261–268. doi:10.4103/0971-5916.166582
62. Schiano V, Laurenzano E, Brevetti G, et al. Omega-3 polyunsaturated fatty acid in peripheral arterial disease: effect on lipid pattern, disease severity, inflammation profile, and endothelial function. Clin Nutr. 2008;27(2):241–247. doi:10.1016/j.clnu.2007.11.007
63. Mackay I, Ford I, Thies F, Fielding S, Bachoo P, Brittenden J. Effect of Omega-3 fatty acid supplementation on markers of platelet and endothelial function in subjects with peripheral arterial disease. Atherosclerosis. 2012;221(2):514–520. doi:10.1016/j.atherosclerosis.2011.12.041
64. Usharani P, Kishan PV, Fatima N, Kumar UC. A comparative study to evaluate the effect of highly standardised aqueous extracts of Phyllanthus emblica, withania somnifera and their combination on endothelial dysfunction and biomarkers in subjects with type II diabetes mellitus. IJPSR. 2014;5(7):2687–2697.
65. Harris WS, Ginsberg HN, Arunakul N, et al. Safety and efficacy of Omacor in severe hypertriglyceridemia. J Cardiovasc Risk. 1997;4(5–6):385–391. doi:10.1097/00043798-199710000-00011
66. Koski RR. Omega-3-acid Ethyl Esters (Lovaza) For severe hypertriglyceridemia. Pharm Ther. 2008;33(5):271–303.
67. Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RA, Soni PN. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol. 2011;108(5):682–690. doi:10.1016/j.amjcard.2011.04.015
68. Kataoka Y, Uno K, Puri R, Nicholls SJ. Epanova(®) and hypertriglyceridemia: pharmacological mechanisms and clinical efficacy. Future Cardiol. 2013;9(2):177–186. doi:10.2217/fca.13.4
69. Cicero AF, Rosticci M, Morbini M, et al. Lipid-lowering and anti-inflammatory effects of omega 3 ethyl esters and krill oil: a randomized, cross-over, clinical trial. Arch Med Sci. 2016;12(3):507–512. doi:10.5114/aoms.2016.59923
70. Sawada T, Tsubata H, Hashimoto N, et al. Effects of 6-month eicosapentaenoic acid treatment on postprandial hyperglycemia, hyperlipidemia, insulin secretion ability, and concomitant endothelial dysfunction among newly - diagnosed impaired glucose metabolism subjects with coronary artery disease. An open label, single blinded, prospective randomized controlled trial. Cardiovasc Diabetol. 2016;15(1):121.
71. Brown TJ, Brainard J, Song F, et al. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. BMJ. 2019;366:l4697. doi:10.1136/bmj.l4697
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
