Back to Journals » Infection and Drug Resistance » Volume 17
Evaluation of Nanopore Sequencing for Diagnosing Pulmonary Tuberculosis Using Negative Smear Clinical Specimens
Authors Yu G , Shen Y , Yao L , Xu X
Received 24 October 2023
Accepted for publication 13 February 2024
Published 19 February 2024 Volume 2024:17 Pages 673—682
DOI https://doi.org/10.2147/IDR.S442229
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Guocan Yu,1 Yanqin Shen,2 Liwei Yao,2 Xudong Xu1
1Zhejiang Tuberculosis Diagnosis and Treatment Center, Hangzhou Red Cross Hospital, Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Nursing, Hangzhou Red Cross Hospital, Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China
Correspondence: Liwei Yao; Xudong Xu, Email [email protected]; [email protected]
Purpose: This study aimed to evaluate the efficacy of nanopore sequencing for diagnosing pulmonary tuberculosis (PTB) using smear-negative clinical specimens.
Methods: We conducted a retrospective study based on a review of patient medical records to assess the accuracy of nanopore sequencing as a diagnostic tool for smear-negative PTB. Compared with clinical diagnosis, we determined the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC) of nanopore sequencing.
Results: A total of 647 patients were evaluated. Nanopore sequencing demonstrated an overall sensitivity of 91.7%, specificity of 85.3%, PPV of 95.1%, NPV of 76.4%, and AUC of 0.88. Notably, the overall diagnostic accuracy of nanopore sequencing was significantly higher than that of Mycobacterium tuberculosis (MTB) culture technique.
Conclusion: Nanopore sequencing exhibited satisfactory overall diagnostic accuracy for smear-negative PTB, regardless of MTB culture status. Therefore, if conditions permit, nanopore sequencing is recommended as a diagnostic method for smear-negative PTB.
Keywords: nanopore sequencing, smear-negative specimens, pulmonary tuberculosis, diagnostic accuracy
Introduction
Tuberculosis (TB) is a highly contagious disease caused by Mycobacterium tuberculosis (MTB), which continues to pose threats in the field of global health, as indicated by recent statistical data.1 In 2022, 10.6 million new TB diagnoses and 1.3 million TB deaths were recorded worldwide.1 Consequently, TB ranked among the top 10 causes of death attributed to a single infectious disease.2 The most prevalent form of TB is pulmonary TB (PTB), accounting for approximately 70–85% of all TB cases.3 PTB serves as the primary source of TB transmission.4 Therefore, controlling the source of infection is crucial for reducing the spread of TB. Effective detection and treatment of PTB are key for controlling the source of infection effectively.
Acid-fast bacilli (AFB) smear is the most widely used clinical method for diagnosing PTB.5 However, it exhibits low sensitivity, indicating that a large proportion of patients present with a negative AFB smear.6 Smear-negative PTB cases are easily overlooked, resulting in PTB transmission.2 Improving the diagnostic accuracy of PTB using smear-negative clinical specimens remains a major challenge. Although MTB culture technique is essential for PTB diagnosis, it requires a long time and cannot facilitate early diagnosis.7 Moreover, smear-negative PTB specimens may exhibit lower MTB content than smear-positive PTB specimens, resulting in a reduced culture positivity rate.8 Consequently, relying solely on MTB culture technique for diagnosing smear-negative PTB may have limited efficacy.9
The development of molecular diagnostic techniques in recent years has led to significant advancements in the rapid diagnosis of infectious diseases, including TB.10,11 Xpert MTB/RIF is the most widely used molecular test for rapid diagnosis of TB recommended by the World Health Organization, also in China.12 However, its accuracy in paucibacillary TB diagnosis still needs to be further improved.13 Genetic sequencing is widely used in the field of infectious diseases and offers unique advantages for the rapid diagnosis of TB.13,14 The utilization of next-generation sequencing (NGS) in TB has demonstrated remarkable diagnostic efficacy, thereby greatly improving TB diagnosis.15 Nanopore sequencing is a unique and scalable technology that monitors changes in the current of nucleic acids as they pass through nanopore proteins. The resulting signal is decoded to provide specific DNA or RNA sequences.16 Nanopore sequencing offers portability, real-time analysis, and long read lengths compared with other NGS techniques.17,18 Its ability to directly detect and decode genetic material with high accuracy and efficiency will undoubtedly revolutionize various fields of research and significantly contribute to advancements in personalized medicine and molecular biology. Its application in rapid TB diagnosis is gradually increasing and has demonstrated excellent performance, particularly in PTB, the most common form of TB.19–21 However, studies evaluating its diagnostic accuracy for smear-negative PTB in specimens with low bacterial content are lacking. Therefore, this study aimed to assess the efficacy of nanopore sequencing for diagnosing PTB using smear-negative clinical specimens.
Materials and Methods
Study Design
This retrospective study was conducted at a TB diagnosis and treatment center in Zhejiang Province, China, from October 2021 to October 2022. This study included patients who were hospitalized at our center and had suspected PTB with negative-smear specimens. The index test was nanopore sequencing, and the control test was MTB culture technique. The measured outcomes included sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC) of the relevant test for diagnosing smear-negative PTB.
To identify patients with suspected smear-negative PTB, a set of criteria was followed: a) the presence of symptoms such as cough, fever, and other related clinical indications; b) imaging tests conducted to detect signs of pneumonia, tree-in-bud patterns, and cavity formation; c) positive tuberculin purified protein derivative (PPD) test and/or TSPOT.TB; d) negative AFB smear test in clinical specimens; and e) no other confirmed pathogenic microbial infections. The inclusion criteria were patients of any age with suspected smear-negative PTB who had undergone both nanopore sequencing and MTB culture technique using clinical respiratory specimens. Exclusion criteria included patients who did not undergo both tests or lacked complete information despite undergoing relevant tests. In this study, clinical specimens referred to fresh sputum and bronchoalveolar lavage fluid (BALF) samples. Sputum was obtained through spontaneous excretion, and BALF was collected via fiberoptic bronchoscopy after the lavage of lung lesions. The study protocol was approved by the Ethics Committee of Hangzhou Red Cross Hospital (2022–039); it is in accordance with the guidelines of Declaration of Helsinki. This is a retrospective study conducted on already available data and will not have any impact on patients; therefore, our ethics committee waived informed consent from patients.
Based on the Health Industry Standard of the People’s Republic of China - Diagnosis for pulmonary tuberculosis (WS 288–2017),19 we categorized all suspected smear-negative PTB into three groups:
Group A (confirmed PTB): Positive MTB culture in clinical specimens. In this study, it refers to smear-negative and culture-positive PTB.
Group B (probable PTB): Negative MTB culture, positive TB-related symptoms and imaging results, positive PPD regardless of TSPOT.TB, positive results of other nucleic acid amplification tests (such as Xpert MTB/RIF), and favorable response to aggressive anti-TB treatment. In this study, it refers to smear- and culture-negative PTB.
Group C (non-PTB): No evidence of TB-related disease, confirmed diagnosis of other infectious diseases, disease improvement without anti-TB treatment, or ineffective response to anti-TB treatment.
Groups A and B represented clinical diagnosis of PTB cases and received anti-TB treatment. Two investigators independently classified the patients based on retrospectively obtained patient information. In case of disagreement, a third investigator participated in the discussion to determine the final grouping.
Diagnostic Specimen Collection and Handling
We collected fresh sputum or bronchoalveolar lavage fluid (BALF) for relevant tests before diagnosis. Sputum was the first sputum after gargling in the early morning, and BALF was obtained by fiberoptic bronchoscopy irrigation of the lung lesion site. Each sample was divided equally for MTB culture and nanopore sequencing. Fresh specimens were obtained and placed in a 4°C incubator and used directly in the assays within 6 hours.
MTB Culture Technique
For MTB culture technique, a freshly collected sample (1 mL) was utilized. Preparation of clinical specimens involved digestion and decontamination, which was performed using N-acetyl-l-cysteine–NaOH. The samples were decontaminated with an equal amount of 4% N-acetyl-l-cysteine–NaOH pretreatment solution for 15–20 minutes at room temperature. Phosphate buffer saline was added to the decontaminated specimen and then centrifuged at 3500 g for 15–20 minutes, the supernatant was discarded and the precipitate was retained.22 Then, the retained specimens were inoculated into two different growth media: Lowenstein–Jensen solid medium and BACTEC MGIT 960 liquid medium (BD Diagnostic Systems in Sparks, MD). Each MGIT tube was supplemented with 0.8 mL PANTA. Subsequently, the samples were incubated at an optimal temperature of 35°C–37°C.12 All tubes were incubated until flagging positive.
Nanopore Sequencing
Nanopore sequencing of sputum and BALF samples involved several steps. First, sputum samples were liquefied in NaOH solution and then centrifuged to remove the supernatant. The resulting pellet was washed with phosphate-buffered saline and was resuspended in a lysis solution. Similarly, BALF samples underwent centrifugation and resuspension. Both samples were then physically ground with grinding beads, and lysozyme solution was added to facilitate further lysis. DNA was subsequently extracted from the lysates using a QIAamp DNA Microbiome Kit (Cat. No. 51707, Qiagen, Hilden, Germany). Polymerase chain reaction (PCR) amplification was performed using specific primers (Rpo5’ [5’-TCAAGGAGAAGCGCTACGA-3’] and Rpo3’ [5’-GGATGTTGATCAGGGTCTGC-3’]) targeting the rpoB gene of Mycobacterium spp. PCR was performed based on the touchdown method.23 Starting with a high-temperature denaturation step and gradually decreasing the annealing temperature to facilitate effective amplification. The steps included denaturation at 98°C for 3 min, followed by six cycles of amplification at 95°C for 15s, annealing at 66°C for 60s (with a decrease of 1°C per cycle), and elongation at 72°C for 30s. After the initial 6 cycles, the annealing temperature was lowered to 61°C for the remaining 29 cycles. Finally, an extension step was performed at 72°C for 5 min.
After purifying and labeling the amplified product using barcode technology, it was sequenced using the GridION platform (Oxford Nanopore Technologies). Amplified nucleic acid samples were added to the sample cassette and inserted into the GridION platform for fully automated sequencing according to the platform instrument instructions.19 The MinKnow v3.6.5 software developed by Oxford Nanopore Technologies was utilized to gather the real-time sequencing data.19 The paired raw sequencing reads were initially subjected to a quality filtration process to eliminate any low-quality data. The quality of sequencing data was evaluated in terms of the length of reads, coverage of target genes, and depth of sequencing. Following this, further analysis was performed on the high-quality reads. During the subsequent analysis, any sequenced fragments shorter than 200 base pairs (bp) were excluded from the dataset to ensure the reliability and accuracy of the sequencing results. The resulting sequences underwent alignment with reference sequences of MTB (NC_000962.3) and Mycobacterium (txid 1763) using MiniMap 2 (Version 2.17) software.24 To ensure the absence of contamination, host DNA reads were removed by aligning them with the human reference genome (GRCh38). Overall, this process provided results within 48 h.
Data Processing and Statistical Analysis
To evaluate the accuracy of diagnostic methods used in this study, various statistical techniques were employed. Mean values, quartiles, standard deviations, and true positive (TP), false positive (FP), false negative (FN), and true negative (TN) values were calculated using IBM Corp.’s SPSS 24.0 software. Diagnostic accuracy indices, including sensitivity, specificity, PPV, NPV, and AUC with 95% confidence intervals, were calculated using MedCalc Statistical v15.2.2 software. These calculations relied on the four values of TP, FP, FN, and TN. Paired data were analyzed using McNemar’s test, whereas chi-square or Fisher’s exact tests were used to compare two ratios. Differences between two AUC values were compared using the Z-test. p-values of <0.05 were considered to indicate statistical significance for all tests.
Results
During the study period, 670 patients with smear-negative PTB underwent nanopore sequencing. However, 23 patients were excluded from the analysis due to incomplete data, such as missing MTB culture results. Therefore, the final sample size was 647, which met the inclusion criteria outlined in the study protocol. The screening process and diagnostic classification of the included patients are illustrated in Figure 1.
 |
Figure 1 Inclusion of patient screening process and diagnostic classification. Abbreviations: PTB, pulmonary tuberculosis; MTB, Mycobacterium tuberculosis; NS, nanopore sequencing. |
The average age of the patients was 49.5 ± 18.6 years, with males accounting for 57.3% (371/647) of the cases. Among the included patients, PTB was diagnosed in 491 patients, of whom 160 patients had confirmed PTB (smear-negative and culture-positive), 331 patients had probable PTB (smear-negative and culture-negative), and 156 patients had non-PTB. A total of 160 patients tested positive for MTB culture, whereas 473 patients showed positive nanopore sequencing results. The overlap and distribution of positive results between these two tests and the resulting diagnosis of PTB are shown in Figure 2. The nanopore sequencing reads ranged from 1 to 125,840, with most patients having reads in the range of 1–7000 (Figure 3). The average length of reads ranged from 2001 to 2956bp. The coverage of target gene ranged from 95.1% to 100%, and the depths of sequencing ranged from 402X to 1199X. No cases of HIV infection were identified in this study.
 |
Figure 3 Distribution of nanopore sequencing reads for all patients. |
Among the 160 confirmed PTB cases, all patients tested positive for MTB culture, whereas 152 cases were identified via nanopore sequencing. Among the 331 probable PTB cases, no patients tested positive for MTB culture, but 298 patients showed positive nanopore sequencing results. In the non-PTB group, 23 patients tested positive for nanopore sequencing, with read counts ranging from 1 to 19. Notably, these patients had no other evidence of TB infection, showed disease improvement without anti-TB treatment, and exhibited no recurrence of lesions during follow-up. The average number of reads obtained via nanopore sequencing in the PTB group was significantly higher than that in the non-PTB group (P < 0.001). The difference in read counts is presented in Figure 4.
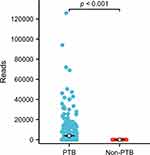 |
Figure 4 The distribution and comparison of reads for pulmonary tuberculosis and non-pulmonary tuberculosis of positive nanopore sequencing patients. |
Comparison of the Diagnostic Efficacy of Culture Technique and Nanopore Sequencing
The diagnostic accuracy of culture technique and nanopore sequencing for cases of total PTB, confirmed PTB, and probable PTB is presented in Table 1. In case of total PTB, culture technique exhibited significantly lower sensitivity and NPV than nanopore sequencing, resulting in a significantly lower overall diagnostic accuracy than that of nanopore sequencing (P < 0.001, Table 1). For confirmed PTB, which refers to smear-negative and culture-positive PTB, culture technique served as the gold standard for diagnosis and demonstrated the highest diagnostic efficacy, and nanopore sequencing also achieved good diagnostic accuracy. In case of probable PTB, which refers to smear- and culture-negative PTB, culture technique was ineffective for diagnosis, whereas nanopore sequencing maintained good diagnostic accuracy, significantly outperforming culture technique (P < 0.001, Table 1). The diagnostic accuracy of nanopore sequencing in confirmed and probable PTB cases was comparable, with no significant difference between them (P > 0.05, Table 1).
 |
Table 1 Diagnostic Accuracy of Nanopore Sequencing and Tuberculosis Culture Technique for Pulmonary Tuberculosis Using Smear-Negative Clinical Specimens |
Discussion
Smear-negative PTB represents an important source of infection in TB transmission, and its significance should not be overlooked despite the low bacterial content in clinical specimens.25 Patients with smear-negative PTB often present with symptoms similar to those with smear-positive PTB, such as coughing and difficulty breathing, but may not test positive for TB bacteria until the later stages of the disease.26 This delay in diagnosis and treatment increases the risk of transmission to others and leaves patients susceptible to ongoing infection.26 Therefore, the effective detection of smear-negative PTB is crucial for providing timely treatment and implementing appropriate protective measures, playing a vital role in TB control.
Despite advancements in medical technology, the early detection of smear-negative PTB remains a major challenge.27 Traditional AFB smear, which relies on the presence of AFB in samples, is not effective for the rapid diagnosis of smear-negative PTB. MTB culture technique, on the other hand, is an essential diagnostic tool for PTB. However, its main limitation is the long duration required to obtain results, often spanning several weeks.28 This delay is problematic because rapid identification and treatment of PTB are vital for preventing the spread of the disease.
The delay in diagnosis caused by relying solely on culture technique can result in the worsening of lung disease and increased exposure of individuals to infection. Furthermore, smear-negative PTB is usually considered as paucibacillary TB,29 the MTB load in smear-negative PTB specimens is lower than that in smear-positive PTB specimens; this lowers the positive MTB culture rate of smear-negative PTB specimens, which is unfavorable for its diagnosis. For these reasons, MTB culture is not a perfect reference standard in smear-negative PTB and can significantly reduce specificity. Clinical diagnostic criteria include MTB culture, nucleic acid detection tests (such as Xpert), and other relevant results, resulting in a comprehensive evaluation that is a more appropriate reference standard for paucibacillary TB,30 and therefore clinical diagnosis was used as the gold standard in our study.
In this study, only 32.6% of all smear-negative PTB specimens tested positive for MTB culture. This result was consistent with previous studies,2,31,32 which demonstrated the unsatisfactory sensitivity of this method for diagnosing smear-negative PTB, regardless of its rate limitations. Additionally, 78% of patients with negative culture results were ultimately diagnosed with PTB. These findings suggested that the MTB content in smear-negative PTB specimens is low and that the traditional MTB assay has limitations in diagnosing smear-negative PTB. Therefore, there is an urgent need to develop a more superior assay for accurate diagnosis.
Molecular diagnostic technology is a popular method for diagnosing TB and has been extensively used for both PTB and extra-pulmonary TB, showing great potential for widespread application.33 In case of smear-negative PTB, molecular diagnostic techniques play a crucial role in enhancing the diagnostic yield. However, the sensitivity of these molecular techniques in smear-negative PTB remains relatively low.34 Previous studies have reported sensitivities of 57.1% for Xpert MTB/RIF and 53.4% for CapitalBio Mycobacterium RT–PCR for detecting smear-negative PTB,2 indicating the need for further improvement. Although the sensitivity of Xpert MTB/RIF ultra for smear-negative PTB has improved,31 it is still unsatisfactory. Meanwhile in China Xpert ultra has not been universally applied except in a few large infectious disease centers, limiting its application.
Nanopore sequencing is a highly effective molecular diagnostic test that has gained significant popularity in various fields, including infectious diseases.35 It has emerged as a valuable tool for the rapid diagnosis of TB and drug susceptibility testing, particularly for drug-resistant TB.36 Although nanopore sequencing plays a crucial role in the rapid diagnosis of PTB, there is limited research focusing on its application in smear-negative PTB, particularly with large sample sizes. This study evaluated the diagnostic efficacy of nanopore sequencing in smear-negative PTB using a larger sample size, thereby providing a more robust and effective diagnostic tool for smear-negative PTB. In this study, targeted PCR amplification was performed on specimens prior to nanopore sequencing to improve detection for paucibacillary TB. The PCR amplification was based on rpoB. The ability of rpoB to efficiently amplify Mycobacterium spp. and perform strain identification has been fully evaluated by previous studies.37–39
Our evidence suggested that nanopore sequencing demonstrated a satisfactory ability to detect smear-negative PTB, accurately identifying 91.3% of PTB cases. However, the results of this study were inferior to those of previous studies,19 which included a large number of smear-positive specimens. In contrast, our study focused solely on smear-negative specimens. Consequently, the correlation of nanopore sequencing results with smear-positive specimens was found to be superior to that with smear-negative specimens. Despite the FP rate of 14.7% in non-PTB patients, the number of reads observed in FP results was significantly lower than that observed in TP results. This suggested that when interpreting nanopore sequencing results, it was important to consider not only the presence of positive results but also the number of obtained reads. A higher number of reads corresponds to increased confidence in the results. The overall diagnostic accuracy of nanopore sequencing was significantly superior to that of MTB culture technique. This could be attributed to the fact that nanopore sequencing detects specific fragments of MTB DNA, regardless of bacterial viability, and utilizes PCR amplification, which can enhance detection. In contrast, culture technique relies on the growth of intact live MTB, resulting in a limited positive rate.
In smear-negative and culture-positive PTB, nanopore sequencing demonstrated superior diagnostic efficacy, detecting 95% of PTB cases, which was highly satisfactory. The number of patients with smear- and culture-negative PTB was significantly larger due to the lower MTB load in these specimens, resulting in a lower culture positivity rate. Diagnosing this type of PTB is even more challenging, and traditional detection methods remain ineffective.
In our study, nanopore sequencing achieved similar diagnostic efficacy in both smear-negative, culture-negative PTB and in smear-negative, culture-positive PTB. This finding suggested that the impact of culture results on the performance of nanopore sequencing is minimal. Overall, nanopore sequencing exhibited excellent diagnostic accuracy regardless of the culture status, highlighting its applicability in the diagnosis of PTB.
The current study has several limitations. First, as a retrospective study, there is a possibility of selection bias in the inclusion of patients, which may have affected the generalizability of the findings. Additionally, the data used in this study were predominantly derived from areas with a high burden of TB, limiting its applicability to regions with different TB burdens. Another limitation is that nanopore sequencing in this study does not include drug resistance results, which is crucial for the diagnosis and treatment of TB. Furthermore, this study was focused primarily on the diagnosis of PTB and did not include any data on non-TB mycobacteria, which is another significant cause of respiratory disease. Therefore, the findings of this study should be interpreted considering these limitations.
Conclusion
The overall diagnostic accuracy of nanopore sequencing for smear-negative PTB was satisfactory, regardless of the MTB culture status. If conditions permit (availability of laboratories to perform nanopore sequencing and good financial status of patients), nanopore sequencing is recommended as a diagnostic method for smear-negative PTB.
Data Sharing Statement
Data will be available from the corresponding author on reasonable request.
Compliance with Ethical Standards
The study was approved by the Human Research Ethics Committee of Hangzhou Red Cross Hospital. This is a retrospective study conducted on already available data and will not have any impact on patients; therefore, our ethics committee waived informed consent from patients.
Acknowledgments
We would like to express our gratitude to the patients and colleagues in our department.
Funding
This work was supported by Hangzhou Science and Technology Bureau, http://kj.hangzhou.gov.cn. Guocan Yu, 20201203B183. The funder does not have a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. World Health Organization. Global Tuberculosis Report 2023. World Health Organization; 2023.
2. Zheng H, Zhong F, Yu G, Shen Y. Comparison of the diagnostic efficacy of the CapitalBio Mycobacterium real-time polymerase chain reaction detection test and Xpert MTB/RIF in smear-negative pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis. 2021;40(5):969–977. doi:10.1007/s10096-020-04113-1
3. Shen Y, Fang L, Xu X, Ye B, Yu G. CapitalBio Mycobacterium real-time polymerase chain reaction detection test: rapid diagnosis of Mycobacterium tuberculosis and nontuberculous mycobacterial infection. Int J Infect Dis. 2020;98:1–5. doi:10.1016/j.ijid.2020.06.042
4. Qiu X, Zheng S, Yang J, Yu G, Ye Y. Comparing mycobacterium tuberculosis RNA accuracy in various respiratory specimens for the rapid diagnosis of pulmonary tuberculosis. Infect Drug Resist. 2022;15:4195–4202. doi:10.2147/IDR.S374826
5. Elbrolosy AM, El Helbawy RH, Mansour OM, Latif RA. Diagnostic utility of GeneXpert MTB/RIF assay versus conventional methods for diagnosis of pulmonary and extra-pulmonary tuberculosis. BMC Microbiol. 2021;21(1):144. doi:10.1186/s12866-021-02210-5
6. Rasheed W, Qureshi R, Jabeen N, Shah HA, Naseem Khan R. Diagnostic accuracy of high-resolution computed tomography of chest in diagnosing sputum smear positive and sputum smear negative pulmonary tuberculosis. Cureus. 2020;12(6):e8467. doi:10.7759/cureus.8467
7. Yu G, Wang L, Shen Y, et al. Comparison of the diagnostic accuracy of xpert MTB/RIF and CapitalBio Mycobacterium RT-PCR detection assay for tuberculous pericarditis. Infect Drug Resist. 2022;15:2127–2135. doi:10.2147/IDR.S360064
8. Bohlbro AS, Mendes AM, Sifna A, Gomes V, Rudolf F, Wejse C. Incidence of pulmonary tuberculosis in suburban Bissau, Guinea-Bissau between 2004 and 2020: a prospective cohort study. Infection. 2022;51(4):955–966. doi:10.1007/s15010-022-01958-w
9. Wu Z, Shi J, Zhou Y, et al. The diagnostic value of the thermostatic amplification of ribonucleic acid in bronchoalveolar lavage fluid in smear-negative pulmonary tuberculosis. Front Public Health. 2022;10:830477. doi:10.3389/fpubh.2022.830477
10. Yu G, Zhong F, Zhao W, Ye B, Xu K, Chen G. Head-to-head comparison of the diagnostic value of five tests for constrictive tuberculous pericarditis: five tests for constrictive TBP. Int J Infect Dis. 2022;120:25–32. doi:10.1016/j.ijid.2022.04.018
11. Yu G, Shen Y, Ye B, Shi Y. Diagnostic accuracy of Mycobacterium tuberculosis cell-free DNA for tuberculosis: a systematic review and meta-analysis. PLoS One. 2021;16:6.
12. Shen Y, Fang L, Ye B, Xu X, Yu G, Zhou L. The role of core needle biopsy pathology combined with molecular tests in the diagnosis of lymph node tuberculosis. Infect Drug Resist. 2022;15:335–345. doi:10.2147/IDR.S350570
13. Yu G, Wang X, Zhu P, Shen Y, Zhao W, Zhou L. Comparison of the efficacy of metagenomic next-generation sequencing and Xpert MTB/RIF in the diagnosis of tuberculous meningitis. J Microbiol Methods. 2021;180:106124. doi:10.1016/j.mimet.2020.106124
14. Zhou Y, Shi W, Wen Y, Mao E, Ni T. Comparison of pathogen detection consistency between metagenomic next-generation sequencing and blood culture in patients with suspected bloodstream infection. Sci Rep. 2023;13(1):9460. doi:10.1038/s41598-023-36681-5
15. Jin Y, Hu S, Feng J, Ni J. Clinical value of metagenomic next-generation sequencing using spinal tissue in the rapid diagnosis of spinal tuberculosis. Infect Drug Resist. 2023;16:3305–3313. doi:10.2147/IDR.S410914
16. Dippenaar A, Goossens SN, Grobbelaar M, et al. Nanopore sequencing for mycobacterium tuberculosis: a critical review of the literature, new developments, and future opportunities. J Clin Microbiol. 2022;60(1):e0064621. doi:10.1128/JCM.00646-21
17. Luo W, He Y, Xu J, et al. Comparison of third-generation sequencing technology and traditional microbiological detection in pathogen diagnosis of lower respiratory tract infection. Discov Med. 2023;35(176):332–342. doi:10.24976/Discov.Med.202335176.34
18. Liu Z, Yang Y, Wang Q, Wang L, Nie W, Chu N. Diagnostic value of a nanopore sequencing assay of bronchoalveolar lavage fluid in pulmonary tuberculosis. BMC Pulm Med. 2023;23(1):77. doi:10.1186/s12890-023-02337-3
19. Yu G, Shen Y, Zhong F, et al. Diagnostic accuracy of nanopore sequencing using respiratory specimens in the diagnosis of pulmonary tuberculosis. Int J Infect Dis. 2022;122:237–243. doi:10.1016/j.ijid.2022.06.001
20. Yang J, Ye W, Zhang C, et al. Accuracy of nanopore sequencing as a diagnostic assay for pulmonary tuberculosis versus smear, culture and xpert MTB/RIF: a head-to-head comparison. Trop Med Infect Dis. 2023;8(9). doi:10.3390/tropicalmed8090441
21. Zou X, Zhu Y, Qin Y, et al. Value analysis of next-generation sequencing combined with Xpert in early precise diagnosis of pulmonary tuberculosis. Diagn Microbiol Infect Dis. 2023;107(1):115921. doi:10.1016/j.diagmicrobio.2023.115921
22. Bhat J, Selvakumar N, Rao VG, Gopi PG, Yadav R, Wares DF. Yield of culture of Mycobacterium tuberculosis complex in sputum samples transported from tribal areas. Int J Tuberc Lung Dis. 2011;15(4):478–482. doi:10.5588/ijtld.10.0234
23. Green MR, Sambrook J. Touchdown Polymerase Chain Reaction (PCR). Cold Spring Harb Protoc. 2018;2018:5.
24. Li H, Birol I. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34(18):3094–3100. doi:10.1093/bioinformatics/bty191
25. Marais BJ, Gie RP, Schaaf HS, et al. The clinical epidemiology of childhood pulmonary tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8(3):278–285.
26. Hasen Badeso M, Sani Kalil F, Mohammed Ahmed Y, Abdulkadir Godie S, Habtamu Regesu A. Trends of tuberculosis disease from 2013–2018 in Bale Zone, Oromia Region, Ethiopia. Retrospective Review. Infect Drug Resist. 2022;15:6723–6730.
27. Petnak T, Eksombatchai D, Chesdachai S, et al. Diagnostic accuracy of interferon-gamma release assays for diagnosis of smear-negative pulmonary tuberculosis: a systematic review and meta-analysis. BMC Pulm Med. 2022;22(1):219. doi:10.1186/s12890-022-02013-y
28. Buckwalter SP, Connelly BJ, Louison LK, et al. Description, validation, and review of a decade of experience with a laboratory-developed PCR test for detection of Mycobacterium tuberculosis complex in pulmonary and extrapulmonary specimens. J Clin Tuberc Other Mycobact Dis. 2022;29:100340. doi:10.1016/j.jctube.2022.100340
29. Wang G, Wang S, Jiang G, et al. Xpert MTB/RIF Ultra improved the diagnosis of paucibacillary tuberculosis: a prospective cohort study. J Infect. 2019;78(4):311–316. doi:10.1016/j.jinf.2019.02.010
30. Yu G, Zhong F, Ye B, Xu X, Chen D, Shen Y. Diagnostic accuracy of the xpert MTB/RIF assay for lymph node tuberculosis: a systematic review and meta-analysis. Biomed Res Int. 2019;2019:4878240. doi:10.1155/2019/4878240
31. Chien JY, Lin CK, Yu J, Hsueh PR. Usefulness of xpert MTB/RIF ultra to rapidly diagnose sputum smear-negative pulmonary tuberculosis using bronchial washing fluid. Front Microbiol. 2020;11:588963. doi:10.3389/fmicb.2020.588963
32. Sun X, Song J, Leng X, et al. A preliminary evaluation of targeted nanopore sequencing technology for the detection of Mycobacterium tuberculosis in bronchoalveolar lavage fluid specimens. Front Cell Infect Microbiol. 2023;13:1107990. doi:10.3389/fcimb.2023.1107990
33. Liu Y, Ren W, Xue Z, et al. Real-time recombinase-aided amplification assay for rapid amplification of the IS1081 gene of Mycobacterium tuberculosis. Eur J Clin Microbiol Infect Dis. 2023;42(8):963–972. doi:10.1007/s10096-023-04626-5
34. Lodha L, Mudliar SR, Singh J, et al. Diagnostic performance of multiplex PCR for detection of mycobacterium tuberculosis complex in presumptive pulmonary tuberculosis patients and its utility in smear negative specimens. J Lab Physicians. 2022;14(4):403–411. doi:10.1055/s-0042-1757231
35. Zhao X, Ge Y, Zhang Y, et al. Pathogen diagnosis value of nanopore sequencing in severe hospital-acquired pneumonia patients. Infect Drug Resist. 2023;16:3293–3303. doi:10.2147/IDR.S410593
36. Nilgiriwala K, Rabodoarivelo MS, Hall MB, et al. Genomic sequencing from sputum for tuberculosis disease diagnosis, lineage determination, and drug susceptibility prediction. J Clin Microbiol. 2023;61(3):e0157822. doi:10.1128/jcm.01578-22
37. Wang HY, Kim H, Kim S, Kim DK, Cho SN, Lee H. Performance of a real-time PCR assay for the rapid identification of Mycobacterium species. J Microbiol. 2015;53(1):38–46. doi:10.1007/s12275-015-4495-8
38. Fu Y, Chen Q, Xiong M, et al. Clinical performance of nanopore targeted sequencing for diagnosing infectious diseases. Microbiol Spectr. 2022;10(2):e0027022. doi:10.1128/spectrum.00270-22
39. Ong CS, Ngeow YF, Yap SF, Tay ST. Evaluation of PCR-RFLP analysis targeting hsp65 and rpoB genes for the typing of mycobacterial isolates in Malaysia. J Med Microbiol. 2010;59(Pt 11):1311–1316. doi:10.1099/jmm.0.021139-0
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

