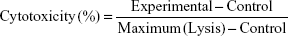Back to Journals » Drug Design, Development and Therapy » Volume 10
Evaluating the cytotoxic effects of the water extracts of four anticancer herbs against human malignant melanoma cells
Authors Ling BB, Michel D, Sakharkar MK , Yang J
Received 7 August 2016
Accepted for publication 20 September 2016
Published 1 November 2016 Volume 2016:10 Pages 3563—3572
DOI https://doi.org/10.2147/DDDT.S119214
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Binbing Ling,1,2 Deborah Michel,1 Meena Kishore Sakharkar,1 Jian Yang1
1Drug Discovery and Development Research Group, College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, SK, 2Department of Biomedical and Molecular Sciences, Queen’s University, Cancer Biology & Genetics Division, Queen’s Cancer Research Institute, Kingston, ON, Canada
Abstract: Malignant melanoma (MM) is the most dangerous type of skin cancer, killing more than 1,100 people each year in Canada. Prognosis for late stage and recurrent MM is extremely poor due to insensitivity to chemotherapy drugs, and thus many patients seek complementary and alternative medicines. In this study, we examined four commonly used anticancer herbs in traditional Chinese medicine, Hedyotis diffusa, Scutellaria barbata, Lobelia chinensis, and Solanum nigrum, for their in vitro antitumor effects toward human MM cell line A-375. The crude water extract of S. nigrum (1 g of dry herb in 100 mL water) and its 2-fold dilution caused 52.8%±13.0% and 17.3%±2.7% cytotoxicity in A-375 cells, respectively (P<0.01). The crude water extract of H. diffusa caused 11.1%±12.4% cytotoxicity in A-375 cells with no statistical significance (P>0.05). Higher concentrated formulation might be needed for H. diffusa to exert its cytotoxic effect against A-375 cells. No cytotoxicity was observed in A-375 cells treated with crude water extract of S. barbata and L. chinensis. Further high performance liquid chromatography-tandem mass spectroscopy analysis of the herbal extracts implicated that S. nigrum and H. diffusa might have adopted the same bioactive components for their cytotoxic effects in spite of belonging to two different plant families. We also showed that the crude water extract of S. nigrum reduced intracellular reactive oxygen species generation in A-375 cells, which may lead to a cytostatic effect. Furthermore, synergistic effect was achieved when crude water extract of S. nigrum was coadministered with temozolomide, a chemotherapy drug for skin cancer.
Keywords: herbal extract, malignant melanoma, cytotoxicity, HPLC-MS/MS, reactive oxygen species
Introduction
Malignant melanoma (MM) is a less common but more dangerous type of skin cancer. It accounts for about 75% of skin cancer-related deaths.1 In 2015, Canadian Cancer Statistics reported that the incidence rate of MM was increased at 2.3% per year in men and 2.9% per year in women, respectively, between 2001 and 2010.2 It was estimated that there would be 6,800 new MM cases and 1,170 MM deaths in Canada in 2015.2 Like other types of cancer, early diagnosis is the key prognostic factor for MM. The 5-year relative survival rate is higher than 90% for early diagnosed MM patients (stages IA and IB); however, prognosis is extremely poor for deeper (>4 cm) and metastatic MM partially due to resistance development toward chemotherapy drugs.3 Thus, many advanced or recurrent MM patients seek complementary and alternative medicines, expecting to achieve better therapeutic efficacy, reduced chemotherapy-related side effects, and/or a boost to the immune system.4–6
Oral and topical administrations of herbal extracts have been widely used to treat skin diseases including MM in traditional medicines for thousands of years even without much knowledge of the active ingredients.7,8 Furthermore, these practices are rarely documented in English and usually lack sufficient quality control. In recent years, a significant amount of research effort has been put into identifying and isolating anticancer components from medicinal plants, such as paclitaxel that was isolated from the bark of the Pacific yew tree.9,10 In fact, more than 70% of the anticancer drugs approved worldwide are natural products or their mimetic analogs.11 However, this research effort did not reduce herbal usage in cancer patients.12 Recently, the US Food and Drug Administration (FDA) has noticed increased herbal usage in cancer patients, updated its regulatory protocols and approved several herbal extracts, such as BZL101 and PHY906, for clinical trials.13–15
Hedyotis diffusa, Scutellaria barbata, Lobelia chinensis, and Solanum nigrum are four commonly used anticancer herbs, classified as nontoxic for both oral and topical administrations, in traditional Chinese medicine (TCM). H. diffusa is a major component in several TCM formulations. It has been used to treat liver, lung, colon, brain, and pancreatic cancers.16 Our previous studies showed that the water extract of H. diffusa was highly cytotoxic toward human breast cancer MCF7 cells.17 However, coadministration of the water extract of H. diffusa diminished the anticancer activity of three chemotherapy agents, doxorubicin, cyclophosphamide, and docetaxel.18 Triterpenes and polysaccharides have been identified to be the active components in H. diffusa.16,19 S. barbata is another widely used herb to treat lung, liver, breast, and gastric cancers in TCM.20 Its water extract, BZL101, has been approved by FDA for clinical trials and has shown promising efficacy against metastatic breast cancer.13,14 L. chinensis is traditionally used to treat snake bites and skin abscess. Recently, the water extract of L. chinensis was shown to possess antitumor activity against lung, colon, and liver cancers and several types of alkaloids have been identified as the active ingredients.21–24 S. nigrum is traditionally used to treat liver disorders, chronic skin ailments, inflammations, and diarrhea. Both water and alcohol extracts of S. nigrum exhibited anticancer activities against liver, colorectal, breast, and cervical cancers.25–28 The water extract of S. nigrum was also reported to inhibit the metastasis of mouse melanoma B16-F1 cells.29 Various active components, including glycoalkaloids, polyphenols, polysaccharides, glycoproteins, and peptides, have been isolated from S. nigrum.30–34 Nevertheless, neither the water extracts of these four commonly used herbs nor their respective active components have been examined for anticancer activity against human MM. In the current study, we evaluated whether the water extracts of the herbs possess any antitumor activity and exhibit synergistic/additive effects with temozolomide, an oral analog of dacarbazine (DTIC) used for stage IV melanoma, against human MM A-375 cells.
Materials and methods
Materials
Dried whole plants of H. diffusa, S. barbata, L. chinensis, and S. nigrum were purchased from a TCM store (Calgary, AB, Canada). All chemicals and fetal bovine serum used in the current study were purchased from Sigma-Aldrich (Oakville, ON, Canada). Human MM cell line A-375 was purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Cell culture medium, Dulbecco’s Modified Eagle’s Medium (DMEM), was purchased from Cedarlane Laboratories (Burlington, ON, Canada). Cytotoxicity assay kit, CytoTox96 non-radioactive cytotoxicity assay, which quantitatively measures lactate dehydrogenase (LDH), was purchased from Promega Corporation (Madison, WI, USA).
Water extract preparation
For each herb, the crude water extract was prepared by boiling 1 g of the chopped herb in 100 mL deionized water for 1.5 hr. The water solution was allowed to cool down at room temperature (~23°C) for at least 2 hr before the supernatant was collected. The supernatant was then diluted serially up to 16-fold with deionized water. Both the supernatant and its serial dilutions were used for cell treatments within 24 hr.
Cell culture
Human MM cell line A-375 was cultured in T-75 culture flasks under ATCC-recommended culture conditions (DMEM media with 10% fetal bovine serum and 1% penicillin/streptomycin) at 37°C under a humidified atmosphere (5% CO2) in a Forma™ series II water-jacketed CO2 incubator purchased from ThermoFisher Scientific Inc. (Waltham, MA, USA). Cell culture media were changed every 2–3 days.
Cytotoxicity assay
All experiments except the high performance liquid chromatography-tandem mass spectrometry analysis were carried out in triplicate in the current study. For the cytotoxicity assay, human MM A-375 cells were collected from the T-75 cell culture flasks, resuspended in the culture media, and plated in 96-well culture plates with each well containing about 10,000 cells. The cells were allowed to attach and grow for 24 hr (reaching 70%–80% confluence) before being treated with the water extractions (1 g/100 mL) of H. diffusa, S. barbata, L. chinensis, and S. nigrum, as well as their respective serial-diluted solutions (2-, 4-, 8-, and 16-fold), for another 24 hr, which was the optimal treatment time predetermined from a pilot study. Cytotoxicity of the herbal water extracts was measured using the CytoTox 96® non-radioactive cytotoxicity assay. Cells treated with the culture media were used as negative control. The cytotoxicity was calculated using the following equation:
|
High performance liquid chromatography-tandem mass spectroscopy (HPLC-MS/MS) analysis
HPLC-MS/MS analysis of the water extracts of H. diffusa, S. barbata, L. chinensis, and S. nigrum was performed in both positive and negative ionization modes with spectra acquired in the mass range of 100–1,500 m/z using an Agilent 1200 HPLC system (Mississauga, ON, Canada) interfaced to an AB Sciex 4000 QTRAP® hybrid triple quadrupole/linear ion trap mass spectrometer equipped with the TurbolonSpray™ interface (Concord, ON, Canada). Applied Biosystems/MDS Sciex Analyst Software (Version 1.6.0) was used for system control and analysis. The reverse-phase chromatography was performed using an Agilent ZORBAX Eclipse C18 column (5 μm, 4.6×150 mm2) at 40°C. For each herbal water extract, 5 μL sample was injected into the C18 column using the Agilent 1200 Autosampler (set to 4°C) and delivered with a gradient mobile phase. The column was equilibrated with 90% water acidified with 0.1% formic acid and 10% acetonitrile acidified with 0.1% formic acid at a flow rate of 0.2 mL/min. The gradient was run for 20 min to 10% water acidified with 0.1% formic acid and 90% acetonitrile acidified with 0.1% formic acid. Then, the column was returned back to 90% water acidified with 0.1% formic acid and 10% acetonitrile acidified with 0.1% formic acid over 2 min and held under the final condition for 1 min. Total run time was 23 min. For the tandem mass spectrometric analysis, the condition was set to source temperature at 500°C, ion spray voltage at 5,500 V in positive ionization mode and −4,500 V in negative ionization mode, curtain gas at 40 psi, nebulizer gas (GS1) at 40 psi, heater gas (GS2) at 40 psi, collision cell exit potential at 10 V, and declustering potential at 40 V in positive ionization mode and −40 V in negative ionization mode. Nitrogen was used as the gas for all cases and the interface heater was on.
Reactive oxygen species (ROS) measurement
The intracellular ROS level in human MM A-375 cells was measured using modified published protocol for the dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay.35 Briefly, A-375 cells harvested from the T-75 flasks were plated on a black flat-bottom 96-well plate at 10,000 cells per well and incubated at 37°C for 24 hr before being treated with the water extract of S. nigrum and its serial diluted solutions (2-, 4-, 8-, and 16-fold) for another 24 hr. Cells treated with cell culture media were used as negative control. At the end of treatment, 5 μL DCFH-DA working solution (concentration: 0.1 mM) was added to each well and allowed to react with the cells for 30 min before being aspirated out. The cells were then washed twice with 200 μL 1× phosphate buffered saline (PBS) buffer; and finally 100 μL 1× PBS buffer was added into each well and fluorescence was read at excitation of 485 nm and emission of 528 nm using an Agilent 8453E UV-visible spectroscopy system.
Cytotoxicity of the water extract of S. nigrum and temozolomide combination
Human MM A-375 cells were plated in 96-well culture plates at 10,000 cells per well and allowed to attach and grow for 24 hr (reaching 70%–80% confluence) before being treated with temozolomide (final concentration: 200 μM) and its combination with the water extract of S. nigrum (1 g/100 mL) for another 24 hr. Treatment with dimethyl sulfoxide (DMSO), in which temozolomide solution was prepared, was used as negative control. Cytotoxicity of each treatment was measured using the CytoTox 96® non-radioactive cytotoxicity assay.
Statistical analysis
The experimental data were processed using Microsoft Excel 2013 and presented as mean ± standard deviation. Unpaired t-test was performed for statistical analyses using GraphPad QuickCalcs. A P-value <0.05 was considered to be statistically significant.
Results and discussion
Cytotoxicity of the herbal water extracts
H. diffusa, S. barbata, L. chinensis, and S. nigrum are four commonly used herbal plants to treat various types of cancer, such as breast, liver, and colorectal cancers, in TCM. However, there is limited literature available regarding their applications against human MM. Only two previous studies reported that water extract of S. nigrum inhibited the metastasis of mouse melanoma B16-F1 cells and the ethanol extract of H. diffusa suppressed the growth of mouse melanoma B16-F10 cells.29,36 To evaluate the applicability of these four herbs in treating MM, we measured the cytotoxicity of their water extracts toward MM cell line A-375. As shown in Figure 1, only S. nigrum exhibited a substantial and concentration-dependent cytotoxic effect against the A-375 cells. The water extract of S. nigrum and its 2-fold dilution increased the cytotoxicity by 52.8%±13.0% (P<0.01) and 17.3%±2.7% (P<0.01) compared to the control, respectively. As the water extract of S. nigrum was further diluted, no cytotoxicity was observed. The water extract of H. diffusa also increased the cytotoxicity by 11.1%±12.4% compared to the control; however this increase was not statistically significant (P=0.23). The clinical dose for H. diffusa is normally 2–5 times of that for S. nigrum in TCM; thus higher concentrated formulation of the water extract of H. diffusa may exhibit cytotoxic effect against A-375 cells. The water extracts of S. barbata and L. chinensis did not show any cytotoxic activity toward the A-375 cells. Higher concentrated formulation of the water extract of S. barbata or L. chinensis might still not be cytotoxic to the A-375 cells as their clinical doses are comparable to that for S. nigrum. Since ethanol extraction is also a common practice in TCM, further studies are warranted to investigate whether the ethanol extracts of these four herbs are cytotoxic toward human MM cells.
HPLC-MS/MS analysis of the herbal water extracts
A major challenge in TCM is identification and quality control of biologically active ingredients in medicinal herbs, as the herbs are usually formulated in Fufang (combination of two or more herbs) with each herb containing hundreds of components. Recently, HPLC-MS, which allows fast separation, quantification, and even identification of compounds in a mixture, has been widely applied in analyzing herbal components.37 Several classes of biologically active compounds including alkaloids, glycosides, flavonoids, polyphenols terpenoids, and saponins have been identified and characterized.37 To identify why only S. nigrum was active against the MM A-375 cells, we analyzed the components of the four anticancer herbs using HPLC-MS/MS in both positive and negative ionization modes. In the positive ionization mode, the total ion current (TIC) chromatogram showed that a peak region with retention time between 1.8 to 5.5 min was only present in the water extracts of S. nigrum and H. diffusa (Figure 2A). We subsequently examined the ions in this peak region (Figure 2B and C). Surprisingly, we discovered that most of the abundant ions (m/z at 102.6, 279.6, 295.7, 352.9, 369.8, 761.3, 773.2, 775.2, 785.3, 801.3, 803.3, 809.3, and 817.3) were shared by both herbs, suggesting that S. nigrum and H. diffusa, which belong to different plant families (Solanaceae versus Rubiaceae), might use the same bioactive components for their cytotoxic effects against MM A-375 cells. The major difference in ion composition was that S. nigrum contained ions with m/z of 702.2, 747.2, 821.3, and 833.3, whereas H. diffusa contained ions with m/z at 704.3, 759.2, 839.2, and 855.5. α-Solanin and solanidine, two major bioactive alkaloids identified in S. nigrum, were confirmed not to present or with minimal quantity in the fraction eluted between 1.8 and 5.5 min upon comparing with their fragmentation pattern reported previously.38 We hypothesized that components eluted from the C18 column within this retention time range were responsible for the cytotoxicity of S. nigrum and more concentrated water extract of H. diffusa would be cytotoxic toward A-375 cells. Studies have already been initiated to prove our hypothesis and confirm the identities of the components. Furthermore, the purpose of coadministration of S. nigrum and H. diffusa in TCM might be to increase the concentration of bioactive components. As for the negative ionization mode, we observed a unique peak region with retention time between 5.5 and 6.8 min for S. nigrum from the TIC chromatogram (Figure 3A). The ions in this peak region were smaller in size compared to those in the positive ionization mode (Figure 3B), with the abundant ions having m/z of 128.3, 165.3, 175.3, 197.4, 204.4, 227.4, 279.4, 281.5, 311.6, 317.5, 331.4, 347.4, 395.7, and 451.5. Further studies are warranted to elucidate whether components eluted from the C18 column at retention time between 5.5 and 6.8 min also contribute to the cytotoxic effect of S. nigrum.
Water extract of S. nigrum reduced intracellular ROS level in A-375 cells
Excessive ultraviolet (UV) exposure has been identified as a major risk factor for MM.39 UVA (320–400 nm) radiation can raise intracellular ROS generation and cause melanin-dependent oxidative DNA damages in melanocytes.40,41 In fact, MM cells maintain a much higher level of intracellular ROS than most other types of cancer.42–44 Furthermore, ROS are generally believed to promote MM invasion and metastasis.45,46 Therefore, we investigated whether the water extract of S. nigrum has any effects on ROS generation. Cultured MM A-375 cells were treated with the water extract of S. nigrum and its serial dilutions for 24 hr and the intracellular ROS level was measured using the DCFH-DA assay. As shown in Figure 4, the ROS level in A-375 cells was reduced to 24.7%±5.0% (P<0.01) upon treatment with the water extract of S. nigrum compared to the control. The 2- and 16-fold dilutions of the S. nigrum water extract also reduced the ROS level in A-375 cells to 79.1%±36.3% (P=0.11) and 83.9%±35.9% (P=0.21) compared to the control, respectively, however these ROS reductions were not statistically significant. Interestingly, the intracellular ROS level in A-375 cells was increased to 122.1%±17.2% (statistically significant, P=0.03) and 110.9%±32.5% (P=0.37) upon treatment with the 4- and 8-fold dilutions of the S. nigrum water extract, respectively. Thus, we concluded that the water extract of S. nigrum possessed cytostatic effect via reducing intracellular ROS generation in additional to its cytotoxic effect toward human MM A-375 cells. Further studies, including the expression of key ROS-related enzymes such as catalase, glutathione peroxidase, and superoxide dismutase, are warranted to fully understand how the water extract of S. nigrum reduces the intracellular ROS generation.
Synergistic effect between the water extract of S. nigrum and temozolomide
Advanced MM generally does not respond to chemotherapy and DTIC is the only commonly used chemotherapy drug with a response rate of about 15%.47 Temozolomide, an oral analog of DTIC, has been extensively evaluated in treating advanced MM.48–52 However, its new drug application (NDA 21-051) for melanoma was denied by FDA due to a lack of evidence showing therapeutic advantage over DTIC. A randomized phase III trial showed that coadministration of temozolomide and interferon α2b (INF-α2b) increased the response rate from 13% to 24% and overall survival time from 8.4 to 9.7 months.53 Since various herbal water extracts have been reported to potentiate the therapeutic efficacy of chemotherapy drugs,25,26,54–56 we evaluated whether the water extract of S. nigrum exhibits synergistic/additive effect with temozolomide against human MM A-375 cells. As illustrated in Figure 5, the cytotoxicity of the coadministered temozolomide and water extract of S. nigrum was increased statistically significantly to 97.0%±3.6% from 31.0%±7.5% for temozolomide (P<0.01) and 55.0%±17.6% for the water extract of S. nigrum (P=0.03), implicating synergistic effect was achieved between temozolomide and the water extract of S. nigrum against human MM A-375 cells. Our current study suggests that oral administration of temozolomide and the water extract of S. nigrum or oral administration of temozolomide in combination with topical wash with the water extract of S. nigrum is worthy of further investigation using a patient-derived xenograft or a mouse xenograft melanoma model.
Conclusion
In this study, we evaluated the cytotoxicity of the water extracts of H. diffusa, S. barbata, L. chinensis, and S. nigrum toward human MM A-375 cells. S. nigrum was the only cytotoxic herb at extraction condition of 1 g herb in 100 mL water. Our HPLC-MS/MS analysis showed that S. nigrum and H. diffusa might adopt the same bioactive components for their cytotoxic functions and higher concentrated formulation of the water extract of H. diffusa would exhibit cytotoxic effect against A-375 cells. The water extract of S. nigrum was also shown to possess cytostatic activity toward MM A-375 cells through decreasing intracellular ROS generation. Furthermore, synergistic effect was observed between the water extract of S. nigrum and temozolomide. Based on the current results, we hypothesized that coadministration of the water extract of S. nigrum could improve the therapeutic efficacy of temozolomide, as well as DTIC, against human MM. Further studies are warranted to prove our hypothesis using a patient-derived xenograft or a mouse xenograft melanoma model.
Acknowledgment
This work was supported by an internal research grant from the College of Pharmacy and Nutrition, University of Saskatchewan.
Disclosure
The authors report no conflicts of interest in this work.
References
American Cancer Society. Cancer Facts & Figures. Atlanta; 2014. | ||
Canadian Cancer Society. Canadian Cancer Statistics. Canada: Public Health Agency of Canada; 2015. | ||
Helmbach H, Rossmann E, Kern MA, Schadendorf D. Drug-resistance in human melanoma. Int J Cancer. 2001;93:617–622. | ||
HemaIswarya S, Doble M. Potential synergism of natural products in the treatment of cancer. Phytother Res. 2006;40(4):239–249. | ||
Cheng KC, Li YX, Cheng JT. The use of herbal medicine in cancer-related anorexia/cachexia treatment around the world. Curr Pharm Des. 2012;18(31):4819–4826. | ||
Yang C, Chien LY, Tai CJ. Use of complementary and alternative medicine among patients with cancer receiving outpatient chemotherapy in Taiwan. J Altern Complement Med. 2008;14(4):413–416. | ||
Shenefelt PD. Herbal treatment for dermatologic disorders. In: Benzie IFF, Wachtel-Galor S, editors. Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed. Boca Raton: CRC Press/Taylor & Francis; 2011:383–404. | ||
Zink A, Traidl-Hoffmann C. Green tea in dermatology – myths and facts. J Dtsch Dermatol Ges. 2015;13(8):768–775. | ||
Riondel J, Jacrot M, Picot F, Beriel H, Mouriquand C, Potier P. Therapeutic response to taxol of six human tumors xenografted into nude mice. Cancer Chemother Pharmacol. 1986;17(2):137–142. | ||
Slichenmyer WJ, Von Hoff DD. Taxol: a new and effective anti-cancer drug. Anticancer Drugs. 1991;2(6):519–530. | ||
Wachtel-Galor S, Benzie IFF. Herbal medicine: an introduction to its history, usage, regulation, current trends, and research needs. In: Benzie IFF, Wachtel-Galor S, editors. Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed. Boca Raton: CRC Press/Taylor & Francis; 2011:1–10. | ||
Damery S, Gratus C, Grieve R, et al. The use of herbal medicines by people with cancer: a cross-sectional survey. Br J Cancer. 2011;104(6):927–933. | ||
Rugo H, Shtivelman E, Perez A, et al. Phase I trial and antitumor effects of BZL101 for patients with advanced breast cancer. Breast Cancer Res Treat. 2007;105(1):17–28. | ||
Perez AT, Arun B, Tripathy D, et al. A phase 1B dose escalation trial of Scutellaria barbata (BZL101) for patients with metastatic breast cancer. Breast Cancer Res Treat. 2010;120(1):111–118. | ||
Saif MW, Li J, Lamb L, et al. First-in-human phase II trial of the botanical formulation PHY906 with capecitabine as second-line therapy in patients with advanced pancreatic cancer. Cancer Chemother Pharmacol. 2014;73(2):373–380. | ||
Shao J, Gong G, Trombetta L. An evidence-based perspective of Hedyotis diffusa or Oldenlandia diffusa (spreading Hedyotis) for cancer patients. In: Cho WCS, editor. Evidence Based Anticancer Material Medica. Berlin: Springer; 2011:179–192. | ||
Ling B, Dong Q, Sun W, et al. Evaluating the anticancer activity of Hedyotis diffusa water extract against human breast cancer MCF7 cells. Open Nat Prod J. 2013;6(1):1–4. | ||
Dong Q, Ling B, Gao B, Maley J, Sammynaiken R, Yang J. Hedyotis diffusa water extract diminished the cytotoxic effects of chemotherapy drugs against human breast cancer MCF7 cells. Nat Prod Commun. 2014;9:699–700. | ||
Li C, Zhao Y, Guo Z, Zhang X, Xue X, Liang X. Effective 2D-RPLC/RPLC enrichment and separation of micro-components from Hedyotis diffusa Willd and characterization by using ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J Pharm Biomed Anal. 2014;99:35–44. | ||
Ye L, Jia Y, Ji KE, et al. Traditional Chinese medicine in the prevention and treatment of cancer and cancer metastasis. Oncol Lett. 2015;10(3):1240–1250. | ||
Chen MW, Chen WR, Zhang JM, Long XY, Wang YT. Lobelia chinensis: chemical constituents and anticancer activity perspective. Chin J Nat Med. 2014;12(2):103–107. | ||
Han SR, Lv XY, Wang YM, et al. A study on the effect of aqueous extract of Lobelia chinensis on colon precancerous lesions in rats. Afr J Tradit Complement Altern Med. 2013;10(6):422–425. | ||
Santosa MH, Herzog R, Voelter W. Antitumor activity of the hot water extract of Lobelia chinensis. Planta Med. 1986;(6):555. | ||
Yang S, Shen T, Zhao L, et al. Chemical constituents of Lobelia chinensis. Fitoterapia. 2014;93:168–174. | ||
Wang CK, Lin YF, Tai CJ, et al. Integrated treatment of aqueous extract of Solanum nigrum-potentiated cisplatin- and doxorubicin-induced cytotoxicity in human hepatocellular carcinoma cells. Evid Based Complement Alternat Med. 2015;2015:675270. | ||
Tai CJ, Wang CK, Tai CJ, et al. Aqueous extract of Solanum nigrum leaves induces autophagy and enhances cytotoxicity of cisplatin, doxorubicin, docetaxel, and 5-fluorouracil in human colorectal carcinoma cells. Evid Based Complement Alternat Med. 2013;2013:514719. | ||
Lai YJ, Tai CJ, Wang CW, et al. Anti-cancer activity of solanum nigrum (AESN) through suppression of mitochondrial function and epithelial-mesenchymal transition (EMT) in breast cancer cells. Molecules. 2016;21(5):pii E553. | ||
Li J, Li Q, Feng T, Li K. Aqueous extract of Solanum nigrum inhibit growth of cervical carcinoma (U14) via modulating immune response of tumor bearing mice and inducing apoptosis of tumor cells. Fitoterapia. 2008;79(7–8):548–556. | ||
Wang HC, Wu DH, Chang YC, Li YJ, Wang CJ. Solanum nigrum Linn. water extract inhibits metastasis in mouse melanoma cells in vitro and in vivo. J Agric Food Chem. 2010;58(22):11913–11923. | ||
Ding X, Zhu F, Yang Y, Li M. Purification, antitumor activity in vitro of steroidal glycoalkaloids from black nightshade (Solanum nigrum L.). Food Chem. 2013;141(2):1181–1186. | ||
Yang MY, Hsu LS, Peng CH, Shi YS, Wu CH, Wang CJ. Polyphenol-rich extracts from Solanum nigrum attenuated PKC alpha-mediated migration and invasion of hepatocellular carcinoma cells. J Agric Food Chem. 2010;58(9):5806–5814. | ||
Li J, Li QW, Gao DW, Han ZS, Lu WZ. Antitumor and immunomodulating effects of polysaccharides isolated from Solanum nigrum Linne. Phytother Res. 2009;23(11):1524–1530. | ||
Heo KS, Lee SJ, Ko JH, Lim K, Lim KT. Glycoprotein isolated from Solanum nigrum L. inhibits the DNA-binding activities of NF-kappaB and AP-1, and increases the production of nitric oxide in TPA-stimulated MCF-7 cells. Toxicol In Vitro. 2004;18(6):755–763. | ||
Jeong JB, De Lumen BO, Jeong HJ. Lunasin peptide purified from Solanum nigrum L. protects DNA from oxidative damage by suppressing the generation of hydroxyl radical via blocking fenton reaction. Cancer Lett. 2010;293(1):58–64. | ||
Ling B, Gao B, Yang J. Evaluating the effects of tetrachloro-1,4-benzoquinone, an active metabolite of pentachlorophenol, on the growth of human breast cancer cells. J Toxicol. 2016;2016:8253726. | ||
Kuo YJ, Yang JS, Lu CC, Chiang SY, Lin JG, Chung JG. Ethanol extract of Hedyotis diffusa willd upregulates G0/G1 phase arrest and induces apoptosis in human leukemia cells by modulating caspase cascade signaling and altering associated genes expression was assayed by cDNA microarray. Environ Toxicol. 2015;30(10):1162–1177. | ||
Li M, Hou XF, Zhang J, Wang SC, Fu Q, He LC. Applications of HPLC/MS in the analysis of traditional Chinese medicines. J Pharm Anal. 2011;1(2):81–91. | ||
Jensen PH, Juhler RK, Nielsen NJ, et al. Potato glycoalkaloids in soil-optimising liquid chromatography-time-of-flight mass spectrometry for quantitative studies. J Chromatogr A. 2008;1182(1):65–71. | ||
Lo JA, Fisher DE. The melanoma revolution: from UV carcinogenesis to a new era in therapeutics. Science. 2014;346(6212):945–949. | ||
Swalwell H, Latimer J, Haywood RM, Birch-Machin MA. Investigating the role of melanin in UVA/UVB- and hydrogen peroxide-induced cellular and mitochondrial ROS production and mitochondrial DNA damage in human melanoma cells. Free Radic Biol Med. 2012;52(3):626–634. | ||
Kamenisch Y, Baban TS, Schuller W, et al. UVA-irradiation induces melanoma invasion via enhanced Warburg effect. J Invest Dermatol. 2016;136(9):1866–1875. | ||
Sander CS, Hamm F, Elsner P, Thiele JJ. Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br J Dermatol. 2003;148(5):913–922. | ||
Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51(3):794–798. | ||
Gidanian S, Mentelle M, Meyskens FL Jr, Farmer PJ. Melanosomal damage in normal human melanocytes induced by UVB and metal uptake – a basis for the pro-oxidant state of melanoma. Photochem Photobiol. 2008;84(3):556–564. | ||
Lin X, Zheng W, Liu J, et al. Oxidative stress in malignant melanoma enhances tumor necrosis factor-α secretion of tumor-associated macrophages that promote cancer cell invasion. Antioxid Redox Signal. 2013;19(12):1337–1355. | ||
Joosse A, De Vries E, van Eijck CH, Eggermont AM, Nijsten T, Coebergh JW. Reactive oxygen species and melanoma: an explanation for gender differences in survival? Pigment Cell Melanoma Res. 2010;23(3):352–364. | ||
Finn L, Markovic SN, Joseph RW. Therapy for metastatic melanoma: the past, present, and future. BMC Med. 2012;10:23. | ||
Azzabi A, Hughes AN, Calvert PM, et al. Phase I study of temozolomide plus paclitaxel in patients with advanced malignant melanoma and associated in vitro investigations. Br J Cancer. 2005;92(6):1006–1012. | ||
Bafaloukos D, Tsoutsos D, Kalofonos H, et al. Temozolomide and cisplatin versus temozolomide in patients with advanced melanoma: a randomized phase II study of the Hellenic Cooperative Oncology Group. Ann Oncol. 2005;16(6):950–957. | ||
Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18(1):158–166. | ||
Alrwas A, Papadopoulos NE, Cain S, et al. Phase I trial of biochemotherapy with cisplatin, temozolomide, and dose escalation of nab-paclitaxel combined with interleukin-2 and interferon-α in patients with metastatic melanoma. Melanoma Res. 2014;24(4):342–348. | ||
Beasley GM, Speicher P, Augustine CK, et al. A multicenter phase I dose escalation trial to evaluate safety and tolerability of intra-arterial temozolomide for patients with advanced extremity melanoma using normothermic isolated limb infusion. Ann Surg Oncol. 2015;22(1):287–294. | ||
Kaufmann R, Spieth K, Leiter U, et al. Temozolomide in combination with interferon-alfa versus temozolomide alone in patients with advanced metastatic melanoma: a randomized, phase III, multicenter study from the dermatologic cooperative oncology group. J Clin Oncol. 2005;23(35):9001–9007. | ||
Sharma G, Tyagi AK, Singh RP, Chan DC, Agarwal R. Synergistic anti-cancer effects of grape seed extract and conventional cytotoxic agent doxorubicin against human breast carcinoma cells. Breast Cancer Res Treat. 2004;85(1):1–12. | ||
Xu WW, Li B, Lai ET, et al. Water extract from Pleurotus pulmonarius with antioxidant activity exerts in vivo chemoprophylaxis and chemosensitization for liver cancer. Nutr Cancer. 2014;66(6):989–998. | ||
Chen NC, Chyau CC, Lee YJ, Tseng HC, Chou FP. Promotion of mitotic catastrophe via activation of PTEN by paclitaxel with supplement of mulberry water extract in bladder cancer cells. Sci Rep. 2016;6:20417. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.