Back to Journals » International Journal of General Medicine » Volume 14
Evaluation of Empirical Meropenem Bolus Protocol in Pseudomonas aeruginosa: A Three-Year Analysis in Tertiary Intensive Care Unit
Authors Suranadi IW, Panji PAS, Budayanti NNS, Senapathi TGA , Susatya AB
Received 26 September 2021
Accepted for publication 21 October 2021
Published 9 November 2021 Volume 2021:14 Pages 7861—7867
DOI https://doi.org/10.2147/IJGM.S341423
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
I Wayan Suranadi,1 Putu Agus Surya Panji,1 Ni Nyoman Sri Budayanti,2 Tjokorda Gde Agung Senapathi,1 Arif Budiman Susatya1
1Department of Anesthesiology and Intensive Care, Faculty of Medicine, Universitas Udayana/Sanglah General Hospital, Denpasar, Bali, 80113, Indonesia; 2Department of Clinical Microbiology, Faculty of Medicine, Universitas Udayana, Denpasar, Bali, 80113, Indonesia
Correspondence: I Wayan Suranadi
Department of Anesthesiology and Intensive Care, Faculty of Medicine, Universitas Udayana/Sanglah General Hospital, Diponegoro Street, Dauh Puri Klod, Denpasar, 80113, Indonesia
Tel/Fax +62361-227911
Email [email protected]
Purpose: To describe meropenem empirical use, susceptibility trend, and associated factors for acquired nonsusceptibility in P. aeruginosa in the intensive care unit.
Patients and Methods: This study was conducted in the intensive and high care unit of a tertiary care hospital in Indonesia to evaluate empirical meropenem bolus administration protocol. All patients admitted during the 3 year study period from January 2018 through January 2021 with culture-confirmed P. aeruginosa infection were included in the study. Primary data were collected from hospital database electronic medical record and series of local biannual report of microorganism susceptibility pattern.
Results: The data suggested that there was increasing trend in meropenem nonsusceptibility and multidrug-resistance rates. A total of 135 patients with various primary diagnoses and comorbidities were studied. P. aeruginosa isolates were mostly (73.4%) obtained from sputum specimen. Empirical meropenem therapy was administrated in 24.4% of patients with standard- and high-dose as indicated. Nonsusceptibility was acquired in 37% patients who mostly received empirical therapy. Multivariable analysis revealed protocol being evaluated as a statistically significant risk factor for nonsusceptibility in P. aeruginosa (PR = 30.65; p < 0.001).
Conclusion: Empirical meropenem administration protocol in this study was an independent determinant of nonsusceptibility acquisition in P. aeruginosa. These findings proved that empirical therapeutic strategy modification is indispensable and routine evaluation practice should be promulgated.
Keywords: antibiotic resistance, critical care, empirical therapy, Indonesia, meropenem, Pseudomonas aeruginosa
Introduction
Ever-growing consumption of antibiotics all over the world gives rise to the concern of its judicious use and expected efficacy. Global estimation of antibiotic consumption reported a 65% increase during 15 years observation up to 2015, followed by further 15% projected increment by the end of the year 2030. Low- and middle-income countries (LMICs) as the main driver of this phenomenon are likely to face the impending danger of unresolved infectious disease burden compounded by resistant infections.1 Resistance also poses increased mortality risk and economic burden hence resulting in unavailing effort.2,3 The intricate problem of antimicrobial resistance adds an overwhelming complexity to critical care management in which timely targeted therapy is imperative. While antibiotics were administered in almost three-fourths of intensive care unit (ICU) patients, unnecessary use was indicated in up to half of the cases.4
Among the notorious nosocomial pathogens in the ICU referred to as the ESKAPE group (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter sp.), multidrug-resistant Pseudomonas aeruginosa emerged as a formidable pathogen with level of resistance exceeding 50% in various LMICs.5 Similarly, unequivocal decreasing susceptibility of P. aeruginosa to last resort antibiotics which are intended to be used prudently such as carbapenems was observed in multiple ICUs in Indonesia alone.6,7 Carbapenems are recommended for empirical therapy in intensive care setting considering the wide array of indications that they cover, particularly meropenem in the context of P. aeruginosa suspicion on the grounds of its relative superiority over other agents within the class.8,9 Hence, prompt investigation is warranted to address the potential of meropenem resistance in P. aeruginosa.
Evaluation at facility-level to improve antibiotics use is one of the core elements of antibiotic stewardship program (ASP) as stated in the latest update by the Centers for Disease Control and Prevention.10 The need for local ICU data integration in constructing national guideline to pursue ASP efforts is further emphasized in American Thoracic Society (ATS) Workshop collaboration.11 However, local data are still underrepresented and recent review only identified two studies from Indonesia.5 Aggregation of multicentral data is expected to assist in the development of national guidelines which in turn would be tailored to fit into local hospital-wide ASP implementation. This study aims to describe meropenem empirical use, susceptibility trend, and associated factors for acquired nonsusceptibility in P. aeruginosa during the three-year observation period.
Patients and Methods
This observational study was conducted from January 2018 through January 2021 in the ICU and high care unit (HCU) of a tertiary care hospital in Indonesia. The 21-bed ICU and 7-bed HCU facilitates critically ill medical and surgical patients. Bacterial isolates collection and subsequent antimicrobial susceptibility testing was done by the microbiology department of a tertiary hospital, of which complete procedure was described in detail elsewhere.7
All patients admitted to the ICU and HCU with culture-confirmed clinical P. aeruginosa infection, regardless of the level of susceptibility, were included in the study. Any duplicate, incomplete data, and meropenem administration beyond 30 days prior to culture specimen collection rendered subject exclusion. Clinical data were extracted from electronic medical record in the hospital database, while microbiological data were derived from routine biannual report of local microorganism susceptibility pattern. Current study protocol conformed with the Declaration of Helsinki and its following amendments and was approved by Universitas Udayana/Sanglah General Hospital research ethics committee (no. LB.02.01/XIV.2.2.1/12961/2021). Patient informed consent was obtained prior to study commencement and data confidentiality was ensured by omitting protected health information.
The working definition of antibiotic stewardship provided by ATS in 2020 was set to be the cornerstone of variables being studied.11 Given a priori-determined drug of interest, time of administration, and targeted pathogen, the remaining variables of dose and duration became the independent variables. Empirical meropenem administration in the ICU was done under the protocol of one- (standard-dose) or two-gram (high-dose) intravenous meropenem in 100 mL of normal saline over 30 minutes to an hour infusion every 8 hours (q8h) for 7 days or until the result of culture and sensitivity testing was issued. High-dose administration involved clinical decision-making based on infection severity and/or overnutrition status (ie, overweight and obesity). Clinical indications for empirical meropenem administration encompassing sepsis, urinary tract infection, and ventilator-associated pneumonia. Antimicrobial exposure was a subset of antimicrobial duration which represented the time from initial administration until culture specimen collection. Resistance or intermediate susceptibility was considered as meropenem nonsusceptibility in this study and the categorization was made according to the Clinical and Laboratory Standards Institute (CLSI) guidance applicable in corresponding year of study period.
Statistical Analysis
Microsoft® Office Excel 2016 for Windows, version 2002 (Microsoft Corporation) and the Statistical Package for Social Sciences version 23 (IBM Corp, Chicago, IL, USA) were used for data organization and analysis, respectively. Descriptive analysis was done to present preliminary data summary. Numerical measures were reported in appropriate central tendency and dispersion based on data distribution, as one of the pairs of median and interquartile range or mean and standard deviation. Categorical variables were reported as absolute number and percentage. Each patient was assigned for single category for all categorical variables except for comorbidity where multiple comorbidities were accounted for. Univariate analysis using chi-square test and independent t-test were done to identify variables with a significance level of less than 0.1 and include them in the multivariate model. Selected variables were then compared with multivariable logistic regression analysis using enter method. Kolmogorov–Smirnov test was employed to determine data normality. A p value of less than 0.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 135 patients were recruited and included in the analysis (Table 1). The mean age was 51.3±17.4 years with predominantly males (68.9%). Most patients were admitted for neurosurgical primary diagnosis (32%) and invasive procedure or surgery in the current episode of care as comorbidity (68.9%). Sputum specimen (73.4%) was obtained markedly more than other specimen materials.
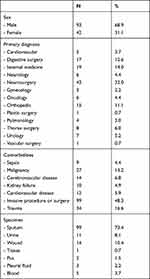 |
Table 1 Demographic and Clinical Characteristics |
Meropenem Use
Table 2 shows the details regarding meropenem use in this study. Meropenem was used as empirical agent in 33 (24.4%) patients to whom mainly (87.9%) standard-dose was administrated, while high-dose was administrated in the remaining 12.1% of patients. The mean exposure and duration of meropenem use were 9.9±4.0 and 12.3±7.1 days, respectively.
 |
Table 2 Meropenem Use |
Meropenem Susceptibility Trend
Direct comparison of ICU and overall hospital trends for meropenem susceptibility rates starting from a year prior to the study period is presented in Figure 1. The first optimal susceptibility rate was achieved in the first semester of 2018 in both observed scales. Susceptibility rate of isolates collected from the ICU then dropped to 0 by the end of 2018, before surpassing (84%) hospital susceptibility rate (78%) in late 2019. The latest ICU susceptibility rate reported (62%) declined approaching the initial optimal rate (61%).
The number of isolates was taken into consideration to interpret the susceptibility trends (Figure 2). Total number of P. aeruginosa isolates collected in the hospital fluctuated while consistently staying above the minimum threshold. The opposite is true for the number of ICU isolates which did not meet the minimum threshold throughout the study period, especially at its lowest point in late 2018 when only 4 isolates were obtained.
Meropenem Nonsusceptibility and Its Associated Factors
An increase in the rate of multidrug-resistant P. aeruginosa occurred concurrently with the reduction of susceptibility rate (Figure 3). The figure rose from 16% to 30.3% in two consecutive periods of late 2016 (data not shown) towards early 2017, and then from 16.8% to 23.2% in the first half of the year 2019 and 2020, respectively.
 |
Figure 3 Trend of multidrug-resistant P. aeruginosa rate. (Dashed trendline represents moving average of two consecutive study periods). |
Nonsusceptibility was acquired in 50 (37%) patients who mostly received meropenem as an empirical therapy (87.9%). Empirical therapy was the only variable with significant association with nonsusceptibility acquisition. Nonetheless, age and sepsis were included in the multivariate model as the level of significance fulfilled the predetermined threshold (Table 3). Meropenem use as empirical therapy (PR = 30.65; p <0.001) was independently associated with its nonsusceptibility in P. aeruginosa on multivariate logistic regression analysis (Table 4).
 |
Table 3 Analysis of Risk Factors for Meropenem Nonsusceptibility |
 |
Table 4 Multivariate Logistic Regression Analysis Identifying Independent Risk Factors for Meropenem Nonsusceptibility |
Discussion
To the best of our knowledge, this was the first study to integrate local meropenem susceptibility data with the review of its empirical use and nonsusceptibility simultaneously. Consolidation of these point of interests as a part of periodic evaluation would bring forth sharper outlook of the gaps to be addressed by ASP program. This local data representing one of the leading referral centers in LMIC was also intended to enrich the landscape of ICU-related antibiotic nonsusceptibility occurrence and determinants at large. The result demonstrated that apt use of meropenem as empirical agent was maintained with standardized protocol. Meanwhile, a propensity for increasing nonsusceptibility and resistance to meropenem and other antibiotics in P. aeruginosa was evident. Current study was done to appraise the implemented protocol in attempt to reinvent prospective treatment strategy.
Major problems of carbapenem empirical therapy in the ICU can be rationalized by the distinct challenges and burdens each clinical indication caused thereof. Proper empirical treatment strategy is pivotal in sepsis, as a multicenter study in Southeast Asia revealed failure in pathogen identification in 52.4% of the adult cases.12 Similarly, causative pathogen was unidentifiable in 23.4% ventilator-associated pneumonia (VAP) and antibiotic susceptibility in identified pathogens was gradually declining.13 Apprehension of escalating resistance rate was notable for urinary tract infection, of which burden due to carbapenem resistance was largely contributed by P. aeruginosa.14
A randomized clinical trial reported comparable clinical, microbiological, and pharmacological outcomes of 1-g and 2-g meropenem infusion q8h over 3 hours administration in sepsis and septic shock patients. However, the pharmacological surrogate outcome of 40% of the time above identified minimum inhibitory concentration (fT > MIC) was not achieved in both doses for MIC of 8 mg/L.15 Moreover, another study on meropenem clinical pharmacokinetics reported that 1-g dose with the same mode of administration and disease targets never achieved 40% fT > 4 mg/mL from the first until the third day of treatment.16 Higher doses of up to 3-g were studied in VAP caused by multidrug-resistant bacteria. The study demonstrated that the clinical success rate was similar between 2-g and 3-g doses over 3 hours infusion, but superiority in sequential organ failure assessment (SOFA) score reduction was found in the latter.17
Various combination of bolus-extended infusion regimens were analyzed and simulated using pharmacokinetics modeling and simulation. Administration of meropenem 1.5-g or 2-g q6-8h preceded by once 0.5-g bolus could achieve and maintain 40% fT > 4 mg/mL until third day of treatment in compartmental analysis and simulation.16 A more profound Monte Carlo simulation was done to assess meropenem administration in meropenem-nonsusceptible strains (MIC ≥ 16 mg/L). Optimal target of ≥90% for probabilities of target attainment (PTA) and cumulative fractions of response (CFR) was set to predict pharmacodynamic attainment. The simulation involved the following doses divided into 39 regimens with or without intravenous bolus (IVB): 0.5-g, 1-g, and 2-g q8h with 0.75-g and 1.5-g q6h being the dosage modifications of the latter two. None of the extended infusion regimens administrated over 4–6 hours achieved the requisite targets, while the combination regimens of 0.5 g (5-min IVB) + 0.25 g (5–6 h), 0.5 g (5-min IVB) + 0.5 g (5–6 h), 1–1.5 g (5-min IVB) + 0.5 g (4–6 h), 0.5–1 g (5-min IVB) + 1 g (4–6 h), and 0.5 g (5-min IVB) + 1.5 g (5–6 h) were predicted to achieve the targets in different P. aeruginosa infections at MIC 16 mg/L.18
When assessing the outcome of antibiotic administration, it is important to consider antibiotic dose and administration mode to compare dosing strategy. Meropenem dosing strategy applied in this study differed notably in exclusive intermittent bolus administration compared to those of latest studies published beyond current study period initiation. Accumulated clinical studies were in favor of extended infusion administration, as demonstrated in a previous meta-analysis,19 with higher doses required especially for nonsusceptible strains. Despite the well-established time-dependent bactericidal activity of meropenem, extended infusion for up to 6 hours was not sufficient and acceleration of peak time achieved through initial bolus dose may be necessary for isolates with higher MIC.18 Large inter-individual variability of meropenem serum concentration may also contribute to the aforementioned phenomenon.20 These data suggested that bolus dose administration held the potential role to allow for dose reduction and counter resistance when administered in adjunction to extended infusion dose.
Antecedent decreasing trend of meropenem susceptibility in P. aeruginosa was reported in isolates obtained from our local ICU. The susceptibility rate reduction from 75% to 64% reported in two previous studies21,22 in 2013 through 2015 continued thereafter to 47% by late 2017. More subtle decline recurred approaching the end of current study period hence yielding a curvilinear graph assuming unimodality in meropenem susceptibility rate (Figure 1). The SENTRY worldwide surveillance program reported higher overall (77.4%) and Asia-Pacific (82.8%) susceptibility rates in 2013–2016 compared to our facility.23 Overall (70.3%) and Asia-Pacific (79.7%) susceptibility rates subsequently reported in the Antimicrobial Testing Leadership and Surveillance (ATLAS) program in 2016–2018 were lower than those of the SENTRY although both were substantially higher than our local data.24 Co-occurring inverted graph discernible in multidrug-resistance rate of P. aeruginosa was in concordance with that of the susceptibility rate. At the end of observation period it can be concluded that there was a tendency for increasing nonsusceptibility and multidrug-resistance. Similar instance manifested at region-level across Asia-Pacific.23,24
The empirical therapy protocol being evaluated was found to increase the risk of nonsusceptibility acquisition and the continuation of which may impact ensuing susceptibility rate. Our facility relied on hospital-wide susceptibility rate to adjudicate empirical use of meropenem in the ICU because the minimum number of isolates required to deem the ICU-wide figure representative was not fulfilled. Forthcoming dose and mode of administration adjustment in the protocol should be accomplished in the light of clinical studies with the highest level of evidence available. Future studies on combined mode of administration to promote dose reduction and efforts to strengthen antibiotic-sparing strategy are vital to supersede meropenem resistance and retain its efficacy.
There were some limitations in this study. Data incompleteness in multidrug-resistant P. aeruginosa rate was inevitable since not all data are routinely included in the biannual report. There may be other potential covariates for nonsusceptibility acquisition that were not otherwise analyzed here due to external data management constraints. Finally, the wide confidence interval range for the primary endpoint signifies small sample size and less precision thus should be interpreted with caution regardless of statistical significance.
Conclusion
Empirical use of meropenem being evaluated in this study was independently associated with nonsusceptibility acquisition in P. aeruginosa. Parallel increase in nonsusceptibility and multidrug-resistance rates provides compelling indication for therapeutic strategy adjustment. Routine evaluation assisted in prompt detection of potentially modifiable cause for impending resistance issue thus the practice should be broadly endorsed.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA. 2018;115(15):E3463–70. doi:10.1073/pnas.1717295115
2. Founou RC, Founou LL, Essack SY. Clinical and economic impact of antibiotic resistance in developing countries: a systematic review and meta-analysis. PLoS One. 2017;12:12. doi:10.1371/journal.pone.0189621
3. Zhen X, Lundborg CS, Sun X, Hu X, Dong H. Economic burden of antibiotic resistance in ESKAPE organisms: a systematic review. Antimicrob Resist Infect Control. 2019;8(1):137. doi:10.1186/s13756-019-0590-7
4. Timsit JF, Bassetti M, Cremer O, et al. Rationalizing antimicrobial therapy in the ICU: a narrative review. Intensive Care Med. 2019;45(2):172–189. doi:10.1007/s00134-019-05520-5
5. Saharman YR, Karuniawati A, Severin JA, Verbrugh HA. Infections and antimicrobial resistance in intensive care units in lower-middle income countries: a scoping review. Antimicrob Resist Infect Control. 2021;10(1):22. doi:10.1186/s13756-020-00871-x
6. Saharman YR, Pelegrin AC, Karuniawati A, et al. Epidemiology and characterisation of carbapenem-non-susceptible Pseudomonas aeruginosa in a large intensive care unit in Jakarta, Indonesia. Int J Antimicrob Agents. 2019;54(5):655–660. doi:10.1016/j.ijantimicag.2019.08.003
7. Budayanti NS, Aisyah DN, Fatmawati NND, Tarini NMA, Kozlakidis Z, Adisasmito W. Identification and distribution of pathogens in a major tertiary hospital of Indonesia. Front Public Heal. 2020;7:395. doi:10.3389/fpubh.2019.00395
8. Khilnani GC, Zirpe K, Hadda V, et al. Guidelines for antibiotic prescription in intensive care unit. Indian J Crit Care Med. 2019;23(Suppl 1):1–63. doi:10.5005/jp-journals-10071-23101
9. Patrier J, Timsit JF. Carbapenem use in critically ill patients. Curr Opin Infect Dis. 2020;33(1):86–91. doi:10.1097/QCO.0000000000000622
10. Centers for Disease Control and Prevention. The core elements of hospital antibiotic stewardship programs: antibiotic stewardship program assessment tool; 2019. Available from: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/assessment-tool-P.pdf.
11. Wunderink RG, Srinivasan A, Barie PS, et al. Antibiotic stewardship in the intensive care unit: an official American Thoracic Society Workshop report in collaboration with the AACN, CHEST, CDC, and SCCM. Ann Am Thorac Soc. 2020;17(5):531–540. doi:10.1513/AnnalsATS.202003-188ST
12. Limmathurotsakul D. Causes and outcomes of sepsis in Southeast Asia: a multinational multicentre cross-sectional study. Lancet Glob Heal. 2017;5(2):e157.
13. Luyt CE, Hékimian G, Koulenti D, Chastre J. Microbial cause of ICU-acquired pneumonia: hospital-acquired pneumonia versus ventilator-associated pneumonia. Curr Opin Crit Care. 2018;24(5):333–338. doi:10.1097/MCC.0000000000000526
14. Shields RK, Zhou Y, Kanakamedala H, Cai B. Burden of illness in US hospitals due to carbapenem-resistant Gram-negative urinary tract infections in patients with or without bacteraemia. BMC Infect Dis. 2021;21(1):572. doi:10.1186/s12879-021-06229-x
15. Lertwattanachai T, Montakantikul P, Tangsujaritvijit V, et al. Clinical outcomes of empirical high-dose meropenem in critically ill patients with sepsis and septic shock: a randomized controlled trial. J Intensive Care. 2020;8:1. doi:10.1186/s40560-020-00442-7
16. Kothekar AT, Divatia JV, Myatra SN, et al. Clinical pharmacokinetics of 3-h extended infusion of meropenem in adult patients with severe sepsis and septic shock: implications for empirical therapy against Gram-negative bacteria. Ann Intensive Care. 2020;10(1):4. doi:10.1186/s13613-019-0622-8
17. Monajati M, Ala S, Aliyali M, et al. Clinical effectiveness of a high dose versus the standard dose of meropenem in ventilator-associated pneumonia caused by multidrugresistant bacteria: a randomized, single-blind clinical trial. Infect Disord Drug Targets. 2021;21(2):274–283. doi:10.2174/1871526520666200227102013
18. Song X, Wu Y, Cao L, Yao D, Long M. Is meropenem as a monotherapy truly incompetent for meropenem-nonsusceptible bacterial strains? A pharmacokinetic/pharmacodynamic modeling with Monte Carlo simulation. Front Microbiol. 2019:2777. doi:10.3389/fmicb.2019.02777
19. Yu Z, Pang X, Wu X, Shan C, Jiang S. Clinical outcomes of prolonged infusion (extended infusion or continuous infusion) versus intermittent bolus of meropenem in severe infection: a meta-analysis. PLoS One. 2018;13:7. doi:10.1371/journal.pone.0201667
20. Uricová J, Kacířová I, Brozmanová H. Meropenem serum concentrations in intensive care patients: a retrospective analysis. Czech Slovak Pharm. 2020;69(5–6):230–236.
21. Hamdiyati R, Pinatih KJP, Fatmawati NND. Microbes and their susceptibility pattern to antibiotics in intensive care unit (ICU) Sanglah Hospital Denpasar Bali at August 2013 until October 2013. E-Jurnal Med. 2016;5(4):1–6.
22. Dharmayanti IGAMP, Sukrama DM. Characteristics of Pseudomonas aeruginosa and its susceptibility pattern to antibiotics in intensive care unit (ICU) of Sanglah General Hospital in November 2014-January 2015. E-Jurnal Med. 2019;8:4.
23. Shortridge D, Gales AC, Streit JM, Huband MD, Tsakris A, Jones RN. Geographic and temporal patterns of antimicrobial resistance in Pseudomonas aeruginosa over 20 years from the SENTRY antimicrobial surveillance program, 1997–2016. Open Forum Infect Dis. 2019;6(Suppl 1):S63. doi:10.1093/ofid/ofy343
24. Piérard D, Stone GG. In vitro antimicrobial susceptibility of clinical respiratory isolates to ceftazidime-avibactam and comparators (2016–2018). BMC Infect Dis. 2021;21(1):600. doi:10.1186/s12879-021-06153-0
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


