Back to Journals » Cancer Management and Research » Volume 12
Evaluation of Alterations to Bile Ducts and Laboratory Values During the First 3 Months After Irreversible Electroporation of Malignant Hepatic Tumors
Authors Bäumler W, Sebald M, Einspieler I , Schicho A , Schaible J, Wiggermann P, Dollinger M, Stroszczynski C, Beyer LP
Received 11 May 2020
Accepted for publication 1 July 2020
Published 14 September 2020 Volume 2020:12 Pages 8425—8433
DOI https://doi.org/10.2147/CMAR.S261838
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Wolf Bäumler,1 Mareike Sebald,2 Ingo Einspieler,1 Andreas Schicho,1 Jan Schaible,1 Philipp Wiggermann,3 Marco Dollinger,1 Christian Stroszczynski,1 Lukas Philipp Beyer4
1Department of Radiology, University Hospital Regensburg, Regensburg, Germany; 2Department of Radiology, Municipal Hospital Landshut, Landshut, Germany; 3Department of Radiology and Nuclear Medicine, Hospital Braunschweig, Braunschweig, Germany; 4Department of Diagnostic and Interventional Radiology, Ernst von Bergmann Hospital, Potsdam, Germany
Correspondence: Wolf Bäumler
Department of Radiology, University Hospital Regensburg, Regensburg 93053, Germany
Tel +49 941 944 7410
Email [email protected]
Purpose: To assess the incidence and evolution of biliary alterations adjacent to the ablation area in patients with hepatic malignancies during the first 3 months after percutaneous irreversible electroporation (IRE) and to investigate associated changes in laboratory values.
Material and Methods: Bile ducts located within a ≤ 1.0 cm radius of the ablation zone were analyzed in 45 patients by preinterventional and postinterventional MRI (1– 3 days, 6 weeks, and 3 months after IRE). Moreover, levels of alkaline phosphatase (AP) and serum bilirubin (SB) were examined for evidence of bile duct injury. Biliary alterations and the presence of postinterventional-elevated laboratory levels were correlated with features of the lesions, patients, ablation procedures, and laboratory values.
Results: A total of 80 bile ducts were located within a 1.0 cm radius of the ablation zone: 59 were encased, 16 were abutting and 5 were located within a radius of 0.1– 1.0 cm of the ablation area. In total, 38 biliary injuries (narrowing, n=22; dilatation, n=14; biloma, n=2) were detected, 3 cases of narrowing occurred for the first time 6 weeks and 3 months after IRE, 21 alterations (dilatation: n=9; narrowing; n=10; biloma: n=2) had resolved during the first 6 weeks, 1 alteration (dilatation: n=1) had resolved by the last follow-up control. Three months after IRE, 19 patients showed elevated levels of AP, whereas SB levels were increased in 10 cases. No significant association between biliary alterations or postinterventional-elevated laboratory values and the investigated characteristics of lesions, patients, ablation procedures or laboratory values could be proven.
Conclusion: Different alterations of bile ducts adjacent to an IRE ablation zone are common, of which dilatation and especially narrowing commonly represent a long-term complication after IRE. Moreover, a definite correlation between the frequently observed prolonged post-ablative elevation of AP- and SB-levels and the postinterventional biliary alterations could not be proven.
Keywords: bile ducts, hepatic tumors, electroporation
Introduction
Despite the continuing evolution of ablation technology, the treatment of hepatic tumors in critical locations such as those adjacent to certain intrahepatic structures (eg, bile ducts) still represents a challenge in local ablation therapy. Because percutaneous thermal ablation techniques such as radiofrequency ablation (RFA) are contraindicated for hepatic tumors in proximity to bile ducts because of the risk of thermal damage,1 the role of irreversible electroporation (IRE) has constantly increased during the last few years.
Causing cell death through the repeated application of high-voltage electrical impulses, which create irreversible damage to the membranes of tumor cells,2 IRE, as a predominantly nonthermal ablative method, represents a viable alternative to thermal ablations. IRE also affects bile ducts but does not damage their architectural integrity.3 As a safe method for the ablation hepatic tumors adjacent to major bile ducts,4 at this point of time little is known about the alterations of bile ducts in proximity to an IRE ablation area. In particular, hardly any information is available in the current literature about the evolution of these alterations.
The aim of the present study was to evaluate the occurrence and the evolution of alterations in bile ducts located in proximity to the IRE ablation zone in patients with hepatic malignancies during the first 3 months after IRE. Moreover, the objective was to determine any potential risk factors associated with detected biliary complications and to analyze a potential correlation between biliary alterations and levels of alkaline phosphatase and serum bilirubin measured before and during the first 3 months after the intervention.
Materials and Methods
Study Design, Participant Selection and Patient Characteristics
To investigate alterations in bile ducts adjacent to the ablation area after percutaneous IRE of malignant liver tumors, MRI images of all ablations performed at University Hospital Regensburg between December 2011 and May 2018 were retrospectively evaluated. The single-center retrospective observational study was approved by the local ethics committee (Ethics Committee of the University of Regensburg approval number 18-1027-104) and followed the guidelines outlined in the Declaration of Helsinki. Each patient provided written informed consent for the acquisition of contrast-enhanced MR images, the ablation procedure, and the anonymous use of the data for scientific purposes. The following inclusion criteria were applied to participate in the study: (I) Primary or secondary liver malignancies. (II) Liver tumors treated by percutaneous IRE. (III) Examination by contrast-enhanced MR images of the entire liver, including the hepatobiliary phase, before the intervention and at three defined moments in time after IRE. These three moments were 1–3 days, 6 weeks, and 3 months after the ablation. (IV) Bile ducts were located within a 1.0 cm radius of the ablation area, measured from the edge of the ablation zone at the follow-up MR imaging examination 1–3 days after the intervention. The location of the bile ducts in relation to the ablation zone was divided into three classifications: encased, that is, completely surrounded by the ablation zone; abutting, that is, directly adjacent to the ablation zone, but not encased; and distant, that is, 0.1–1.0 cm from the ablation zone. A prior treatment of liver malignancies by any other kind of therapy (eg, surgery, systemic chemotherapy) was not an exclusion criterion.
Forty-five patients (34 men and 11 women) aged 68.3 ± 11.6 years who had undergone IRE ablations of 62 hepatic lesions in 53 ablation procedures fulfilled the mentioned conditions and were included in the study (Table 1). The mean tumor diameter was 2.0 cm ± 1.1 (range, 0.4–4.4 cm). Thirty-four of 62 lesions (in 27 patients) were primary liver tumors, 28 of 62 lesions (in 18 patients) were secondary liver tumors (Table 2). In 45 sessions only one tumor was ablated, in seven sessions the ablation of two tumors was performed, and in one session three tumors were ablated, all at once.
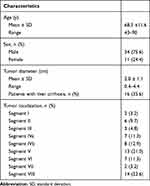 |
Table 1 Baseline Patient and Disease Characteristics |
 |
Table 2 Tumor Types of 45 Patients Treated by Irreversible Electroporation of Malignant Liver Tumors |
The pre- and postinterventional MR images were analyzed by two radiologists with 4 years and 7 years of experience in abdominal imaging. Each MR image was examined for possible biliary alterations, concentrating on dilatation and narrowing of the bile ducts. Moreover, the occurrence of bilomas was observed and noted, as they were considered to be a postablative complication. During several follow-up controls, the evolution of biliary alterations was evaluated. Each detected biliary alteration was categorized based on the Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0.5 All MR scans contained the whole liver and were conducted with a hepatocyte-specific contrast agent (gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid; Primovist; Bayer Schering, Berlin, Germany). The MR protocol included the following sequences: T2 haste, T1 vibe3d in-phase, T1 vibe3d out-of-phase, T1 vibe3d fat-suppressed, T1 vibe3d fat-suppressed contrast-enhanced early arterial phase, T1 vibe3d fat-suppressed contrast-enhanced late arterial phase, T1 vibe3d fat-suppressed contrast-enhanced portal venous phase, T2 blade fat-suppressed contrast-enhanced, diffusion trace contrast-enhanced, T1 vibe3d fat-suppressed delayed phase.
Levels of alkaline phosphatase and serum bilirubin, measured at each of the follow-up controls, were compared with the preinterventional levels, because elevated alkaline phosphatase or bilirubin levels could correlate with bile duct alterations. Because of the biological variation of laboratory values, increases in alkaline phosphatase ≥117 U/L or in serum bilirubin ≥1.0 mg/dL were considered clinically significant and abnormal.
Irreversible Electroporation (IRE)
All IRE were performed percutaneously with deep muscle relaxation using the NanoNife system (AngioDynamics, Latham, New York). The operator used two to six monopolar 18-gauge IRE probes. They were placed around the target tumor. If the distance between two probes was more than 2.0 cm because of the tumor size, one additional probe was placed in the center of the tumor. After that, the pulses were applied consecutively between the probes. The IRE parameters were as follows: pulses per cycle, 70; pulse length, 90 µs; electric field, 1500 V/cm needle distance. A test pulse of 270 V was delivered before the delivery of the 90 therapeutic pulses to confirm sufficient conductivity.
Statistical Analysis
All collected data are presented as frequency counts and percentages. To identify variables for prediction of biliary changes and the postinterventional elevation of laboratory values at the specific follow-up points, a binary logistic regression model was used. Equal correlation of the binary response for individual patients was assumed, implying an exchangeable correlation structure. As effect estimates, maximum-likelihood odds-ratio estimators and 95% confidence intervals are presented. A p-value of ≤0.05 was considered statistically significant. All statistical analyses were performed with SPSS statistic (IBM SPSS Statistics, version 25). The analyzed variables for the prediction of biliary alterations were postinterventional presence of elevated levels of alkaline phosphatase and serum bilirubin, age (<65y vs ≥65y), sex, nature of the bile duct adjacent to the ablation zone (minor vs major), the presence of liver cirrhosis, number of IRE electrodes (≤4 vs >4), localization of the bile duct with regard to the ablation zone (abutting/distant vs encased), tumor type (primary vs secondary hepatic malignancy), ablation volume (≤60 mL vs >60 mL), the presence of hepatic stents or metallic implants and the relative distance from needle to bile duct (≤0.3 cm vs >0.3 cm). Moreover, the potential influence of the ablation volume (≤60 mL vs >60 mL) and the tumor volume (≤1.8 mL vs >1.8 mL) on the postinterventional elevation of alkaline phosphatase or serum bilirubin was evaluated.
Results
In the current study, 80 bile ducts in 45 patients were located within a 1.0 cm radius of the ablation zone. Fifty-nine (73.8%) of the 80 bile ducts were encased by the ablation defect. Sixteen (20.0%) abutted the ablation defect and 5 (6.2%) were located within a radius of 0.1–1.0 cm of the ablation area. During the first 3 months after IRE, alterations were observed in 38 (47.5%; 19 patients) of the 80 bile ducts. An overview of the different types and the development of biliary alterations is presented in Table 3. Narrowing of the bile duct (n=22; 57.9%) was the most frequently observed alteration, followed by dilatation of the bile duct (n=14; 36.8%) and biloma (n=2; 5.3%). Details about the type of bile duct and alterations are illustrated in Table 4. Alterations of major bile ducts were detected in 4 of 12 cases (33.3%), whereas alterations of minor bile ducts were observed in 34 of 68 cases (50.0%). All detected biliary alterations were categorized “grade 1” based on CTCAE Version 5.0. In the binary logistic regression model, no significant risk factors for the occurrence of biliary alteration or postinterventional elevated laboratory values were detected (Tables 5 and 6).
 |
Table 3 Number and Types of Biliary Alterations During the First 3 Months After IRE |
 |
Table 4 Types and Numbers of Bile Ducts Adjacent to the Ablation Zone and Number of Bile Ducts with Alterations with Regard to Their Localization |
 |
Table 5 Results of Binary Logistic Regression Model Predicting Biliary Alterations |
 |
Table 6 Results of Binary Logistic Regression Model Predicting Postinterventional Elevated Values of Alkaline Phosphatase or Serum Bilirubin |
Early Alterations
Alterations were observed in 32 of the 80 (40.0%) bile ducts 1–3 days after IRE. Fourteen of the 32 altered bile ducts were dilatated (36.8%), 16 were narrowed (42.1%) and 2 bilomas (5.3%) were noted (Table 3). In one case of bile duct narrowing, a simultaneous cholestasis was observed at the first follow-up control but had completely resolved after 6 weeks.
Delayed Alterations
Three (7.9%) of the 38 biliary alterations (narrowing: n=3) were noted for the first time 6 weeks after the intervention, whereas 3 (7.9%) alterations (narrowing: n=3) occurred for the first time 3 months after IRE.
Evolution of Alterations
A total of 21 alterations (dilatation: n=9; narrowing; n=10; biloma: n=2) had resolved during the first 6 weeks. The detected bilomas (n=2) had resolved during the first 6 weeks after the intervention without any additional treatment such as abdominal draining. One bile duct dilatation showed spontaneous regression 3 months after the intervention.
Laboratory Values
An overview of the evolution of alkaline phosphatase and serum bilirubin levels after IRE in 53 ablation procedures is presented in Table 7. During the first 3 days after IRE, biliary alterations with simultaneous elevation of alkaline phosphatase levels were detected in seven cases (13.2%) of all 53 ablation procedures, of which elevated alkaline phosphatase levels were pre-existent before IRE in 5 cases (9.4%). Increased levels of serum bilirubin associated with biliary complications were noted in 12 cases (22.6%), of which 3 (5.7%) had already been observed before the intervention. Six weeks after the intervention, the number of cases of abnormal levels of alkaline phosphatase and serum bilirubin decreased from 7 to 4 (7.5%) and 12 to 3 (5.7%), respectively. Three months after IRE, biliary alterations associated with elevated levels of alkaline phosphatase and serum bilirubin were observed in two cases (3.8%) and 2 cases (3.8%), respectively. During the 6-week and the 3-month follow-up control, no newly elevated levels of alkaline phosphatase or serum bilirubin were noted in patients with biliary alterations. In the current study, postinterventional elevated levels of alkaline phosphatase (p= 0.978; OR= 0.98; 95% CI: 0.25–3.78) or serum bilirubin (p= 0.943; OR= 0.99; 95% CI: 0.31–3.12) did not significantly occur more frequently in patients suffering from post-IRE bile duct injuries.
 |
Table 7 Evolution of Laboratory Values During the First 3 Months After IRE in 53 Ablation Procedures |
Discussion
If surgical resection is impossible because the patient suffers from several comorbidities6 or because of the critical tumor localization, ablative methods such as IRE play an important role in the therapy of primary or secondary hepatic malignancies. In particular, IRE has become a viable alternative for the therapy of peribiliary tumors during the last few years because of its predominantly nonthermal mode of action.
Recently, several studies have investigated biliary alterations and changes in laboratory values after hepatic IRE. In animal studies conducted by Rubinsky et al and Lee et al, the architecture of bile ducts remained undamaged, although mild histopathologic changes, eg, wall necrosis and peridochal edema, were observed.3,7 In contrast, Distelmaier et al, who observed their study population for 2 years after IRE of hepatic malignancies, described a temporary, mild to moderate cholestasis in 5 (23.8%) of 21 patients 2–6 weeks after the intervention. No further cholestasis was observed during the remaining 24-month observation period.8 In a review of 16 studies by Scheffer et al, bile duct complications after hepatic IRE were reported in 6 (4.8%) of 129 cases.9 Dollinger et al observed biliary structures for the first 3 days and laboratory levels (alkaline phosphatase and serum bilirubin) for 1–2 months after IRE and detected 15 alterations (27.3%; narrowing: n=8; dilatation: n=7) in 55 bile ducts (24 patients) 1–3 days after IRE of 53 hepatic tumors. All of the 55 bile ducts were located ≤1.0 cm from the ablation zone. The authors also reported one case of long-term increased laboratory levels (alkaline phosphatase), which were presumed to be caused by local tumor recurrence.10
Most of these previous studies do not include any, or at most a short-term follow-up control of the evaluated parameters. Consequently, a potential biliary alteration occurring for the first time at one of the later follow-up controls could not have been detected. Furthermore, the evolution of the observed parameters cannot be evaluated. Some of the studies that included a follow-up control present some limitations because there was no exactly predefined follow-up schedule for the time of data acquisition or because of significant lack of data during the follow-up. The aim of the current study was to evaluate potential biliary alterations and changes of alkaline phosphatase and serum bilirubin levels after IRE at predefined points of time to investigate an exact evolution during the whole follow-up period.
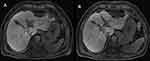
Narrowing of the bile duct can constitute a potential complication of IRE, which has already been observed in an animal study by Choi et al, who reported seven cases of bile duct narrowing during the first 8 weeks after 12 IRE procedures of swine liver.11 In our trial, bile duct narrowing (Figure 1) represented the most frequently observed biliary alteration (n=22) of all 38 alterations, which coincided with the findings of Dollinger et al, who observed bile duct narrowing in 53.3% of all complications.10 Six (27.3%) of all cases of bile duct narrowing were observed for the first time 6 weeks (n=3) and 3 months (n=3) after IRE (Table 3). In 10 cases, bile duct narrowing had resolved 6 weeks after the intervention, whereas in 12 (54.5%) of all 22 cases, it persisted until the 3-month follow-up control. Bile duct narrowing can occur as a result of edematous alterations in the ablated tissue and the adjacent intact liver parenchyma, as reported in several studies.3,12 Moreover, although IRE is believed to be a primarily nonthermal technique, there are some reports of heat development adjacent to the electrodes,13 which could potentially cause thermal coagulation and stenosis of a bile duct that was directly in contact with one of the IRE-electrodes.4 The present study indicated that bile duct narrowing is not only a typical early complication of IRE but also persists during the course of the postablative period.
In contrast to the results of Ueshima et al, who performed endoscopy-guided intraluminal IRE in the common bile duct of six pigs without the occurrence of ensuing bile duct dilatation during the first postinterventional week,14 in our trial bile duct dilatation represented 14 of all 38 biliary alterations (Figure 2). Consequently, it constituted the second most common biliary alteration, similar to the observations made by Dollinger et al10 10 had resolved 6 weeks (n=9) or 3 months (n=1) after IRE, whereas 4 (28.6%) of all 14 dilatations were still present at the last follow-up control. The findings suggest that bile duct dilatation, similar to bile duct narrowing, does not solely represent an early complication of IRE. Reactive choledochitis is presumed to be a potential cause of bile duct dilatation, as reported in a preliminary animal study.7 Although it has been proposed as a possible reason for bile duct narrowing,3,12 peridochal edema is also assumed to cause dilatation of bile ducts adjacent to the ablation zone.7 In the current study, bilomas were a rarely occurring biliary complication (Table 3). All bilomas (n=2) were caused by IRE itself and had resolved 6 weeks after IRE without the need for additional treatment such as abdominal draining. In contrast to the occurrence of narrowing and dilatation of the bile duct, the findings suggested that bilomas seem to be an early complication of IRE. Regarding the time of the first occurrence of all biliary alterations, the results indicate that only bile duct narrowing represented a delayed alteration being detected for the first time 6 weeks (n=3) and 3 months (n=3) after IRE. Especially 3 months after the intervention it has to be discussed whether these alterations can be solely interpreted as a delayed complication of IRE or if there are other potential reasons such recurrent cholangitis or a prior treatment by another kind of therapy, eg radiotherapy.
None of the observed biliary alterations after IRE required an additional intervention or were classified as “severe” or “life-threatening”. They all belonged to the same category “asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated” and were consequently categorized “grade 1” based on CTCAE Version 5.0.5 Regarding preclinical animal studies evaluating blood chemistry after hepatic IRE in pigs, the literature offers diverse findings. Choi et al could not detect elevated levels of serum bilirubin and alkaline phosphatase during the first 8 weeks after the intervention,11 whereas Charpentier et al reported a temporary elevation of serum bilirubin 5 days after IRE, which had normalized by post-ablation day 14.15 In comparison, in human studies small increases in the levels of alkaline phosphatase and serum bilirubin are commonly observed in the first few days after IRE and are probably caused by several factors. Silk et al assumed stasis in bile ducts originated owing to edematous alterations.4 One to three days after IRE, the alkaline phosphatase level was abnormal in 20 of 53 cases (37.7%; pre-existent before IRE: n=15, 28.3%). Six weeks and 3 months after the intervention, an increased level of alkaline phosphatase was observed in 23 (43.4%) and 19 (35.8%) patients, respectively. These findings were in accordance with the results of Froud et al, who ascertained a slow increase in alkaline phosphatase levels with a maximum level approximately 1 month after IRE and a prolonged resolution during the course of observation.16 During the first follow-up control, 31 of 53 cases (58.5%; pre-existent before IRE: n=7, 13.2%) showed elevated levels of serum bilirubin. 6 weeks and 3 months after IRE, abnormal levels of serum bilirubin were observed in 15 (28.3%) and 10 (18.9%) patients, respectively (Table 7). These outcomes were partially consistent with the observations of Froud et al, who described two different patterns of evolution of serum bilirubin after IRE, of which one was an early increase combined with a slow resolution, whereas the rest of the study population showed an early peak of serum bilirubin followed by a rapid decrease.16
The current results of the binary logistic regression models suggest that the occurrence of postinterventional biliary alterations does not increase the risk of suffering from elevated postablative levels of alkaline phosphatase (p= 0.978) or serum bilirubin (p= 0.943). Contrary to the findings of Dollinger et al,10 post-IRE vessel alterations did not occur significantly more frequently in patients older than 64 years (p=0.086). The sex of the patient, nature of bile ducts (minor vs major), presence of liver cirrhosis, number of IRE electrodes (≤4 vs >4) and bile duct location (abutting or distant vs encased) did not show any significant relation to bile duct injury, which was consistent with the results of Dollinger et al.10 The characteristics “tumor type (primary vs secondary hepatic malignancy)”, “ablation volume (≤60 mL vs >60 mL)”, “the presence of hepatic stents or metallic implants” and “the relative distance from needle to bile duct (≤0.3 cm vs >0.3 cm)”, which were not evaluated in the binary logistic regression model of Dollinger et al,10 also did not show any significant influence on bile duct injury.
The current study has several limitations: The first one is the retrospective nature of the study. Moreover, the study group consisted of a heterogeneous patient population with regard to sex and tumor type. Furthermore, a lot of patients had undergone an additional tumor treatment, such as chemotherapy or radiotherapy, which could have had a damaging effect on the observed bile ducts or caused changes in alkaline phosphatase or serum bilirubin levels. Additionally, there was no evaluation of data concerning existing biliary sludge or stones. Because it is not known whether these factors could influence post-IRE bile duct alterations, the lack of these data is another limitation of the present study.
Conclusions
Bile ducts located in proximity to the ablation zone are usually affected by several alterations, of which dilatation and especially narrowing of the bile duct commonly represent a long-term complication after IRE. Furthermore, a definite correlation between the frequently observed prolonged post-ablative elevation of alkaline phosphatase and serum bilirubin levels and the postinterventional biliary alterations could not be proven.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumors. Cardiovasc Intervent Radiol. 2010;33(1):11–17. doi:10.1007/s00270-009-9736-y
2. Ahmed M. Technology Assessment Committee of the Society of Interventional Radiology. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update: supplement to the consensus document. J Vasc Interv Radiol. 2014;25(11):1706–1708. doi:10.1016/j.jvir.2014.09.005
3. Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality - clinical implications. Technol Cancer Res Treat. 2007;6(1):37–48. doi:10.1177/153303460700600106
4. Silk MT, Wimmer T, Lee KS, et al. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J Vasc Interv Radiol. 2014;25(1):112–118. doi:10.1016/j.jvir.2013.10.012
5. U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0; November 27, 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf.
6. Sasson AR, Sigurdson ER. Surgical treatment of liver metastases. Semin Oncol. 2002;29(2):107–118. doi:10.1053/sonc.2002.31676
7. Lee EW, Loh CT, Kee ST. Imaging guided percutaneous irreversible electroporation: ultrasound and immunohistological correlation. Technol Cancer Res Treat. 2007;6(4):287–294. doi:10.1177/153303460700600404
8. Distelmaier M, Barabasch A, Heil P, et al. Midterm safety and efficacy of irreversible electroporation of malignant liver tumors located close to major portal or hepatic veins. Radiology. 2017;285(3):1023–1031.
9. Scheffer HJ, Nielsen K, de Jong MC, et al. Irreversible electroporation for nonthermal tumor ablation in the clinical setting: a systematic review of safety and efficacy. J Vasc Interv Radiol. 2014;25(7):997–1011. doi:10.1016/j.jvir.2014.01.028
10. Dollinger M, Zeman F, Niessen C, et al. Bile duct injury after irreversible electroporation of hepatic malignancies: evaluation of MR imaging findings and laboratory values. J Vasc Interv Radiol. 2016;27(1):96–103. doi:10.1016/j.jvir.2015.10.002
11. Choi JW, Lu DSK, Osuagwu F, Raman S, Lassman C. Assessment of chronological effects of irreversible electroporation on hilar bile ducts in a porcine model. Cardiovasc Intervent Radiol. 2014;37(1):224–230. doi:10.1007/s00270-013-0731-y
12. Lee YJ, Lu DS, Osuagwu F, Lassman C. Irreversible electroporation in porcine liver: acute computed tomography appearance of ablation zone with histopathologic correlation. J Comput Assist Tomogr. 2013;37(2):154–158. doi:10.1097/RCT.0b013e31827dbf9b
13. Faroja M, Ahmed M, Appelbaum L, et al. Irreversible electroporation ablation: is all the damage nonthermal? Radiology. 2012;266:462–470. doi:10.1148/radiol.12120609
14. Ueshima E, Schattner M, Mendelsohn R, et al. Transmural ablation of the normal porcine common bile duct with catheter-directed irreversible electroporation is feasible and does not impact duct patency. Gastrointest Endosc. 2018;87(1):
15. Charpentier KP, Wolf F, Noble L, Winn B, Resnick M, Dupuy DE. Irreversible electroporation of the liver and liver hilum in swine. HPB. 2011;13(3):168–173. doi:10.1111/j.1477-2574.2010.00261.x
16. Froud T, Venkat SR, Barbery KJ, Gunjan A, Narayanan G. Liver function tests following irreversible electroporation of liver tumors: experience in 174 procedures. Tech Vasc Interv Radiol. 2015;18(3):140–146. doi:10.1053/j.tvir.2015.06.004
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.