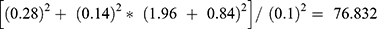Back to Journals » Research and Reports in Tropical Medicine » Volume 14
Evaluation of Renal Function Profile in Human Visceral Leishmaniasis (Kala-Azar) Patients: A Case of Western Tigray, Ethiopia
Authors Asfaw KG, Gizaw ST , Gnanasekaran N
Received 14 March 2023
Accepted for publication 16 June 2023
Published 28 June 2023 Volume 2023:14 Pages 21—33
DOI https://doi.org/10.2147/RRTM.S410137
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario Rodriguez-Perez
Kibrom Gerezgiher Asfaw,1 Solomon Tebeje Gizaw,1 Natesan Gnanasekaran1,2
1Department of Medical Biochemistry, School of Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 2Regan Institute of Metabolic Therapy, Rasipuram Tk, Namakkal Dt, Tamilnadu, India
Correspondence: Solomon Tebeje Gizaw, Tel +251911731148, Email [email protected]
Background: Leishmaniasis is a vector-borne protozoan infection that has a wide clinical spectrum in the tropics and subtropics. Kidney damage is frequently associated with increased morbidity and mortality in visceral leishmaniasis (VL) patients. However, up to date, there is a very limited report on the effect of visceral leishmaniasis on kidney function profiling in Ethiopia.
Objective: To evaluate the renal function profile in human visceral leishmaniasis (kala-azar) patients.
Materials and Methods: Human blood was taken from VL patients (n = 100) and healthy controls (n = 100) attending Kahsay Abera and Mearg Hospitals, Western Tigray of Ethiopia. Serum was separated according to the conventional protocol and kidney function profiling (creatinine, urea, and uric acid) was analyzed by Mindray 200E automated chemistry analyzer. The estimated glomerular filtration rate (eGFR) was also assessed in this study. The obtained data were processed using SPSS Version 23.0. Descriptive statistics, independent-test, and bivariate correlations were used for data analysis. P values < 0.05 were considered statistically significant at a 95% confidence level.
Results: The mean serum creatinine level was found significantly higher, while respective serum urea and eGFR were significantly lower in VL patients compared to healthy controls. Specifically, from 100 VL cases, an increased level of serum creatinine, urea, and uric acid was found in 10%, 9% and 15% VL cases, respectively; meanwhile, a decreased serum urea and eGFR have been reported from 33% to 44% VL cases, respectively.
Conclusion: The finding of this study asserted that visceral leishmaniasis causes derangement in kidney activities characterized by alteration of renal function profile. This may indicate that VL is the determinant factor for developing kidney dysfunction. This study encourages researchers to engage in visceral leishmaniasis and its effect on other organ function profiles in humans and identify potential markers for both prevention and intervention.
Keywords: visceral leishmaniasis, renal function test, creatinine, urea, glomerular filtration rate
Introduction
Leishmaniasis is a group of parasitic diseases caused by more than 20 species of obligate intracellular protozoa of the genus Leishmania that are transmitted between humans and other mammalian hosts by phlebotomine sandflies.1 It occurs in four forms: visceral leishmaniasis (VL, aka kala-azar), post-kala-azar dermal leishmaniasis (PKDL), cutaneous leishmaniasis (CL) and mucocutaneous leishmaniasis.2,3 Leishmania parasites have two basic life stages: promastigote and amastigote. The amastigotes exist in vertebrate hosts and promastigotes in an invertebrate host.4
Among neglected tropical diseases, visceral leishmaniasis is one of the most fatal parasitic diseases that claim approximately 20,000 lives every year. Globally, thousands of deaths are reported in many developing countries including Ethiopia5 and of the three eco-epidemiological hotspots, East Africa (Ethiopia, Eritrea, Kenya, Somalia, South Sudan, Sudan and Uganda), Indian Subcontinent (Bangladesh India and Nepal), and Brazil accounted for 57%, 18%, and 16%, respectively.2
Visceral leishmaniasis is a disease of major public health concern leading to a severe mortality rate worldwide. The disease is endemic in several tropical and subtropical regions and the Mediterranean basin. The estimated annual global burden of VL is approximately 300, 000 new cases and more than 20, 000 deaths,6 of which 95% of new cases reported to the World Health Organization (WHO) occurred in 10 countries: Bangladesh, Brazil, China, Ethiopia, India, Kenya, Nepal, Somalia, and South Sudan.5
In Ethiopia, VL has spread to become endemic in many parts of the country. The disease is prevalent mostly in lowland and arid areas. Most endemic areas are Metema and Humera lowlands of Northwest of Ethiopia which account for 60% of the total burden,7 the Omo plains, the Aba Roba focus and Weyto River Valley in Nationalities and Peoples’ Regional State (SNNPR). The disease was also reported from the Moyale area and Genale river basin in the Oromia regional state, Afder, and Liban zones in Ethiopia’s Somali region, and the Awash Valley in the Afar regional state. The annual burden of VL in Ethiopia is estimated to be between 4500 and 5000 cases, and the population at risk is more than 3.2 million.8 It is endemic in Southern Europe and tropical and subtropical areas of the globe with a worldwide incidence of approximately 0.5 million cases per year.9
VL (kala-azar) is a chronic infectious disease caused by parasites of the Leishmania donovani complex in Ethiopia.10 It is one of the most deadly infectious diseases that can cause various clinical manifestations ranging from irregular and recurrent fever to hepato-splenomegaly, anaemia, leukopenia and thrombocytopenia as well as hypergammaglobulinemia as a consequence of the intense parasitism of the reticular endothelial system by the Leishmania parasite.11,12
Visceral leishmaniasis is diagnosed by serological tests like the rapid diagnostic tests (RDTs) of recombinant 39 amino acid antigen (rk39) and parasitological tests like lymph node aspiration, bone marrow aspiration, and spleen aspiration in combination with clinical signs and symptoms.13
The kidneys are two bean-shaped organs found in vertebrates. They are located on both sides of the spinal column, in the posterior part of the abdomen. The kidney performs various activities such as maintaining fluid and electrolyte homeostasis in the body, urine formation, acid-base balance regulation, protein metabolism waste product excretion, hormonal function, and protein conservation. Glomerular filtration, tubular reabsorption, and tubular secretion are the three basic processes through which the kidneys perform their physiological functions.14
Renal abnormalities caused by Leishmania have been well documented in experimental animal studies and are comprised of interstitial and glomerular abnormalities. Immune complex deposition, T cells and adhesion molecule activation might be responsible for the renal involvement in visceral leishmaniasis.3 Kidney function alteration and interstitial nephritis with glomerular changes can be seen in VL patients. Oxidative stress is a major determinant of various renal diseases and also contributes to the manifestation of glomerulonephritis during the pathogenesis of visceral leishmaniasis. Also, antibodies produced in response to infection can be trapped in glomeruli by different mechanisms, such as immune complexes, and lead to cause damage to the glomerulus of the kidney.13
Serum creatinine (SCr), urea (U), and eGFR concentrations, as well as electrolytes (E), are the most practical measure of renal function.15,16 Measuring the glomerular filtration rate17 is most useful for assessing renal function. A useful and practical surrogate marker for the glomerular filtration rate is creatinine clearance. Creatinine clearance rate (CCr or CrCl) is the volume of blood plasma that is cleared of creatinine per unit of time. GFR can be calculated by measuring any chemical that has a steady level in the blood and is freely filtered but neither reabsorbed nor secreted by the kidneys. The GFR is typically recorded in units of volume per time, eg, millilitres per minute (mL/min).18,19 However, proximal tubular cell secretion of creatinine and endogenous production of creatinine have been identified as drawbacks when serum creatinine concentration (SCr) is used as an estimate for GFR. Creatinine estimates of GFR are also affected by race, body mass index, age, and sex.15,20
The kidney is affected by a different disease like visceral leishmaniasis. Kidney involvement in chronic leishmaniasis is frequent and associated with increased mortality. Kidney disease is closely interrelated with heart and blood vessel disease, with 7% of all cardiovascular deaths attributed to the reduced glomerular filtration rate.21 Importantly, kidney disease also has a strong impact on morbidity and non-fatal outcomes.22 Therefore, a patient suspected of visceral leishmaniasis is better assessed for further kidney function profile and gets early treatment to decrease mortality related to derangement of kidney activities.
Although different studies were performed on the renal profile of kala-azar patients in different parts of the world, up-to-date, there is a very limited report on the effect of visceral leishmaniasis on kidney profiles in Ethiopia. Therefore, this study is intended to evaluate the renal function profile in human visceral leishmaniasis.
Materials and Methods
Study Area
This study was conducted in Kahsay Abera and Meareg Hospitals, Humera and Dansha towns, respectively, in Western Tigray, Ethiopia. Humera is located in Western Tigray, Ethiopia, which is 984 km far from the capital city, Addis Ababa. It is located at a longitude and latitude 14o18’N 36 o37’E with an elevation of 585 meters above sea level. Kahsay Abera Hospital is the district hospital found in the Kala-azar endemic region in Northern Ethiopia with 210 beds and an estimated 742,000 catchment population. Mearg Hospital is the district hospital found in the Kala-azar endemic area in Tsegede Wereda, Western Tigray, which has an estimated 299,594 catchment population including migrant population and 134 beds in emergency, medical, surgical, gynaecology, and paediatric wards.
Study Design and Period
A comparative cross-sectional study design was employed from June to September 2019 G.C.
Study Participant
Two study groups, case and controls, were involved in the current study. VL patients confirmed at Kahsay Abera and Mearg Hospital from June to September 2019 using:
- Physician examination using the most pronounced signs and symptoms of VL, viz., splenomegaly (100%), hepatomegaly (36%), fever (100%), weight loss (65%), jaundice (21%), skin mucosal pallor (72%), diarrhoea (15%), anorexia (52%), abdominal pain (17%), general weakness (85%), and bleeding (67%).
- Serological test, which detects antibodies against leishmania rk39-based rapid diagnostic tests (RDTs).
- Diagnostic tests for VL including splenic aspiration and culture were routinely performed.
Control group: All healthy persons who accompanied the patients at Kahsay Abera and Mearg Hospitals, who were matched with cases in age and sex with not any of the physician and laboratory test findings.
Inclusion and Exclusion Criteria
Inclusion Criteria
Case group: All VL patients were confirmed at Kahsay Abera and Mearg hospital laboratories during the study period.
Control group: All healthy accompanied the patients at Kahsay Abera and Mearg Hospitals, who were matched with cases in age and sex without having VL. Selection of controls was employed by the physician based on the WHO guideline for the diagnosis of VL and rk39 was used to screen the healthy controls.
Exclusion Criteria
Case group: VL patients who have a history of any other chronic disease (kidney disease, liver disease, cancer, HIV/AIDS, DM, hypertension TB, and malaria). Patients under treatment with anti-kala-azar were excluded from the study. Patients with a habit of chronic alcohol drinking and smoking were also excluded from the study.
Control group: Individuals who have a history of any chronic disease (kidney disease, liver disease, cancer, HIV/AIDS, DM, hypertension TB, and malaria). Individuals with a habit of chronic alcohol drinking and smoking were also excluded from the study.
Study Variables
Dependent variables: Creatinine, Urea, Uric acid, and Glomerular filtration rate (eGFR).
Independent variables: Socio-demographic factors (age, sex, residence, educational level and occupation), duration of illness, and body weight.
Sample Size Determination and Sampling Methods
The sample size needed for comparing the means of two normally distributed samples is calculated by using a two-sided test with significance level α and power 1 – β. A 95% confidence level and 80% power were used to calculate the appropriate sample size. From the study conducted in Brazil, mean value of serum creatinine for Kala-azar patients and controls was 0.89 ± 0.28 and 0.99 ± 0.14, respectively (Table 1).
 |
Table 1 The Levels of Mean Serum Creatinine of Kala-Azar Patients Conducted in Brazil (Used for Calculating Sample Size by Comparing Two Means) |
The sample size was calculated by using the mean value of creatinine from the study conducted in Brazil which gives the highest sample size using the following formula.
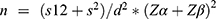 , where n = desired sample size; s1 (standard deviation of case group) = 0.28;
, where n = desired sample size; s1 (standard deviation of case group) = 0.28;
s2 (standard deviation of control group) = 0.14; Zα = 1.96, Zβ = power = 0.84;
Difference between two means (d) = (0.89–0.99)2 = 0.01.
Considering a 10% nonresponse rate (= 0.1*77 = 8), the minimum sample size will be 77 + 8 = 85 for each group. Increasing sample size can give greater power to detect the difference between control and case groups. Consequently, 100 case and 100 control groups’ serum specimens were collected for the current study.
Sampling Method
A convenient sampling technique was employed to select the study participants.
Measurement and Data Collection
Data Collection Procedure
A semi-structured pretested and translated questionnaire was used to collect socio-demographic characteristics. Data collectors fill out the questionnaire by direct interview of the study participants. Information concerning the clinical history was obtained from a clinical log sheet.
Laboratory Analysis
About 5 mL venous blood sample was collected using a serum separator tube from both VL patients and healthy controls. A serum sample was separated by centrifuge at 4000 rpm for 5 minutes and about 1.5–1.8 mL serum sample was stored at a temperature of −20℃ up to −30℃ in a deep freezer before laboratory analysis. The serum sample was transported to Adigrat General Hospital Laboratory department for clinical chemistry analysis.
Renal Function Tests Analysis
Renal function test includes measurement of urea, creatinine, and uric acid. The analysis was done by the principle of spectrophotometry for measuring the absorption spectrum of the analyte at each wavelength. The JOURI LABS Biochemistry reagents and smart, versatile, easy Mindray 200 E automated chemistry analyzer (Mindray Medical India Pvt. Ltd., Gurugram, 122002 Haryana, India) were used to measure the concentration of creatinine, urea, and uric acid from serum. All the tests were performed according to the manufacturer’s protocol.
Data Quality Assurance
Pre-Analytical
The questionnaires were pre-tested on 5% of the study population one week before the actual data collection in Shre town to ensure clarity, length, logical sequence and skip patterns of the questions. A questionnaire was prepared in English and was translated into Tigrigna and Amharic versions, which is easily understandable by the study participant. Experienced laboratory personnel participated in the proper collection, processing, and transportation of the sample. Standard operating procedures (SOPs) were used strictly to ensure labelling with an identification number, proper sample container, and enough volume and test procedures. The collected sample was allowed to clot for 20–25 minutes and centrifuged specimen to separate serum for analysis. The standard working environment (optimized, instrument, high analytical grade reagent, and temperature) was met, and sample hemolysis was checked before analysis.
Analytical
Before sample analysis, both normal and pathological quality controls were analyzed to ensure the proper function, validity and reliability of the instrument. Levy Jennings (LJ) chart was drawn for both controls and interpreted based on the west guard rule to reject or to accept controls. Levy Jennings is a graph that quality control data are plotted on to give a visual indication of whether a laboratory test is working well and the west guard rule can be applied to see whether the results from the samples when the control was done can be released, or if they need re-run. The samples were analyzed after both controls were accepted.
Post-Analytical
The result was properly recorded based on the sample identification number, and the data were interpreted by the principal investigator. The laboratory result was recorded in the logbook for rechecking. Clear and neat test results were reported to the investigator for analysis.
Data Analysis and Interpretation
The obtained data were entered into SPSS version 23 software. Descriptive statistics were used, and the generated data were expressed in numbers and percentages in the form of tables and figures. An Independent Student’s t-test was used to compare the mean difference between the VL cases and control groups. Bivariate correlation analysis was used to check the significant correlation of associated factors with renal function tests of VL cases. A P-value less than 0.05 with a corresponding 95% confidence interval was considered a significant association.
Operational Definition
Kala-azar: Visceral leishmaniasis is a chronic and vector-borne potentially fatal parasitic disease caused by the Leishmania (L.) donovani/L. infantum/L. chagasi complex.
Renal function tests – These are groups of blood tests that are useful in the evaluation and management of patients with kidney dysfunction. Some of the blood tests are urea, creatinine, and uric acid.
Acute kidney injury (AKI): An absolute increase in serum creatinine of more than or equal to 0.3 mg/dL or the percentage increase in serum creatinine of more than or equal to 50%.
Acute nephrotic syndrome: This is a group of symptoms that occur with some disorders that cause inflammation of the glomeruli in the kidney or glomerulonephritis.
Glomerular filtration rate:17 Measures how well your kidneys are cleaning your blood and is approximated by the Creatinine clearance test which determines the overall GFR (Supporting Information, Annex VII).
Case: Are individuals who have confirmed visceral leishmaniasis.
Control: Are individuals who are healthy and unlikely to share visceral leishmaniasis.
Chronic disease: Is a human health condition or disease that is long-lasting in its effect which includes hypertension, heart disease, kidney disease, liver disease, HIV/AIDS, cancer, diabetes Mellitus, etc.
Normal: Is the test value lies within the established reference ranges.
Low: Is the value of the test lie below the established reference ranges.
High: Is the value of the test lie above the established reference ranges.
Result
Socio-Demographic Characteristics of Study Participants
This study enrolled 100 VL-confirmed patients (88 of them were males) and 100 healthy control people (90 of them were males). Without any further analysis, the infection was found highly pronounced in males; and the study was conducted almost between age-matched VL and control groups. The mean age of VL patients and healthy controls were 29.38 ± 9.72 years and 28.95 ± 10.00 years, respectively. The majority of the VL patients and healthy controls were within the age range of 15–30 years. There was no significant difference in the mean age of the VL patients and the healthy control subjects. About 44% of VL cases and 43% of healthy controls were single. Around 57% of VL cases and 71% of healthy controls attended either primary or secondary schools. Besides, the majority of the patients (89%) were from the rural and kala-azar endemic areas (Table 2).
 |
Table 2 Socio-Demographic Characteristics of VL Patients (n = 100) and Healthy Controls (n = 100) |
Renal Function Test results of Visceral Leishmaniasis Patients Deviated from Established Reference Ranges
For the current study, we established renal marker ranges based on JOURI LABS reference values (for men and women) as: lower limit normal creatinine (<0.5 mg/dL), urea (<18 mg/dL), uric acid (<2.6 mg/dl), and eGFR (<90 mL/min) and the upper limit normal creatinine (>1.3 mg/dL), urea (>45 mg/dL); uric acid (>7.2 mg/dL), and eGFR (>150 mL/min) (Supporting Information Annex VII). While the percentage (absolute number) of VL patients having decreased serum analyte levels from the lower limit normal was found to be 33% urea (12.48 ± 3.75 mg/dL), 5% uric acid (2.54 ± 0.06 mg/dL), and 44% eGFR (71.91 ± 11.66 mL/min), the percentage (absolute number) of VL patients having elevated serum analyte levels from the upper limit normal was found to be 10% creatinine (1.43 ± 0.11 mg/dL), 4% urea (54.0 ± 2.16 mg/dL), 15% uric acid (9.56 ± 1.72 mg/dL), and 2% eGFR (155.18 ± 2.69 mL/min).
Comparison of Renal Function Tests Between VL and Controls
The mean value of serum creatinine was significantly higher in VL patients (0.935 ± 0.229 mg/dL) when compared to those of healthy controls (0.709 ± 0.119 mg/dL) with p < 0.001. Even though the mean value of serum uric acid shows a slight increase in VL patients than controls, there was no statistical significance between VL cases (5.16 ± 2.25 mg/dL) and healthy controls (4.87 ± 1.74 mg/dL) with p = 0.31. The mean value of serum urea was significantly lower in VL patients (23.11 ± 10.72 mg/dL) when compared to those of healthy controls (29.19 ± 9.32 mg/dL) with p < 0.001. Similarly, the mean value of eGFR was significantly lower in VL patients (90.95 ± 22.15 mg/dL) when compared to those of healthy controls (128.36 ± 21.06 mL/min) with p < 0.001 (Figure 1).
Comparison of Urea/Creatinine and Uric Acid/Creatinine Ratios Between VL Patients and Controls
Calculating the ratio of urea to creatinine and uric acid to creatinine levels is important to predict or indicate how well the kidneys function. The principle behind this ratio is the fact that both urea and creatinine are freely filtered by the glomerulus; however, urea reabsorbed by the tubules can be regulated, whereas creatinine reabsorption remains the same. The ratio of the mean values of serum urea to creatinine (urea/creatinine) was significantly lower in VL patients (26.3± 15.2) when compared to those of healthy controls (42.1± 14.5) with p < 0.001. Similarly, the serum uric acid to creatinine ratio was significantly lower in VL patients (5.8± 2.94) when compared to those of healthy controls (7.04 ±2.75) with p<0.01 (Table 3).
 |
Table 3 Comparison of Urea/Creatinine and Uric Acid/Creatinine Ratios Between VL Cases (n = 100) and Controls (n = 100) |
Comparison of Renal Function Tests Between VL Cases with a Duration of Illness
Of a total of 100 patients having visceral leishmaniasis, 51% of them had a duration of illness of more than 4 weeks. The mean value of serum creatinine was significantly higher in VL patients with a duration of illness of more than 4 weeks (1.05 ± 0.23 mg/dL) when compared to those of VL patients with a duration of illness less than 4 weeks (0.82 ± 0.16 mg/dL) with p < 0.001.
The mean value of eGFR was significantly lower in VL patients with a duration of illness of more than 4 weeks (79.35 ± 17.3 mg/dL) when compared to those of VL patients with a duration of illness 0–4 weeks (103.04 ± 20.21 mg/dL) with p < 0.001. Urea and uric acid levels were not shown any significant difference with the duration of the illness.
Other Factors and Renal Function Test Results of VL Cases
Among visceral leishmaniasis patients, there was a statistically significant positive correlation between serum creatinine level and duration of illness (rs = 0.52, p < 0.001). On the other hand, the eGFR level showed a statistically significant inverse association with the duration of illness (rs = −0.58, p < 0.001) in VL patients. Correlation analyses also showed that there was a significant negative association between age and serum urea (rs = −0.21* p < 0.05).
However, other factors or patients’ history such as sex, age, educational level, residence, and BMI were not shown any significant correlation with renal function tests of VL cases as shown in Table 4.
 |
Table 4 Correlation Analysis Between Other Factors and Renal Function Test Result of VL Cases (n = 100) |
Discussion
The present study evaluates the effect of visceral leishmaniasis on renal injury profiles (serum creatinine, uric acid, urea, and eGFR) between VL patients and healthy controls. These parameters have been used as the most indicators for renal damage.16,23 Our study showed that, while the mean value of serum creatinine was found significantly higher in VL patients, serum urea and eGFR were found significantly lower in VL patients, when compared to the healthy controls. However, the mean value of serum uric acid was not shown any statistical significance.
Our finding revealed that the mean serum creatinine level which was found significant increase in VL patients compared to healthy controls is in corroboration with the previous studies conducted on renal function profile among VL cases, and reported AKI and progressive glomerulonephritis found an increase in serum creatinine level.24–26 This may be attributed to the level of immune complex deposition, T cells and adhesion molecule activation during the inflammatory processes observed in active VL disease resulting in renal dysfunction.27,28 The production of reactive oxygen species (ROSs) from the activated macrophage may also contribute to the alterations of renal function.28,29 The disease itself and the hemodynamic disturbs in the context of the disease (anaemia, hypotension, hypoalbuminemia) can be involved in renal damage.25 In contrast, Lima et al reported that serum creatinine levels did not show any significant difference between VL cases and controls.23 This difference may be attributed to disease progression, diet effect, temperature, diurnal variation and sample size.
The mean serum urea level was significantly lower in VL cases compared to the healthy controls. This finding is in agreement with a study conducted in Brazil.23 The decrease in urea level may be attributed to the low protein intake and reduced production in the liver due to VL infection. VL causes morphological and functional disturbance in the liver and the dysfunction may be caused directly by the protozoa itself or indirectly by the effect related to the immune response of the parasite.30 However, the present study is not incongruous with studies conducted by Efstratiadis et al and Alcântara et al, which reported a high level of serum urea for VL patients.24,27 The difference may be attributed to the duration of dehydration (with an environmental difference), diet and severity of infection.31
Even though 15% of VL cases showed an increase in uric acid, there was no statistically significant difference in the mean uric acid levels between VL cases and healthy controls. In contrast, the study conducted in Brazil reported decreased uric acid levels in VL patients.32,33 This difference may be due to genetic variabilities, diet effects, temperatures, and diurnal variation.
Approximately half of the patients with kala-azar were found decreased eGFR. The mean value of eGFR was significantly lower in VL cases than in healthy controls. In contrast, another study in which the level of eGFR did not show any significant difference between VL cases and controls.23 This difference may be attributed to extrarenal fluid losses (diarrhoea, vomiting, and sweating) in patients with kala-azar, disease progression, and means of estimating glomerular filtration rate.
The urea-to-creatinine ratio is one of the common laboratory tests used to distinguish between pre-renal and acute tubular necrosis.34 High serum urea to creatinine ratio (UCR) is associated with pre-renal injury. The hemodynamic instability which leads to reduced GFR accounts for pre-renal injury.35 In our study, we found that the ratio of serum urea to creatinine and serum uric acid to creatinine was decreased in VL cases when compared to controls. A low urea-to-creatinine ratio suggests protein malnutrition and reduced urea synthesis as in advanced liver disease.35 However, different studies showed that the urea-to-creatinine ratio (UCR) was not a reliable parameter to distinguish prerenal acute kidney injury from other forms of acute kidney injury.36,37 Extrarenal factors can affect the blood levels of these two markers. Serum uric acid to creatinine ratio might be a better predictor of incident chronic kidney disease than serum UA alone.38 According to our study, the mean value of serum uric acid to creatinine ratio is significantly lower in VL cases when compared to controls. This may be attributed to the significantly elevated mean serum creatinine level of VL patients, but the uric acid level did not show any significant difference. Besides, factors such as diet, genetic variance, and underlying medical conditions can also affect the blood level of these two markers.
We have also assessed the association between the duration of illness and renal function test results of VL patients. This study revealed that the duration of illness had a moderate positive correlation with serum creatinine (rs = 0.52; p < 0.05) in VL patients. Serum creatinine was found elevated in patients with more than 4 weeks of the duration of illness. This is in concordant with the previous study conducted by Silva Junior et al.3 They reported that serum creatinine was found to increase in patients with a longer time between symptom onset and hospital admission. Correlation analysis also showed that there was a significant moderate negative association between the duration of illness and eGFR level (rs = −0.58; p < 0.05). VL patients with more than 4 weeks of illness were found to decrease in eGFR compared to those with less than 4 weeks of illness. This may be attributed to the disease progresses; the occurrence of glomerulonephritis is inevitable resulting in decreased eGFR.39–41
Our study also went through assessing the association between patients’ history (age, gender, education, marital status, and occupation) and the renal function test results of VL patients and found that the levels of serum creatinine have no significant association with gender and age. This finding is not in agreement with the study conducted in Australia, in which the serum creatinine concentration increased steadily with age.42 Similarly, eGFR was not shown a significant association with gender and age. This finding is not in agreement with the study conducted in Brazil and London. Both reports were shown that the level of GFR declined with age and the decrease in eGFR is less pronounced in males as they were getting aged.43–45 Correlation analysis also showed that age had a weak correlation with serum urea (rs = −0.21* p < 0.05). These results are in trajectory with a study done by Musch et al and Seki et al. Both researchers reported a direct correlation between age and urea.46,47 This discordant may be attributed to genetic variabilities, environmental factors (temperature, altitude and climatic changes), lifestyle (smoking, physical activities, and diet), and hormonal effects.
We believe our study answered important questions about whether renal markers are significantly altered in VL patients. Despite that, our study has a limitation: viz., different tests like electrolyte, protein, albumin and endocrine tests were not done due to resource constraints; literature for this area study was limited to construct discussions at intended; and the study is only cross-sectional.
Conclusion
Visceral leishmaniasis (kala-azar) patients showed quite significant alterations in renal function profiles when compared to healthy controls. This may indicate that VL is the determinant factor for developing kidney dysfunction. Further investigation is required and will not be a layaway task for researchers, particularly in this field of study and the scientific community.
Data Sharing Statement
All data pertaining to this study are contained and presented in this document and in the Supplementary Files. Anyone interested in the full data in Excel format can have the data by writing to [email protected].
Ethics Approval, Consent to Participate and Publication
Before starting data collection, an ethical clearance letter with reference number SOM/BCHM/121/011 was obtained from the Departmental Research and Ethics Review Committee, Department of Biochemistry, School of Medicine, College of Health Sciences, Addis Ababa University. A collaboration letter for data collection was also obtained from the Tigray Regional Health Bureau and the Administrators of Kahsay Abera and Mearg hospitals. Samples and data were collected after written informed consent had been obtained from study participants. For child participants (below the age of 18), written formal consent was obtained from the parents who accompanied them and the confidentiality of any information obtained from each participant was coded for the samples and results. Further permission was also obtained from Adigrat General Hospital to perform the clinical chemistry tests. Also, written informed consent for data collection and publication was already obtained (Supporting Information). The study was performed by the ethical standards of the Declaration of Helsinki and approved by the Ethics Committee of Addis Ababa University (Informed consent was obtained from all patients).
Acknowledgments
We are extremely grateful to the study participants for their valuable time and their willingness to participate in this study and to Addis Ababa University (AAU) for partial funding for this research project. The authors would like to acknowledge the staff members of Adigrat Referral Hospital. This paper is based on the thesis “Evaluation of renal function profile in human visceral leishmaniasis (kala-azar) patients by Kibrom Gerezgiher Asfaw.48 It has been published on the Addis Ababa University website: http://etd.aau.edu.et/handle/123456789/23992.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research project is partly funded by Addis Ababa University. The funding organization has no role in data collection, data analysis, data interpretation and manuscript writing.
Disclosure
The authors have declared that no competing interests exist.
References
1. Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63(12):e202–e64. doi:10.1093/cid/ciw670
2. World Health Organization. Global leishmaniasis surveillance: 2019–2020, a baseline for the 2030 roadmap. Wkly Epidemiol Rec. 2021;96(35):401–420.
3. Silva Junior G, Barros EJG, Daher EDF. Kidney involvement in leishmaniasis—a review. Braz J Infect Dis. 2014;18:434–440. doi:10.1016/j.bjid.2013.11.013
4. Kobets T, Grekov I, Lipoldova M. Leishmaniasis: prevention, parasite detection and treatment. Curr Med Chem. 2012;19(10):1443–1474. doi:10.2174/092986712799828300
5. Mondiale de la Santé O; World Health Organization. Global leishmaniasis update, 2006–2015: a turning point in leishmaniasis surveillance–Le point sur la situation mondiale de la leishmaniose, 2006–2015: un tournant dans la surveillance de la maladie. Wkly Epidemiol Rec. 2017;92(38):557–565.
6. World Health Organization. Leishmaniasis. World Health Organization; 2018. Available from: https://www.who.int/leishmaniasis/en/.
7. Hailu A, Gebre-Micheal T, Berhe N, Balkew M. Epidemiology and Ecology of Health and Disease in Ethiopia: Leishmaniasis in Ethiopia. Addis Ababa: Shama Press; 2006:15–634.
8. Leta S, Dao THT, Mesele F, Alemayehu G. Visceral leishmaniasis in Ethiopia: an evolving disease. PLoS Negl Trop Dis. 2014;8(9):e3131. doi:10.1371/journal.pntd.0003131
9. Dantas-Torres F, Brandão-Filho SP. Visceral leishmaniasis in Brazil: revisiting paradigms of epidemiology and control. Rev Inst Med Trop Sao Paulo. 2006;48:151–156. doi:10.1590/S0036-46652006000300007
10. Gelanew T, Kuhls K, Hurissa Z, et al. Inference of population structure of Leishmania donovani strains isolated from different Ethiopian visceral leishmaniasis endemic areas. PLoS Negl Trop Dis. 2010;4(11):e889. doi:10.1371/journal.pntd.0000889
11. Tesfaye EFK, Terefe B, Enawgaw B. Haematological abnormalities in visceral leishmaniasis patients attending Gondar University Hospital; Retrospective Study. Int J HIV AIDS Prev Educ Behav Sci. 2017;3(5):48. doi:10.11648/j.ijhpebs.20170305.11
12. Van Griensven J, Diro E. Visceral leishmaniasis. Infect Dis Clin. 2012;26(2):309–322. doi:10.1016/j.idc.2012.03.005
13. Kumar R, Nylén S. Immunobiology of visceral leishmaniasis. Front Immunol. 2012;3:251. doi:10.3389/fimmu.2012.00251
14. Ogedegbe HO. Renal function tests: a clinical laboratory perspective. Lab Med. 2007;38(5):295–304. doi:10.1309/RWG5DY7RG1CYBUR7
15. Rosner MH, Bolton WK. Renal function testing. Am J Kidney Dis. 2006;47(1):174–183. doi:10.1053/j.ajkd.2005.08.038
16. Norris KC, Smoyer KE, Rolland C, Van Der Vaart J, Grubb EB. Albuminuria, serum creatinine, and estimated glomerular filtration rate as predictors of cardio-renal outcomes in patients with type 2 diabetes mellitus and kidney disease: a systematic literature review. BMC Nephrol. 2018;19(1). doi:10.1186/s12882-018-0821-9
17. Langfred CW, Rockmann KW. The push and pull of autonomy: the tension between individual autonomy and organizational control in knowledge work. Group Organ Manag. 2016;41(5):629–657. doi:10.1177/1059601116668971
18. Barrett K, Barman S, Boitano S, Brooks H. General principles and energy production in medical physiology. In: Ganong’s Review of Medical Physiology.
19. Scanlon VC, Sanders T. Essentials of Anatomy and Physiology. FA Davis; 2018.
20. Jones CA, McQuillan GM, Kusek JW, et al. Serum creatinine levels in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 1998;32(6):992–999. doi:10.1016/S0272-6386(98)70074-5
21. Eknoyan G, Lameire N, Eckardt KU, et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3:5–14.
22. Abubakar I, Tillmann T, Banerjee A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171.
23. Lima Verde FA, Lima Verde IA, Silva Junior GB, Daher EF, Lima Verde EM, Lima Verde EM. Evaluation of renal function in human visceral leishmaniasis (kala-azar): a prospective study on 50 patients from Brazil. J Nephrol. 2007;20(4):430–436.
24. Alcântara SD, Santana LRL, Evangelista PD, Teixeira AC, Silva Junior GBD, Daher EDF. Renal dysfunction in Leishmaniasis and Chagas disease coinfection: a case report. Rev Inst Med Trop Sao Paulo. 2018;60. doi:10.1590/s1678-9946201860073
25. Clementi A, Battaglia G, Floris M, Castellino P, Ronco C, Cruz DN. Renal involvement in leishmaniasis: a review of the literature. Clin Kidney J. 2011;4(3):147–152. doi:10.1093/ndtplus/sfr008
26. Daher EF, Evangelista LF, Silva Júnior GB, et al. Clinical presentation and renal evaluation of human visceral leishmaniasis (kala-azar): a retrospective study of 57 patients in Brazil. Braz J Infect Dis. 2008;12(4):154.
27. Efstratiadis G, Boura E, Giamalis P, et al. Renal involvement in a patient with visceral leishmaniasis. Nephrol Dial Transplant. 2006;21(1):235–236. doi:10.1093/ndt/gfi157
28. Salgado FN, Ferreira TMA, Costa JM. Involvement of the renal function in patients with visceral leishmaniasis (kala-azar). Rev Soc Bras Med Trop. 2003;36(2):215.
29. Reis AB, Martins-Filho OA, Teixeira-Carvalho A, et al. Systemic and compartmentalized immune response in canine visceral leishmaniasis. Vet Immunol Immunopathol. 2009;128(1–3):87–95. doi:10.1016/j.vetimm.2008.10.307
30. Bates I, Ekem I. Haematological aspects of tropical diseases. Postgrad Haematol. 2007;2007:979–993.
31. Higgins C. Urea and the clinical value of measuring blood urea concentration. Acutecaretesting Org. 2016;22:1–6.
32. De Francesco Daher E, Martins Dos Santos G, Daher EDF, Santos GMD, Neto AS, Verde EML. Renal tubular dysfunction in human visceral leishmaniasis (Kala-azar). Clin Nephrol. 2009;71(5):492–500. doi:10.5414/CNP71492
33. Verde FAL, Verde FA, Veronese FJV, Neto A, Fuc G, Verde EML. Hyponatremia in visceral leishmaniasis. Rev Inst Med Trop Sao Paulo. 2010;52:253–258. doi:10.1590/S0036-46652010000500006
34. Lieberthal W, Nigam SK. Acute renal failure. I. Relative importance of proximal vs. distal tubular injury. Am J Physiol Ren Physiol. 1998;275(5):F623–F32. doi:10.1152/ajprenal.1998.275.5.F623
35. Macedo E, Mehta RL. Prerenal failure: from old concepts to new paradigms. Curr Opin Crit Care. 2009;15(6):467. doi:10.1097/MCC.0b013e328332f6e3
36. Manoeuvrier G, Bach-Ngohou K, Batard E, Masson D, Trewick D. Diagnostic performance of serum blood urea nitrogen to creatinine ratio for distinguishing prerenal from intrinsic acute kidney injury in the emergency department. BMC Nephrol. 2017;18(1):1–7. doi:10.1186/s12882-017-0591-9
37. Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant. 2013;28(2):254–273. doi:10.1093/ndt/gfs380
38. Gu L, Huang L, Wu H, Lou Q, Bian R. Serum uric acid to creatinine ratio: a predictor of incident chronic kidney disease in type 2 diabetes mellitus patients with preserved kidney function. Diab Vasc Dis Res. 2017;14(3):221–225. doi:10.1177/1479164116680318
39. Brito DE, Neto VA, Duarte IS, Penna DO, Neto VA. Glomerular involvement in human kala-azar. A light, immunofluorescent, and electron microscopic study based on kidney biopsies. Am J Trop Med Hyg. 1975;24(1):9–18. doi:10.4269/ajtmh.1975.24.9
40. Kaplan AA, Kohn OF. Fractional excretion of urea as a guide to renal dysfunction. Am J Nephrol. 1992;12(1–2):49–54. doi:10.1159/000168417
41. Weisinger JR, Pinto A, Velazquez GA, et al. Clinical and histological kidney involvement in human kala-azar. Am J Trop Med Hyg. 1978;27(2 Pt 1):357–359. doi:10.4269/ajtmh.1978.27.357
42. Tiao JY-H, Semmens JB, Masarei JRL, Lawrence-Brown MMD. The effect of age on serum creatinine levels in an aging population: relevance to vascular surgery. Cardiovasc Surg. 2002;10(5):445–451. doi:10.1177/096721090201000501
43. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419.
44. MachasNúñez JF, Cameron JS, Oreopoulos DG. The Aging Kidney in Health and Disease. Springer; 2008.
45. Oliveira MJ, Júnior GBS, Abreu KLS, et al. Risk factors for acute kidney injury in visceral leishmaniasis (Kala-Azar). Am J Trop Med Hyg. 2010;82(3):449. doi:10.4269/ajtmh.2010.09-0571
46. Musch W, Verfaillie L, Decaux G. Age-related increase in plasma urea level and decrease in fractional urea excretion: clinical application in the syndrome of inappropriate secretion of antidiuretic hormone. Clin J Am Soc Nephrol. 2006;1(5):909–914. doi:10.2215/CJN.00320106
47. Seki M, Nakayama M, Sakoh T, et al. Blood urea nitrogen is independently associated with renal outcomes in Japanese patients with stage 3–5 chronic kidney disease: a prospective observational study. BMC Nephrol. 2019;20(1):1–10. doi:10.1186/s12882-019-1306-1
48. Gerezgiher K. Evaluation of Renal Function Profile in Human Visceral Leishmaniasis (Kala-Azar) Patients. Addis Ababa, Ethiopia: Addis Ababa University; 2020.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.