Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
Evaluation of Antiemetic Therapy for Hepatic Arterial Infusion Chemotherapy with Oxaliplatin, Fluorouracil, and Leucovorin
Authors Zhao Y, He M, Liang R, Li Q , Shi M
Received 21 September 2020
Accepted for publication 14 December 2020
Published 22 January 2021 Volume 2021:17 Pages 73—77
DOI https://doi.org/10.2147/TCRM.S283192
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Yang Zhao, MinKe He, RunBin Liang, QiJiong Li, Ming Shi
Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China
Correspondence: Ming Shi
Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, People’s Republic of China
Tel/Fax +86-020-87343938
Email [email protected]
Purpose: Our aim was to compare the antiemetic efficacy of the triple combination of aprepitant, dolasetron and dexamethasone with the combination of dolasetron and dexamethasone for chemotherapy-induced nausea and vomiting (CINV) in hepatocellular carcinoma (HCC) patients receiving hepatic arterial infusion chemotherapy (HAIC) with oxaliplatin, fluorouracil and leucovorin (FOLFOX).
Patients and Methods: This was a retrospective study. In the dolasetron plus dexamethasone group (D group), the patients received dolasetron (100 mg, i.v., on day 1) and dexamethasone (10 mg, i.v., on day 1) 30 min before starting administration of chemotherapeutic drugs. In the aprepitant plus dolasetron and dexamethasone group (AD group), the patients received dolasetron and dexamethasone as described above, and aprepitant (125 mg, p.o.) on day 1 followed by 80 mg on days 2 and 3. The primary endpoint was the complete response rate (CR, defined as no emetic episodes and no rescue medication use) during the first cycle of hepatic arterial infusion chemotherapy.
Results: Between January 2018 and August 2019, 302 eligible patients were included: 197 in AD group and 105 in D group. Patients in AD group had significantly higher complete response rates than those in D group during the first cycle (85.8% vs 71.4%, P = 0.003) and all cycles (73.6% vs 49.5%, P< 0.001). Patients in AD group had lower rescue therapy (1.5% vs 26.7%, P< 0.001) and lower incidence of disruption related to chemotherapy-induced nausea and vomiting (0.5% vs 6.7%, P = 0.002) than patients in D group.
Conclusion: Aprepitant, dolasetron plus dexamethasone is more effective to prevent chemotherapy-induced nausea and vomiting in hepatocellular carcinoma patients treated with FOLFOX-HAIC therapy than dolasetron plus dexamethasone.
Keywords: aprepitant, chemotherapy-induced nausea and vomiting, hepatocellular carcinoma, hepatic arterial infusion chemotherapy
Introduction
FOLFOX-HAIC therapy has been reported to achieve favorable results for hepatocellular carcinoma (HCC), and chemotherapy-induced nausea and vomiting (CINV) is one of the most frequent adverse events.1–4 Uncontrolled CINV can not only discontinue chemotherapy but also markedly impairs the patient’s quality of life.5 In recent antiemetic guidelines, FOLFOX therapy was classified as having moderately emetogenic chemotherapy (MEC) risk, and two-drug combination therapy with a 5-hydroxytryptamine 3 (5-HT3) receptor antagonist and dexamethasone is the recommended antiemetic therapy for patients treated with MEC.6–8 Even though these patients were treated with a two-drug combination of dolasetron (a 5-HT3 receptor antagonist) and dexamethasone, the rates of nausea and vomiting were still high, ranging from 79.8–82.9% and 42.9–59.7%, respectively.4,9,10
Recently, aprepitant was reported to be a potent and selective oral non-peptide antagonist of the neurokinin-1 (NK1)-receptor that inhibits the binding of substance P to NK1-receptors of the vomiting center in the nervous system.11 Large phase III trials have shown that three-drug combination antiemetic therapy that included aprepitant was associated with a decreased rate of vomiting and increased complete response and complete protection.12,13 Several studies have suggested that the addition of aprepitant to 5-HT3 receptor antagonist plus dexamethasone is effective for oxaliplatin-based chemotherapy-induced nausea and vomiting.14,15 However, it is unclear whether the addition of aprepitant to 5-HT3 receptor antagonist plus dexamethasone could improve antiemetic efficacy for patients receiving FOLFOX-HAIC.
There is no standard treatment at present for CINV caused by HAIC. More research is required to establish antiemetic prophylaxis guidelines for FOLFOX-HAIC. The purpose of this study was to compare the antiemetic efficacy of the triple-drug combination of aprepitant, dolasetron, and dexamethasone with that of the two-drug combination of dolasetron and dexamethasone for CINV in HCC patients receiving FOLFOX-HAIC therapy.
Patients and Methods
The present study was carried out in accordance with the Declaration of Helsinki and approved by the Institutional Research Ethics Committee of Sun Yat-sen University Cancer Center. Due to the nature of this research, it involves no more than minimal risk, and the waiver of informed consent will not adversely affect the rights and welfare of the participants. Whenever appropriate, participants will be provided with additional pertinent information after their participation. This study was a retrospective analysis of the data for the period between January 2018 and August 2019. Eligible patients were 18 years or older with HCC confirmed by pathological biopsy or two imaging techniques and received FOLFOX-HAIC therapy. Other eligibility criteria were as follows: an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2, a life expectancy of 3 months or more, and normal liver and renal function. All the patients treated with hepatic artery infusion chemotherapy were selected with liver function classification Child-Pugh class A. Exclusion criteria consisted of a previous history of other systemic chemotherapy; complications that induced nausea and/or vomiting (e.g. symptomatic brain metastases, opioid dose change within 120 h following chemotherapy); a known medical history of HIV infection; pregnancy or breastfeeding; and other invasive malignant diseases. Patients who had changed their antiemetic regimen were screened out.
HAIC treatment cycles were repeated every 21 days as our previous studies described.4,9 FOLFOX was administered via hepatic artery: oxaliplatin, 85 mg/m2, from hour 0 to 2 on day 1; leucovorin, 400 mg/m2, from hour 2 to 3 on day 1; fluorouracil, 400 mg/m2, bolus at hour 3, and 2400 mg/m2 over 46 hours on days 1 and 2. In the D group, the patients received the two-drug combination with a single dose of dolasetron (100 mg, i.v., on day 1) and dexamethasone (10 mg, i.v., on day 1) 30 min before starting administration of chemotherapeutic drugs. In the AD group, the patients received the triple-drug combination with a single dose of dolasetron (100 mg, i.v., on day 1) and dexamethasone (10 mg, i.v., on day 1) 30 min before starting administration of chemotherapeutic drugs, and aprepitant 125 mg orally 60 min before starting administration of chemotherapeutic drugs on day 1 followed by the oral administration of 80 mg of aprepitant on days 2 and 3.
Uncontrolled CINV events were identified through records of nausea, vomiting, and rescue medications. The CINV was not classified into the acute (< 24 hours), delayed (25–120 hours), and protracted (>5 days) phases. Nausea and vomiting were classified using the National Cancer Institute Common terminology Criteria for adverse events v4.0.3.
All data were retrospectively collected from the electronic medical record system. They included age, sex, height, weight, body surface area, courses of HAIC, tumor size, history and episodes of CINV, and whether antiemetic agents were also used. The primary endpoint of this study was the complete response (CR) rate during the first cycle defined as no emetic episodes, no rescue medication use during the first cycle of FOLFOX-HAIC therapy.7 The secondary endpoints were CR rate during all cycles, rescue medication rate, and HAIC disruption due to CINV.
All analyses were performed using SPSS statistics software, version 25.0 (IBM Corp., Armonk, NY, USA). The CINV parameters (i.e. nausea grade, vomiting grade, rescue medication rate) for the two antiemetic protocols were compared using the Chi square test. All statistical tests used in this study were two-sided, and a P value <0.05 was considered significant.
Results
A total of 302 patients were identified who had been treated with a first-generation 5-HT-receptor antagonist (dolasetron) plus dexamethasone (n=105), or aprepitant with dolasetron plus dexamethasone (n=197) (Figure 1). The median age of the two groups was 51 and 49 (P = 0.561), respectively. There were 168 males and 29 females in AD group, and 90 male patients and 15 female patients in D Group (P = 0.919). There was no significant difference in BMI (body mass index) and tumor stage (BCLC) (Table 1). Patients in the AD group received more courses of HAIC than those in the D group (P<0.001).
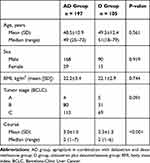 |
Table 1 Patients’ Characteristics |
The results for the endpoints are listed in Table 2. Patients in the AD group had significantly higher CR rates than those in the D group during the first cycle (85.8% vs 71.4%, P = 0.003) and all cycles (73.6% vs 49.5%, P<0.001) of FOLFOX-HAIC. Patients in the AD group also had a lower incidence rate of nausea than those in the D group in the first cycle (23.9% vs 38.1%, P = 0.009) and all cycles (43.1% vs 56.2%, P = 0.031). Patients in the AD group had a lower incidence rate of grade 3 vomiting than in the D group in all cycles (5 [2.6%] vs 8 [7.6%], P = 0.04). Most nausea or vomiting was in grade 1 or 2, and no grade 4 nausea or vomiting was observed.
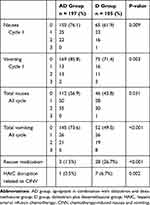 |
Table 2 Comparison of Complete Response Between the Two Groups and Rescue Therapy |
More patients in the D group required rescue therapy than in the AD group (26.7% vs 1.5%, P<0.001). There were 31 patients receiving rescue medication in total. Of the 31, 23 received palonosetron as a rescue medication, and the other 8 received metoclopramide. Patients in the AD group had a lower incidence of HAIC disruption due to CINV than patients in the D group (0.5% vs 6.7%, P=0.002). No adverse events possibly related to aprepitant administration were encountered.
Discussion
To the best of our knowledge, there is no antiemetic guideline for HAIC, and the present study is the first to report the more favorable efficacy of antiemetic prophylaxis with dolasetron, dexamethasone, and aprepitant for FOLFOX-HAIC. This study achieved the primary endpoint: the CR rate in patients receiving aprepitant, dolasetron plus dexamethasone was higher than that in patients receiving dolasetron plus dexamethasone during the first cycle. Furthermore, the CR rate of vomiting and nausea in the AD group was higher than that in the D group during the first cycle and all cycles of FOLFOX-HAIC. Meanwhile, lower incidence of rescue therapy and lower incidence of HAIC disruption were observed in the AD group than in the D group.
The above results suggest that the addition of aprepitant to dolasetron and dexamethasone augments the antiemetic effect for patients receiving HAIC. The enhancement in the antiemetic effect may be due to the synergistic antiemetic interactions between 5-HT-receptor antagonists (dolasetron) and NK1-receptor antagonists (aprepitant): First, significant electrophysiological and biochemical findings suggest that receptor cross-talk occurs between serotonergic 5-HT(3)- and tachykininergic NK(1)-receptors in which co-activation of either receptor by ineffective doses of their corresponding agonists (serotonin [5-HT] or substance P, respectively) potentiates the activity of the other receptor to produce a response.16 Second, first-generation 5-HT-receptor antagonists mainly reduced acute emesis, and NK1-receptor antagonists not only reduced acute emesis but also helped in the reduction of delayed emesis.17 Finally, NK-1 receptor antagonists have potent and usually long-lasting anti-emetic activity against a broad spectrum of central and peripheral emetic agents, whereas 5-HT3 antagonists have a more restricted spectrum of activity with efficacy mostly against peripheral emetogens.18–20
Moreover, most nausea and vomiting in both groups were in grade 1 or 2, and the incidence of nausea and vomiting was consistent with that observed in previous studies.4,9,10 This indicates that CINV caused by HAIC was slight and manageable. However, the 3-drug regimen with dolasetron, dexamethasone, and aprepitant was still necessary for HAIC because no adverse events possibly related to aprepitant administration were encountered and lower incidence of HAIC disruption and grade 3 vomiting was observed in the AD group than in the D group. The difference in the incidence of HAIC disruption due to CINV may be one of the reasons why patients in the AD group received more courses of HAIC than patients in the D group.
There were some limitations in this study. First, this was a retrospective study conducted at a single center. Nonetheless, the baseline characteristics were well balanced except that patients in the AD group received more courses of HAIC. Second, the CINV was not classified into acute (< 24 hours), delayed (25–120 hours), and protracted (>5 days) phases. However, it is difficult because of the retrospective nature. A randomized controlled trial is needed to verify our results. Third, the major adverse events during antiemetic therapy, such as constipation, headache, and diarrhea, could not be compared in the present study. Moreover, factors that might affect the result (for example, motion sickness) were not assessed because of the retrospective design.
In conclusion, this study demonstrates that aprepitant, dolasetron plus dexamethasone is more effective to prevent CINV in HCC patients treated with FOLFOX-HAIC than dolasetron plus dexamethasone. Prophylactic use of these drugs has the potential to reduce CINV during FOLFOX-HAIC therapy for HCC patients.
Funding
This work was supported by National Key R&D Program of China (2017YFA0505803), National Natural Science Foundation of China (No. 81625017, No.81572385), National Science and Technology Major Project of China (2018ZX10302205).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rathore R, Safran H, Soares G, et al. Phase I study of hepatic arterial infusion of oxaliplatin in advanced hepatocellular cancer: a brown university oncology group study. Am J Clin Oncol. 2010;33(1):43–46. doi:10.1097/COC.0b013e31819d8668
2. Lyu N, Lin Y, Kong Y, et al. FOXAI: a Phase II trial evaluating the efficacy and safety of hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin for advanced hepatocellular carcinoma. Gut. 2018;67(2):395–396. doi:10.1136/gutjnl-2017-314138
3. Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69(1):60–69. doi:10.1016/j.jhep.2018.02.008
4. He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–960. doi:10.1001/jamaoncol.2019.0250
5. Oo TH, Hesketh PJ. Drug insight: new antiemetics in the management of chemotherapy-induced nausea and vomiting. Nat Clin Pract Oncol. 2005;2(4):196–201. doi:10.1038/ncponc0132
6. Berger MJ, Ettinger DS, Aston J, et al. NCCN guidelines insights: antiemesis, version 2.2017. J Natl Compr Canc Netw. 2017;15(7):883–893. doi:10.6004/jnccn.2017.0117
7. Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl5):v119–v133. doi:10.1093/annonc/mdw270
8. Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35(28):3240–3261. doi:10.1200/JCO.2017.74.4789
9. He MK, Zou RH, Li QJ, et al. Phase II study of sorafenib combined with concurrent hepatic arterial infusion of oxaliplatin, 5-fluorouracil and leucovorin for unresectable hepatocellular carcinoma with major portal vein thrombosis. Cardiovasc Intervent Radiol. 2018;41(5):734–743. doi:10.1007/s00270-017-1874-z
10. He MK, Le Y, Li QJ, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer. 2017;36(1):83. doi:10.1186/s40880-017-0251-2
11. Aapro M, Carides A, Rapoport BL, Schmoll HJ, Zhang L, Warr D. Aprepitant and fosaprepitant: a 10-year review of efficacy and safety. Oncologist. 2015;20(4):450–458. doi:10.1634/theoncologist.2014-0229
12. Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin–the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21(22):4112–4119. doi:10.1200/JCO.2003.01.095
13. Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97(12):3090–3098. doi:10.1002/cncr.11433
14. Aridome K, Mori SI, Baba K, et al. A Phase II, randomized study of aprepitant in the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapies in colorectal cancer patients. Mol Clin Oncol. 2016;4(3):393–398. doi:10.3892/mco.2015.724
15. Nishimura J, Satoh T, Fukunaga M, et al. Combination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy (SENRI trial): a multicentre, randomised, controlled Phase 3 trial. Eur J Cancer. 2015;51(10):1274–1282. doi:10.1016/j.ejca.2015.03.024
16. Darmani NA, Chebolu S, Amos B, Alkam T. Synergistic antiemetic interactions between serotonergic 5-HT3 and tachykininergic NK1-receptor antagonists in the least shrew (Cryptotis parva). Pharmacol Biochem Behav. 2011;99(4):573–579. doi:10.1016/j.pbb.2011.05.025
17. Rojas C, Raje M, Tsukamoto T, Slusher BS. Molecular mechanisms of 5-HT(3) and NK(1) receptor antagonists in prevention of emesis. Eur J Pharmacol. 2014;722:26–37. doi:10.1016/j.ejphar.2013.08.049
18. Andrews PL, Bhandari P. The 5-hydroxytryptamine receptor antagonists as antiemetics: preclinical evaluation and mechanism of action. Eur J Cancer. 1993;29A(Suppl 1):S11–S16. doi:10.1016/S0959-8049(05)80254-0
19. Tattersall FD, Rycroft W, Hill RG, Hargreaves RJ. Enantioselective inhibition of apomorphine-induced emesis in the ferret by the neurokinin1 receptor antagonist CP-99,994. Neuropharmacology. 1994;33(2):259–260. doi:10.1016/0028-3908(94)90018-3
20. Tattersall FD, Rycroft W, Francis B, et al. Tachykinin NK1 receptor antagonists act centrally to inhibit emesis induced by the chemotherapeutic agent cisplatin in ferrets. Neuropharmacology. 1996;35(8):1121–1129. doi:10.1016/S0028-3908(96)00020-2
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

