Back to Journals » Journal of Pain Research » Volume 16
Evaluating the Effect of Delayed Diagnosis on Disease Outcome in Fibromyalgia: A Multi-Center Cross-Sectional Study
Authors Moshrif A , Mosallam A, Abu-Zaid MH, Gouda W
Received 13 July 2022
Accepted for publication 25 November 2022
Published 22 April 2023 Volume 2023:16 Pages 1355—1365
DOI https://doi.org/10.2147/JPR.S381314
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alaa Abd-Elsayed
Abdelhfeez Moshrif,1 Ahmed Mosallam,1 Mohammed Hassan Abu-Zaid,2 Wesam Gouda1
1Rheumatology Department, Faculty of Medicine, Al Azhar University, Assiut, Egypt; 2Rheumatology Department, Faculty of Medicine, Tanta University, Tanta, Egypt
Correspondence: Abdelhfeez Moshrif, Rheumatology Department, Faculty of Medicine, Al Azhar University, Al-Azhar University Square, King Faysal, Assiut, 71524, Egypt, Tel +201062930120, Email [email protected]
Background: The impact of delayed diagnosis (DD) on fibromyalgia (FM) patients’ symptomatology and disease outcome has not yet been systematically studied.
Objective: To analyze the predictors of DD and the influence of DD on FM aggravation and disease evaluation measures.
Methods: 370 FM patients were interviewed prospectively for this study. The following information was obtained: DD, widespread pain index (WPI), symptom severity scale (SSS), polysymptomatic distress scale (PDS) scale (SSS + WPI), and tender points. We identified three groups of patients: early diagnosis (ED: 2 years; 83 patients), late diagnosis (LD: > 2– 7 years; 198 patients), and very late diagnosis (VLD: > 7 years; 89 patients).
Results: The patients’ average age was 33.9 (9.8) years, and 79% were female. The SSS, PDS, and tender point means were 7.8 (1.6), 16.46 (4.1), and 14.31 (2.3), respectively. The correlation between DD and SSS (r = 0.14), the PDS scale (r = 0.37), and FM tender points (r = 0.16) was significant, but not with WPI (r = 0.06). When the three groups were examined, the SSS mean was 7.54 (1.6), 7.73 (1.4), and 8.25 (1.7), respectively (P 0.008), while the PDS mean was 15 (3.8), 15.95 (3.8), and 18.96 (4.4), respectively (P 0.008). (P 0.001).
Conclusion: Early FM diagnosis is associated with lower SSS, total severity scale, and FM tender points, indicating a less severe condition.
Keywords: fibromyalgia, early diagnosis, diagnostic delay, widespread pain, tender points
Introduction
Fibromyalgia (FM) is a chronic musculoskeletal illness that affects over five million Americans and around 3% of the general population, with a significant female predominance.1,2 It has been estimated to be the second most common rheumatic condition after osteoarthritis.3 In addition to the constitutional and musculoskeletal manifestations such as pain, stiffness, fatigue, and weakness, patients with FM may report neuropsychiatric symptoms like dizziness, headaches, depression, anxiety, and memory changes.4 These persistent features may greatly impact the patient’s quality of life and impair social ties with family, friends, and employers,5 with significant financial constraints on both the patient and society.6 Neuroimaging studies have linked central nervous system neurochemical imbalances to central amplification of pain perception, resulting in allodynia and hyperalgesia, as well as inappropriate pain processing, supporting a neurogenic basis for FM’s most notable symptoms.7
The process of diagnosing FM is still challenging for clinicians, especially general practitioners, by whom most care for FM is provided.8,9 In the same context, the subjective nature of the disease and the absence of validated diagnostic tests negatively impact its management and lead to more delayed diagnosis (DD) with more socioeconomic impairment.10 However, there is still a lack of studies about the effect of DD on the disease status and patients’ symptomatology and quality of life.
In this cross-sectional multi-center study, we aimed to evaluate the impact of DD on the severity and outcome of FM depending on a proposed classification of early and late disease and to assess the predictors of DD.
Patients and Methods
Study Design and Participants
This cross-sectional study was performed between May 2017 and December 2019. A total of three hundred and seventy patients with FM, classified according to the 2016 ACR criteria,11 were enrolled in the study. It was carried out at the Rheumatology departments of Al-Azhar University Hospital and Tanta University Hospital in Egypt.
Demographics, and Clinical Characteristics
A structured, predesigned form was prepared and applied for the gathering of data about the details of the disease. The data included the patients’ age, sex, body mass index (BMI), age at onset of symptoms, and age at diagnosis, in addition to the assessment of the widespread pain index (WPI; 0–19), symptom severity scale (SSS; 0–12), the presence or absence of headache, depression, and the degree of somatic symptoms (0–3). The polysymptomatic distress scale (PDS), score range: (0–31) was also evaluated. Based on the PDS, which is the summation of WPI and SSS, the disease severity could be classified as none (0–3), mild (4–7), moderate (8–11), severe (12–19), and very severe (20–31).12
The Revised Fibromyalgia Impact Questionnaire (FIQR)13 includes other symptoms such as cognitive issues, pain, balance, and environmental sensitivity. FIQR’s total score spans from 0 to 100 and is determined as the sum of three domains: function (upper limit of 30), overall impact (upper limit of 20), and symptom domain (upper limit of 50). The function domain has nine elements, the overall impact domain contains two objects, and the symptom domain contains ten items. The number of FM tender points according to the old 1990 ACR criteria14 was also assessed for each participant. Patients were then classified as having an early diagnosis (<2 years), a late diagnosis (2–7 years), or a very late diagnosis (> 7 years) based on the proposed grading.
Statistical Analysis
SPSS v. 20 for Windows was used to analyze the data (IBM, Armonk, NY, USA). Means (standard deviation), median, and range were used to convey quantitative data, whereas numbers and percentages were used to express qualitative variables. A Spearman rho was done for the correlation of DD with the revised FIQR, PDS, the mean FM tender points, and other non-parametric variables. Further assessment with linear regression was performed for the DD with the highly correlated disease severity parameters. An independent sample t-test was used for comparing DD between groups based on gender and disease severity. The Mann–Whitney U-test was used for the assessment of the association of DD with the presence of headache, depression, and lower abdominal pain as well as with the two groups of disease severity, while the Kruskal–Wallis test was used for the association with the degrees of fatigue, sleep disturbance, awakened unrefreshed, and cognitive dysfunction. The one-way ANOVA test has been used to compare the disease severity scales among the three categories of early diagnosis, late diagnosis, and very late diagnosis. A P-value of < 0.05 was considered significant.
Results
The mean age of patients was 33.97 ± 9.8 years, and the female to male ratio was 3.9:1. According to the PDS, 291 patients (78.6%) had severe FM, while 79 (21.4%) had very severe disease. The demographic and clinical features are summarized in Table 1. There was no difference in DD between men and women (r = 0.048, p = 0.354, 95% CI = −1.189, −0.640). The DD has been found to correlate with the patients’ age (r = 0.199, p = 0.001), BMI (r = 0.159, p = 0.002), FIQR (r = 0.880, p = 0.001), SSS (r = 0.61, p = 0.001), PDS (r = 0.117, p = 0.002), fatigue (r = 0.233, p <0.001), cognitive dysfunction (r = 0.142, p = 0.006), and the overall increased disease severity (r = 0.119, p = 0.002) in addition to the mean FM tender points (r = 0.206, p <0.001). The correlations between DD and the different features and severity parameters of FM are shown in Table 2 and Figures 1–5.
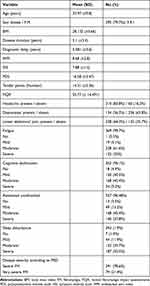 |
Table 1 Demographic and Clinical Features of FM Patients |
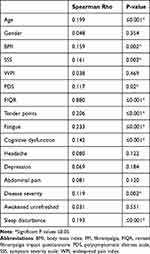 |
Table 2 Correlations of the Overall Mean Diagnostic Delay with FM Features and Severity |
 |
Figure 1 Correlation between PDS and DD in FM. |
 |
Figure 2 Correlation between FIQR and DD in FM. |
 |
Figure 3 Correlation between SSS and DD in FM. |
 |
Figure 4 Correlation between mean FM tender points and DD in FM. |
 |
Figure 5 Correlation between WPI and DD in FM. |
The mean SSS in the ED, LD, and VLD groups was 7.5 (1.6), 7.7 (1.4), and 8.25 (1.7), respectively (p = 0.008), whereas the mean total PDS scale was 15 (3.8), 15.95 (3.8), and 18.96 (4.4), respectively (p = 0.001), and the mean FM tender points were 13.7 (2.3), 14.35 (2.1), and 14.77 (2.8), respectively (p = 0.01). The mean WPI in the ED, LD, and VLD groups was 7.45 (2.8), 7.8 (3.6), and 7.2 (4.8), respectively (p = 0.4). Table 3. For further assessment of the predictors of DD, linear regression analysis was done, and the values are shown in Table 4.
 |
Table 3 Fibromyalgia Quantitative Measures of Severity and Categories of Delayed Diagnosis |
 |
Table 4 The Predictive Effect of Diagnostic Delay on the Disease Severity Parameters and Some Features of FM |
Discussion
FM is a diverse syndrome that generates severe subjective complaints and is often mistaken for several clinical disorders characterized by persistent, widespread pain.15 This intersection with other musculoskeletal conditions, in addition to the absence of objective diagnostic tests, might hamper the early diagnosis of FM. Early detection of inflammatory musculoskeletal disorders is critical for successful therapy and a better prognosis. It also has an indirect impact on the patient’s quality of life.16 Nevertheless, early diagnosis is still challenging for most of these diseases. The time between the beginning of symptoms and diagnosis and the start of successful treatment in rheumatoid arthritis patients, for example, was determined to be 12 weeks.17 Feldtkeller et al showed that the DD in 59% of individuals with spondyloarthropathy was more than ten years.18 Moshrif et al observed a comparable DD for ankylosing spondylitis with an average of 9 years in Egyptian patients.19 Delays in FM diagnosis have been attributed to a greater economic burden, increased healthcare demand, and poor treatment response.20,21 However, its influence on patient symptomatology and disease severity remains underappreciated.
The purpose of this study was to assess the influence of DD on the severity and outcome of FM patients in an initial cohort. The average age of FM patients at diagnosis was 33.9 years, which is consistent with previous research demonstrating that the prevalence of FM is greater in middle age.22,23 There was a significant female predominance where the female to male ratio was 3.9:1, which is in accordance with previously published studies.24–26
The mean DD in our analysis was 5.581 (3.6) years, but in Choy et al’s study of 800 FM patients from six European countries, Mexico, and South Korea, the mean time to get a definitive FM diagnosis was 2.3 years, with most countries lying between 2.1 and 2.7 years. The anomalies were Spain (3.7 years) and South Korea (0.6 years). Similarly, the results reported here differ from those of a previous survey conducted by Clark et al on 900 FM patients from Latin America and Europe; Meanwhile, patients in Latin America and Europe waited 3.5 and 2.5 years, respectively, for an accurate diagnosis of FM.27 Two studies from the United States, however, show that patients frequently wait 5 years before getting an accurate diagnosis of FM.28,29 There was no significant difference between male and female regarding the DD, which is consistent with our recently published work evaluating the gender difference in FM patients considering the different ACR classification criteria.30
Increased age was substantially linked with DD in our FM patients in the current study, which is similar to previously published findings by Gendelmans et al.31 Furthermore, increasing BMI was associated with a longer DD in FM patients; these findings are consistent with those provided by Gota et al.32 The DD was associated with greater disease severity, fatigue, and tender points. We also found that SSS, WPI, and PDS were greater in patients with DD.
Our FM patients’ mean WPI (8.68) and SS (7.88) scores were lower than previously reported by Galvez et al in a survey of 102 patients with FM from Spain. The mean score of WBI was 17.84, and 10.05 for SS.33 In another previously mentioned study by Wolfe et al, the mean scores were 11.4 for WPI and 8 for SS.2 Similarly, in Spanish FM patients, median scores of 15 for WPI and 8 for SS have been observed.34 One possible theory for this discrepancy is that our FM sample was gathered using ACR criteria from 2016. This supports the notion that the 2016 criteria set is more sensitive than those of 2010 and 1990, where more severely presented patients are classified.33
The mean PDS score in this study was 16.58 ± 3.47, which is similar to the findings of Bidari et al on a survey of 169 Iranian FM patients, where the mean PDS score was 15.39 ± 5.43.35 These results were higher than those reported in the study by Srinivasan et al,8 in which the mean score of PDS was 6.1 ±5.1 for FM patients in the USA.
To the best of our knowledge, this is the first study to assess the impact of DD on the severity of FM. Nonetheless, this study has some limitations. First, our sample size is quite modest given the multicentric nature. The cross-sectional design of the study is another limitation. Furthermore, the dependence on the reported disease onset as the time of appearance of symptoms might be biased by the cognitive and memory disturbances common in patients with FM. Therefore, more large-scale, multicentric studies are needed to support our observations.
Conclusions
Our findings show that early FM diagnosis is related with lower SSS, total severity scale, and FM tender points, indicating a less severe condition.
Data Sharing Statement
Data or materials are available from the authors upon request.
Ethical Approval
The study was approved by the ethical committee of Al Azhar University School of Medicine (Az 063-12-019) in May 2017, all participants were informed about the study procedures and signed an informed consent form. The study was conducted in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 1983. No financial support was received for this study.
Acknowledgment
The abstract of the study was presented in EULAR 2020 Virtual Annual Meeting. The poster’s abstract was published in “Poster Abstracts” in Annals of the Rheumatic Diseases 81(Suppl 1):1721.1–1721, DOI: 10.1136/annrheumdis-2022-eular.5146. The article processing charge for this paper was funded by NewBridge Pharmaceuticals FZ LLC.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare no conflicts of interest with respect to the authorship and/or publication of this article.
References
1. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. “SUISJUJT3IFVN”. Arthritis Rheum. 2008;58(1):26–35). doi:10.1002/art.23176
2. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American college of rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62:600–610. doi:10.1002/acr.20140
3. Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA. 2004;292(19):2388–2395. doi:10.1001/jama.292.19.2388
4. Stewart JA, Mailler-Burch S, Muller D, et al. Rethinking the criteria for fibromyalgia in 2019: the ABC indicators. J Pain Res. 2019;12:2115–2124. doi:10.2147/JPR.S205299
5. Arnold LM, Crofford LJ, Mease PJ, et al. Patient perspectives on the impact of fibromyalgia. Patient Educ Couns. 2008;73(1):114–120. doi:10.1016/j.pec.2008.06.005
6. Goldenberg D, Schaefer C, Ryan K, Chandran A, Slateva G. What is the true cost of fibromyalgia to our society: results from a cross-sectional survey in the United States.
7. Nebel MB, Gracely RH. Neuroimaging of fibromyalgia. Rheum Dis Clin North Am. 2009;35(2):313–327. doi:10.1016/j.rdc.2009.06.004
8. Srinivasan S, Maloney E, Wright B, et al. The problematic nature of fibromyalgia diagnosis in the community. ACR Open Rheumatol. 2019;1:43–51. doi:10.1002/acr2.1006
9. Littlejohn G. Fibromyalgia: honing fibromyalgia diagnosis. Nat Rev Rheumatol. 2014;10:267–269. doi:10.1038/nrrheum.2014.48
10. Okifuji A, Hare BD. Management of fibromyalgia syndrome: review of evidence. Pain Ther. 2013;2(2):87–104. doi:10.1007/s40122-013-0016-9
11. Wolfe F, Clauw DJ, Fitzcharles M-A, et al. 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46(3):319–329. doi:10.1016/j.semarthrit.2016.08.012
12. Wolfe F, Wallit B, Rasker JJ, Katz RS, Häuser W. The use of polysymptomatic distress categories in the evaluation of fibromyalgia (FM) and FM severity. J Rheumatol. 2015;42:1494–1501. doi:10.3899/jrheum.141519
13. Bennett RM, Friend R, Jones KD, Ward R, Han BK, Ross RL. The revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties. Arthritis Res Ther. 2009;11(4):R120. Erratum in: Arthritis Res Ther. 2009;11(5):415.PMID: 19664287; PMCID: PMC2745803. doi:10.1186/ar2783
14. Wolfe F, Smythe HA, Yunus MB, et al. The American college of rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum. 1990;33(2):160–172. doi:10.1002/art.1780330203
15. Elboğa G, Akaltun MS, Altındağ Ö, et al. Alexithymia and attention deficit and their relationship with disease severity in fibromyalgia syndrome. Turk J Phys Med Rehabil. 2020;66(2):134–139.
16. Raciborski F, Kłak A, Kwiatkowska B, et al. Diagnostic delays in rheumatic diseases with associated arthritis. Reumatologia. 2017;55(4):169–176. doi:10.5114/reum.2017.69777
17. Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960–977.
18. Feldtkeller E, Khan MA, van der Heijde D, et al. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23(2):61–66. doi:10.1007/s00296-002-0237-4
19. Moshrif A, Mosallam A, Ryan M, et al. characterization of ankylosing spondylitis in Upper Egypt. Int J Clin Rheumatol. 2018;13(1):52–59. doi:10.4172/1758-4272.1000162
20. Hughes G, Martinez C, Myon E, Taïeb C, Wessely S. The impact of a diagnosis of fibromyalgia on health care resource use by primary care patients in the UK: an observational study based on clinical practice. Arthritis Rheum. 2006;54(1):177–183.
21. Choy E, Perrot S, Leon T. A patient survey of the impact of fibromyalgia and the journey to diagnosis. BMC Health Serv Res. 2010;10(1):102. doi:10.1186/1472-6963-10-102
22. Mas AJ, Carmona L, Valverde M, Ribas B. Prevalence and impact of fibromyalgia on function and quality of life in individuals from the general population: results from a nationwide study in Spain. Clin Exp Rheumatol. 2008;26:519e26.
23. Senna ER, De Barros AL, Silva EO, et al. Prevalence of rheumatic diseases in Brazil: a study using the COPCORD approach. J Rheumatol. 2004;31:594–597.
24. Buskila D, Neumann L, Odes LR, Schleifer E, Depsames R, Abu-Shakra M. The prevalence of musculoskeletal pain and fibromyalgia in patients hospitalized on internal medicine wards. Semin Arthritis Rheum. 2001;30(6):411–417. doi:10.1053/sarh.2001.21152
25. Haviland MG, Banta JE, Przekop P. Fibromyalgia: prevalence, course, and co-morbidities in hospitalized patients in the United States, 1999e2007. Clin Exp Rheumatol. 2011;29(6 Suppl 69):S79–S87.
26. Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, Rojas-Villarraga A, Anaya JM. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity. J Autoimmun. 2012;38:J109–J119.
27. Clark P, Paiva ES, Ginovker A, Salomon PA. A patient and physician survey of fibromyalgia across Latin America and Europe. BMC Muscoskelet Disord. 2013;14:188. doi:10.1186/1471-2474-14-188
28. Arnold LM, Clauw DJ, McCarberg BH. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc. 2011;86(5):457–464.
29. Fibromyalgia: facts and statistics; 2013 Available from: http://nationalpainfoundation.org/articles/849/facts-and-statistics.
30. Moshrif A, Shoaeir MZ, Abbas AS, Abdel-Aziz TM, Gouda W. Evaluating gender differences in Egyptian fibromyalgia patients using the 1990, 2011, and 2016 ACR criteria. Open Access Rheumatol. 2022;14:67–74. doi:10.2147/OARRR.S358255
31. Gendelman O, Amital H, Bar-On Y, et al. Time to diagnosis of fibromyalgia and factors associated with delayed diagnosis in primary care. Best Pract Res Clin Rheumatol. 2018;32(4):489–499. doi:10.1016/j.berh.2019.01.019
32. Gota CE, Kaouk S, Wilke M, William S. Fibromyalgia and obesity. J Clin Rheumatol. 2015;21(6):289–295. doi:10.1097/RHU.0000000000000278
33. Galvez-Sánchez CM, de la Coba P, Duschek S, Reyes del paso GA. Reliability, factor structure and predictive validity of the widespread pain index and symptom severity scales of the 2010 American college of rheumatology criteria of fibromyalgia. J Clin Med. 2020;9(8):2460. PMID: 32752048; PMCID: PMC7464133. doi:10.3390/jcm9082460
34. Casanueva B, García-Fructuoso F, Belenguer R, et al. The Spanish version of the 2010 American college of rheumatology preliminary clinical diagnostic criteria for fibromyalgia: reliability and validity assessment. Exp Rheumatol. 2016;34:S55–S58.
35. Bidari A, Ghavidel-Parsa B, Amir Maafi A, et al. Validation of fibromyalgia survey questionnaire and polysymptomatic distress scale in a Persian population. Rheumatol Int. 2015;35(12):2013–2019. doi:10.1007/s00296-015-3340-z
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
