Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Evaluating Risk of Incident Diabetes Between Patients Who Used Lovastatin and Red Yeast Rice Prescriptions (LipoCol Forte): A Retrospective Cohort Study Based on a Real-World Database
Authors Chen TL, Lin CS , Lin JA , Yeh CC, Sung LC , Chang YC, Shih CC, Liao CC
Received 19 July 2019
Accepted for publication 9 December 2019
Published 9 January 2020 Volume 2020:13 Pages 89—98
DOI https://doi.org/10.2147/DMSO.S223833
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Ta-Liang Chen,1,2,* Chao-Shun Lin,2–4 Jui-An Lin,1,2 Chun-Chieh Yeh,5,6 Li-Chin Sung,7 Yi-Cheng Chang,8 Chun-Chuan Shih,9,10,* Chien-Chang Liao2–4,11,12
1Department of Anesthesiology, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan; 2Department of Anesthesiology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan; 3Department of Anesthesiology, Taipei Medical University Hospital, Taipei, Taiwan; 4Anesthesiology and Health Policy Research Center, Taipei Medical University Hospital, Taipei, Taiwan; 5Department of Surgery, China Medical University Hospital, Taichung, Taiwan; 6Department of Surgery, University of Illinois, Chicago, IL, USA; 7Division of Cardiology, Department of Internal Medicine, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan; 8Division of Endocrinology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan; 9School of Chinese Medicine for Post-Baccalaureate, College of Medicine, I-Shou University, Kaohsiung, Taiwan; 10Ph.D. Program in Clinical Drug Development of Herbal Medicine, College of Pharmacy, Taipei Medical University, Taipei, Taiwan; 11Research Center of Big Data and Meta-Analysis, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan; 12School of Chinese Medicine, College of Chinese Medicine, China Medical University, Taichung, Taiwan
*These authors contributed equally to this work
Correspondence: Chien-Chang Liao
Department of Anesthesiology, Taipei Medical University Hospital, 252 Wuxing St., Taipei 11031, Taiwan
Tel +886-2-2737-2181, ext. 8310
Fax +886-2-2736-7344
Email [email protected]
Objective: This study aimed to evaluate the risk of incident diabetes between people who used lovastatin and red yeast rice (RYR) prescriptions.
Methods: A retrospective cohort study was performed to analyze the real-world database of Taiwan’s National Health Insurance. We identified the RYR cohort, which included 34,504 persons age 20 years or older who began their use of a RYR prescription in 2010–2014. A comparison cohort of 34,504 adults beginning the use of lovastatin was selected from the same dataset, which was matched by age and sex. Both cohorts had no diabetes before the use of the medications. Events of incident diabetes in 2000–2015 were ascertained from medical claims. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) of incident diabetes associated with the use of RYR prescriptions were calculated.
Results: The incidences of diabetes for the RYR cohort and the lovastatin cohort were 1.01 and 2.59 per 100 person-years, respectively (P < 0.0001). Compared with the lovastatin cohort, the adjusted HR of incident diabetes was 0.46 (95% CI 0.43–0.50) for people who used RYR prescriptions. The association between reduced incident diabetes and use of RYR prescriptions was significant in various subgroups. There was a dose-response relationship between RYR prescriptions and the reduced risk of incident diabetes.
Conclusion: We raised the possibility that people who used RYR prescriptions may have a lower risk of incident diabetes compared with the lovastatin cohort.
Keywords: diabetes, lovastatin, red yeast rice, risk
Introduction
Due to the increasing number of diabetes patients and its economic burden all over the world,1,2 identifying the risk factors and methods to prevent diabetes has become urgent. Although statin (hydroxymethylglutaryl-CoA reductase inhibitor) therapy is considered a relatively safe and well-tolerated method to effectively improve blood lipid levels and reduce cardiovascular events,3 meta-analyses of randomized trials and cohort studies have suggested that statin therapy slightly increases the risk of new-onset diabetes.4–9 An increased incidence of diabetes with statin therapy by providing evidence of a dose-dependent association has also been investigated.5 Nevertheless, it was widely accepted that the clinical practice in patients with cardiovascular risk or existing cardiovascular disease should not change the treatment plan of using statins.5,10
In the clinical setting of traditional Chinese medicine, red yeast rice (RYR) has been frequently prescribed by physicians to improve blood lipid levels in patients with hyperlipidemia, and its therapeutic effects have been investigated.11,12 Monacolin K (lovastatin) is one of the important components of scientifically processed RYR prescriptions (such as Xuezhikang, HypoCol, and LipoCol Forte) that has been proven to effectively lower levels of total cholesterol and low-density lipoprotein cholesterol.13–15 Although the tolerance and safety of RYR use has been reviewed and studied,16 the risk of diabetes in patients who used RYR has not been adequately evaluated.
There are many studies reporting the risk of diabetes in patients who used lovastatin, but the findings are controversial.6,7,17–22 Some studies suggested lovastatin therapy increased the risk of incident diabetes,6,17–19 while other studies did not find a significant association.7,20,21 Another study suggested a reduced risk of diabetes in patients who used lovastatin.22 However, information was unavailable on the comparison of the risk of incident diabetes between people who used lovastatin and RYR prescriptions. Using reimbursement claims data from Taiwan’s National Health Insurance Program, we conducted a national retrospective cohort study with a real-world database to evaluate the risk of incident diabetes in people who used lovastatin and RYR prescriptions.
Methods
Source of Data
Our study was conducted using reimbursement claims data from Taiwan’s National Health Insurance Program. This insurance program was started in 1995 and currently covers more than 99% of Taiwan’s population (approximately 23 million people). The research data of Taiwan’s National Health Insurance Program is a real-world database record of all the inpatient and outpatient medical services of beneficiaries as well as basic demographics, primary and secondary medical diagnoses, treatment procedures, prescribed medications and medical expenditures. This database has been evaluated previously,23,24 and research articles based on it have been accepted in prominent scientific journals worldwide.15,25
Ethical Approval
For the protection of personal privacy, the reimbursement claims of insurance data used in this study were electronic information that was decoded with regard to patient identification for further academic access. Although the Health and Welfare Data Science Center, Ministry of Health and Welfare exempt such uses from informed consent, because patient identification was decoded and scrambled, this study was reviewed and approved by the Institutional Review Board of Taipei Medical University (TMU-JIRB-201905042; TMUJIRB-201902053; TMUJIRB-201710033).
Study Design
In this real-world database including a retrospective cohort of 23 million insured individuals, we identified 34,504 patients age 20 years or older, with their first use of RYR prescriptions between 2010 and 2014, and without a history of diabetes before the date of RYR use as the RYR cohort. During the same index period, 34,504 people age 20 years or older were matched by age and sex (with case-control ratio=1:1), and they had no history of diabetes before the date of lovastatin use as the lovastatin cohort. Patients with any diagnosis (including primary and secondary diagnoses during inpatient and outpatient care) of diabetes before the use of RYR prescriptions or lovastatin were excluded to ensure that all study participants were free of diabetes at the start of both cohorts. Follow-up started from the use of medication (RYR prescriptions or lovastatin) and lasted until censoring due to death, loss to follow-up, or other causes by December 31, 2015. Therefore, no immortal time bias exists in this study. Those who used RYR prescriptions and lovastatin in the same period were also excluded. We sought to evaluate the risk of incident diabetes between people who used lovastatin and RYR prescriptions during the follow-up period.
Measures and Definitions
By the definition of our previous study,15 we defined people who visited clinics of traditional Chinese medicine and received a physician’s prescription for RYR (LipoCol Forte) under the coverage of Taiwan’s National Health Insurance Program. Unlike biochemical medications, the Chinese herbal medicine LipoCol Forte is an all-natural RYR extract that has been covered by Taiwan’s National Health Insurance Program since 2010. Both physicians of conventional medicine and physicians of Chinese medicine can prescribe LipoCol Forte. Each capsule of LipoCol Forte contains 600 mg RYR, including the index component Monacolin K (lovastatin) 5.7 mg. Under a physician’s standard directions, patients with hyperlipidemia (total cholesterol ≥200 mg/dl and/or low-density lipoprotein cholesterol ≥130 mg/dl and/or triglyceride ≥200 mg/dl) take 2 capsules per day.
Patients’ low income status was defined by qualifying for waived medical copayment as verified by the Ministry of Health and Welfare, Taiwan. We used physicians’ diagnoses and the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) to define coexisting medical conditions and the main outcome of diabetes (ICD-9-CM 250). People who had one visit, two visits, and three visits related to outpatient care for diabetes were defined as diabetes cases in model 1, model 2, and model 3, respectively. In model 4, people admitted for inpatient care of diabetes were defined as diabetes cases. In model 5, people who used antidiabetic drugs were defined as diabetes cases. Coexisting medical conditions were determined from medical claims during the follow-up period and included hypertension (ICD-9-CM 401–405), mental disorders (ICD-9-CM 290–319), chronic obstructive pulmonary disease (ICD-9-CM 490, 491, 496), ischemic heart disease (ICD-9-CM 410–414), heart failure (ICD-9-CM 428), hyperlipidemia (ICD-9-CM 272.0, 272.1, 272.2, 272.4), stroke (ICD-9-CM 430–438), and liver cirrhosis (ICD-9-CM 571.2, 571.5, 571.6). Renal dialysis was defined by administration codes of the insurance program (D8 and D9). Charlson comorbidity index, emergency visits, and number of hospitalizations were also considered important factors of personal medical conditions in this study.
Statistical Analysis
Chi-square tests were used to compare sociodemographic characteristics and coexisting medical conditions, Charlson comorbidity index, emergency visits, and number of hospitalizations between people who used RYR prescriptions and lovastatin. We calculated the hazard ratios (HRs) with 95% CIs for risk of incidence diabetes between the RYR cohort and the lovastatin cohort by adjusting for age, sex, low income, hypertension, mental disorders, hyperlipidemia, ischemic heart disease, heart failure, liver cirrhosis, chronic obstructive pulmonary disease, and renal dialysis in the multivariate Cox proportional hazard models. We conducted sensitivity analyses with different definitions of incident diabetes to calculate the corresponding adjusted HRs of incident diabetes associated with the use of RYR prescriptions. The risk of incident diabetes between people who used RYR prescriptions and lovastatin were also calculated in the subgroups analyses by age, sex, Charlson comorbidity index, number of medical conditions, emergency visits, and hospitalizations.
Results
The baseline characteristics of people who used RYR prescriptions and lovastatin before matching are shown in Table S1. After frequency matching, compared with the lovastatin cohort (Table 1), the RYR cohort had lower proportions of low income (P < 0.0001), ≥3 hospitalizations (P < 0.0001), ≥3 emergency visits (P < 0.0001), hypertension (P < 0.0001), ischemic heart disease (P < 0.0001), liver cirrhosis (P = 0.0286), stroke (P < 0.0001), heart failure (P = 0.0023), and renal dialysis (P < 0.0001). However, high proportions of mental disorders (P < 0.0001), chronic obstructive pulmonary disease (P < 0.0001), and Charlson comorbidity index scores of 0 (P < 0.0001) were found in the RYR cohort compared with the lovastatin cohort. The proportions of use of anti-hypertensive drug (P < 0.0001) and anticoagulant (P < 0.0001) were lower in RYR cohort than in lovastatin cohort.
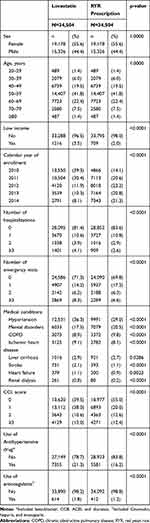 |
Table 1 Baseline Characteristics of People Use RYR Prescription or Lovastatin |
In model 1 (Table 2), people who used RYR prescriptions had a lower risk of incident diabetes compared with people who used lovastatin (HR 0.51, 95% CI 0.49–0.52). The corresponding adjusted HRs of RYR prescriptions associated with incident diabetes in model 2, model 3, and model 4 were 0.49 (95% CI 0.47–0.51), 0.48 (95% CI 0.46–0.50), and 0.30 (95% CI 0.25–0.37), respectively. In model 5, a lower incidence of diabetes (use of antidiabetic drugs) was found in the RYR cohort compared with the lovastatin cohort (1.01 vs 2.59 per 100 person-years, P < 0.0001) during the follow-up period. People who used RYR prescriptions had a lower risk of incident diabetes than those who used lovastatin (HR 0.46, 95% CI 0.43–0.50). In Figure 1, the Kaplan-Meier model shows that the diabetes-free probability in patients who used RYR prescriptions is higher than in patients who used lovastatin (log rank test, P < 0.0001).
 |
Table 2 Risk of Incident Diabetes Between People with Use of RYR Prescription and Lovastatin During the Follow-Up Period |
 |
Figure 1 Kaplan–Meier model for measuring the diabetes-free probability in patients with hyperlipidemia used lovastatin and RYR prescription (log rank test, P < 0.0001). |
The HRs of RYR prescription associated with incident diabetes risk were 0.46 (95% CI0.43–0.50), 0.45 (95% CI 0.42–0.49), and 0.45 (95% CI 0.41–0.48) for excluding incidentdiabetes cases in the one month, three months, and six months of initial follow-up period, respectively.
Under the definition of diabetes as the use of antidiabetic drugs, Table 3 shows that the reduced HRs for incident diabetes associated with RYR prescriptions are 0.46 (95% CI 0.42–0.51) in women and 0.46 (95% CI 0.42–0.51) in men. The association between RYR prescriptions and the reduced risk of incident diabetes was significant in the following age groups: 20–39 (HR 0.32, 95% CI 0.22–0.47), 40–49 (HR 0.34, 95% CI 0.27–0.42), 50–59 (HR 0.46, 95% CI 0.41–0.52), 60–69 (HR 0.48, 95% CI 0.42–0.55), and ≥70 (HR 0.56, 95% CI 0.48–0.66) years. RYR prescriptions were associated with a reduced risk of incident diabetes in subgroups of people with: 0 medical conditions (HR 0.52, 95% CI 0.48–0.57), 1 medical condition (HR 0.38, 95% CI 0.33–0.44), 2 medical conditions (HR 0.43, 95% CI 0.32–0.57), ≥3 medical conditions (HR 0.58, 95% CI 0.37–0.90), 0 emergency visits (HR 0.45, 95% CI 0.42–0.49), 1 emergency visit (HR 0.35, 95% CI 0.27–0.46), ≥2 emergency visits (HR 0.78, 95% CI 0.61–0.99), 0 hospitalizations (HR 0.48, 95% CI 0.44–0.52), 1 hospitalization (HR 0.27, 95% CI 0.20–0.36), and ≥2 hospitalizations (HR 0.70, 95% CI 0.55–0.91). In the subgroups of people with Charlson comorbidity index scores of 0 (HR 0.64, 95% CI 0.58–0.71), Charlson comorbidity index scores of 1 (HR 0.29, 95% CI 0.25–0.34), Charlson comorbidity index scores of 2 (HR 0.32, 95% CI 0.24–0.43), and Charlson comorbidity index scores ≥3 (HR 0.47, 95% CI 0.36–0.62), a reduced risk of incident diabetes was also associated with RYR prescriptions.
 |
Table 3 The Stratified Analysis by Age, Sex, and Medical Conditions for the Association Between Risk of Incident Diabetes and RYR Prescription |
The characteristics of patients with use of RYR prescription and lovastatin before matching were shown in Table S1. The adjusted HRs of incident diabetes were associated with cumulative consumption of RYR prescriptions ≤60 capsules (HR 0.63, 95% CI 0.59–0.68), 61–120 capsules (HR 0.18, 95% CI 0.13–0.24), 121–180 capsules (HR 0.16, 95% CI 0.11–0.25), and ≥181 capsules (HR 0.08, 95% CI 0.05–0.11) during the follow-up period (Table S2). For people who used RYR prescriptions <500 mg, 500–999 mg, 1000–1499 mg, and ≥1500 mg per month on average during the follow-up period, the HRs of incident diabetes were 0.68 (95% CI 0.63–0.74), 0.08 (95% CI 0.05–0.13), 0.09 (95% CI 0.05–0.15), and 0.19 (95% CI 0.16–0.23), respectively, compared with the lovastatin cohort.
Discussion
This nationwide retrospective cohort study is the first study to compare the risk of incident diabetes between people who used RYR prescriptions and lovastatin during the follow-up period. The results showed that use of RYR was probably associated with a reduced risk of incident diabetes, with significant findings regardless of age, gender, or medical conditions. There was a dose-response relationship between RYR prescriptions and the reduced risk of incident diabetes.
In this study, we used some methods and procedures to analyze a real-world database for comparing the risk of incident diabetes in patients who used RYR prescriptions and lovastatin. To reduce the confounding bias, we used multivariate Cox proportional hazard regression to adjust for the potential confounders (age, sex, low income, medical conditions, emergency visits, hospitalizations, and Charlson comorbidity index) before validating the risk of incident diabetes between the RYR cohort and the lovastatin cohort. To validate new-onset diabetes cases, we conducted sensitivity analyses, which included various definitions of incident diabetes according to antidiabetic drugs and physician’s diagnosis of diabetes during outpatient or inpatient medical visits. To evaluate reliability, the stratified analyses were performed to present the significant association between the use of RYR and reduced risk of incident diabetes in several subgroups. To avoid immortal time bias in the RYR cohort and the lovastatin cohort, we calculated the person-years start from the date of intake of medication until the end of study during the follow-up period. By calculating cumulative consumption of RYR prescriptions, we tried to evaluate the possible dose-response relationship between the use of RYR and reduced diabetes risk. However, it was possible that healthy worker effects may have existed in this dose-response relationship, and our study was an observational study that could not provide solid evidence. In previous studies,13–15 RYR prescriptions had no dose-response effects on improving blood lipids and perioperative outcomes.
Cumulative evidence suggested a slightly increased diabetes risk in patients who used statins.4–9 However, how statins increase the risk of diabetes is not completely understood. Some possible mechanisms were suggested by previous studies.26–28 It was suggested that statin treatment decreased ubiquinone (CoQ10) status and cytochrome oxidase activity, which could independently alter insulin secretion.26–28 Statins can also inhibit calcium mediated pancreatic insulin release and decrease expression of the β cell glucose transporters GLUT-2 and GLUT-4.28 Because the therapeutic benefits of decreasing lipids are much more than the scant side effects, patients with cardiovascular risk or existing cardiovascular disease should not change a treatment plan involving the use of statins.5 Individuals with hypercholesterolemia who fail to take their prescribed statin experience a substantially increased risk of fatal stroke.10
Although some studies suggested that RYR was relatively safe,11,13,29 the potential side effects of RYR, such as muscle symptoms, central nervous system complaints, and diabetes, are essentially the same as those for statins.30 However, the risks of diabetes or increased fasting glucose in patients who used RYR were not adequately evaluated previously. Some clinical trials used the combination of RYR and other nutraceuticals, such as garlic, berberine,31,32 chitosan,31 olive,33 phytosterols,34,35 and policosanols;32 thus, the side effects of RYR could not be investigated independently. In addition, it is also not easy to predict or understand the side effect risks of RYR because of the high variability in monacolin content in various commercial RYR dietary supplements.36–38 Unlike the dietary supplement, it was suggested that the content of RYR prescriptions with good manufacturing practice were relatively stable and safe, such as Xuezhikang, HypoCol, and LipoCol Forte.13–15,29 A clinical trial suggested that there were no differences between the HypoCol group and the placebo group with regard to glucose tolerance.13 In a multicenter clinical trial, Xuezhikang decreased fasting blood glucose in patients with or without hyperlipidemia compared to baseline.29 However, these two clinical trials were designed to investigate beneficial effects of lowering blood lipids and did not focus on the risk of incident diabetes in patients who used RYR.
Mechanisms regarding the lower risk of incident diabetes in patients who used RYR are still unclear. There were two possible explanations. First, people with hyperlipidemia who choose RYR prescriptions (with a visit to a traditional Chinese medicine practitioner) may have better knowledge, attitudes and practices regarding disease prevention and health promotion;39 these factors could also contribute to the reduced incidence of diabetes. Second, RYR was effective against obesity-related inflammation, insulin resistance, and nonalcoholic fatty liver disease in mice, irrespective of monacolin K levels.40 This animal study provided some potential evidence to support the hypothesis that the risk of incident diabetes was lower in people who used RYR prescriptions than in people who used lovastatin. However, the findings of our study could not be fully explained by the above reasons. The monacolin K in RYR prescriptions contains gamma-aminobutyric acid and various monacolins, phytosterols, and isoflavones,11 and very little is known about the effects of these constituents on the fasting glucose. RYR prescriptions should be further evaluated carefully before their use as one of the alternative treatments for the management of dyslipidemia in the clinical setting of conventional medicine, although the evidence for the use of RYR prescriptions is in dyslipidemia versus other clinical conditions.11–16
Our study is mainly limited by the unavailable information of the adherence of patients to RYR prescriptions and lovastatin. Although the high validity of Taiwan’s National Health Insurance Program was evaluated,23,24 we did not understand whether the study subjects took all of the medications. Second, because RYR prescriptions (LipoCol Forte) being covered by Taiwan’s National Health Insurance Program since 2010, we could not evaluate the long-term risk of incident diabetes in people who used RYR prescriptions. Third, the incident diabetes and coexisting medical conditions were identified according to the physician’s diagnosis during patients’ visits for outpatient care and/or inpatient care. It is possible that people who had diabetes did not seek medical care. Therefore, we could not exclude the possibility that some people with diabetes were not identified in this study. Fourth, more than 30% of the participants are older than 60 years old who may need to take more than one medication and these medications may alter the level of blood glucose. It is also a study limitation that we could not consider all medications use of participants in this study. In addition, detailed information of socioeconomics, lifestyle (such as smoking and alcohol drinking), physical activity, eating habit, biochemical measures (such as fasting sugar and lipid level), and the severity of comorbid disease and were not available from the insurance database. Therefore, we could not evaluate the effects of these factors on the association between RYR prescriptions and reduced risk of diabetes. The unavailable data of knowledge, attitude, and practice regarding disease prevention were also limitations. Finally, while we have adjusted for various potential confounders in the multiple regression models, residual confounding could not be excluded.
In summary, we raised the possibility that people who used RYR prescriptions may have a lower risk of incident diabetes compared with people who used lovastatin. The reduced risk of incident diabetes varied within people with levels of cumulative consumption of RYR prescriptions. However, we could not infer the causality from the current results because of this study’s limitations. The medication compliance of patients and undiagnosed diabetes were the main limitations that need to be cautiously considered when interpreting our data. Large multicenter trials with long-term evaluation are needed to provide solid evidence for the relationship between RYR prescriptions and incident risk of diabetes. We do not suggest that patients with hyperlipidemia use RYR prescriptions in the clinical setting of conventional medicine unless its safety is acceptable.
Abbreviations
ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; CI, confidence interval; HR, hazard ratio; RYR, red yeast rice.
Author Contributions
TLC, CCL: conception and design, analysis and interpretation of the data, drafting the article, critical revision of the manuscript for important intellectual content and final approval of the version to be published. CSL, JAL, CCY, LCS, YCC, CCS: conception and design, interpretation of the data, critical revision of the manuscript for important intellectual content and final approval of the version to be published. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–1530. doi:10.1016/S0140-6736(16)00618-8
2. American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–928. doi:10.2337/dci18-0007.
3. Hebert PR, Gaziano JM, Chan KS, et al. Cholesterol lowering with statin drugs, risk of stroke, and total mortality: an overview of randomized trials. JAMA. 1997;278(4):313–321. doi:10.1001/jama.1997.03550040069040
4. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–742. doi:10.1016/S0140-6736(09)61965-6
5. Preiss D, Seshasai, SRK, Welsh, P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305(24):2556–2564. doi:10.1001/jama.2011.860
6. Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med. 2012;172(2):144–152. doi:10.1001/archinternmed.2011.625
7. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610. doi:10.1136/bmj.f2610
8. Danaei G, García Rodríguez LA, Fernandez Cantero O, et al. Statins and risk of diabetes: an analysis of electronic medical records to evaluate possible bias due to differential survival. Diabetes Care. 2013;36(5):1236–1240. doi:10.2337/dc12-1756
9. Corrao G, Ibrahim B, Nicotra F, et al. Statins and the risk of diabetes: evidence from a large population-based cohort study. Diabetes Care. 2014;37(8):2225–2232. doi:10.2337/dc13-2215
10. Herttua K, Martikainen P, Batty GD, et al. Poor adherence to statin and antihypertensive therapies as risk factors for fatal stroke. J Am Coll Cardiol. 2016;67(13):1507–1515. doi:10.1016/j.jacc.2016.01.044
11. Becker DJ, Gordon RY, Halbert SC, et al. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med. 2009;150(12):830–839. doi:10.7326/0003-4819-150-12-200906160-00006
12. Yang CW, Mousa SA. The effect of red yeast rice (Monascus purpureus) in dyslipidemia and other disorders. Complement Ther Med. 2012;20(6):466–474. doi:10.1016/j.ctim.2012.07.004
13. Bogsrud MP, Ose L, Langslet G, et al. HypoCol (red yeast rice) lowers plasma cholesterol: a randomized placebo controlled study. Scand Cardiovasc J. 2010;44(4):197–200. doi:10.3109/14017431003624123
14. Moriarty PM, Roth EM, Karns A, et al. Effects of Xuezhikang in patients with dyslipidemia: a multicenter, randomized, placebo-controlled study. J Clin Lipidol. 2014;8(6):568–575. doi:10.1016/j.jacl.2014.09.002
15. Chen TL, Yeh CC, Lin CS, et al. Effects of red yeast rice prescription (LipoCol Forte) on adverse outcomes of surgery. QJM. 2019;112(4):253–259. doi:10.1093/qjmed/hcy278
16. Fogacci F, Banach M, Mikhailidis DP, et al. Safety of red yeast rice supplementation: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2019;143:1–16. doi:10.1016/j.phrs.2019.02.028
17. Olotu BS, Shepherd MD, Novak S, et al. Use of statins and the risk of incident diabetes: a retrospective cohort study. Am J Cardiovasc Drugs. 2016;16(5):377–390. doi:10.1007/s40256-016-0176-1
18. Ma T, Chang M-H, Tien L, et al. The long-term effect of statins on the risk of new-onset diabetes mellitus in elderly Taiwanese patients with hypertension and dyslipidaemia: a retrospective longitudinal cohort study. Drugs Aging. 2012;29(1):45–51. doi:10.2165/11597250-000000000-00000
19. Lee J, Noh Y, Shin S, et al. Impact of statins on risk of new onset diabetes mellitus: a population-based cohort study using the Korean National Health Insurance claims database. Ther Clin Risk Manag. 2016;12:1533–1543. doi:10.2147/TCRM
20. Thakker D, Nair S, Pagada A, et al. Statin use and the risk of developing diabetes: a network meta-analysis. Pharmacoepidemiol Drug Saf. 2016;25(10):1131–1149. doi:10.1002/pds.v25.10
21. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615–1622. doi:10.1001/jama.279.20.1615
22. Ma T, Tien L, Fang C-L, et al. Statins and new-onset diabetes: a retrospective longitudinal cohort study. Clin Ther. 2012;34(9):1977–1983. doi:10.1016/j.clinthera.2012.08.004
23. Cheng C-L, Lee C-H, Chen P-S, et al. Validation of acute myocardial infarction cases in the national health insurance research database in taiwan. J Epidemiol. 2014;24(6):500–507. doi:10.2188/jea.JE20140076
24. Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236–242. doi:10.1002/pds.v20.3
25. Liao CC, Lin CS, Shih CC, et al. Increased risk of fracture and postfracture adverse events in patients with diabetes: two nationwide population-based retrospective cohort studies. Diabetes Care. 2014;37(8):2246–2252. doi:10.2337/dc13-2957
26. Duncan AJ, Hargreaves IP, Damian MS, et al. Decreased ubiquinone availability and impaired mitochondrial cytochrome oxidase activity associated with statin treatment. Toxicol Mech Methods. 2009;19(1):44–50. doi:10.1080/15376510802305047
27. Schaars CF, Stalenhoef AF. Effects of ubiquinone (coenzyme Q10) on myopathy in statin users. Curr Opin Lipidol. 2008;19(6):553–557. doi:10.1097/MOL.0b013e3283168ecd
28. Sampson UK, Linton MF, Fazio S. Are statins diabetogenic? Curr Opin Cardiol. 2011;26(4):342–347. doi:10.1097/HCO.0b013e3283470359
29. Fang Y, Li W. Effect of Xuezhikang on lipid metabolism and islet b cell function in type II diabetes mellitus patients. J Capital Med. 2000;7(2):44–45.
30. Thompson PD, Panza G, Zaleski A, et al. Statin-associated side effects. J Am Coll Cardiol. 2016;67(20):2395–2410. doi:10.1016/j.jacc.2016.02.071
31. Spigoni V, Aldigeri R, Antonini M, et al. Effects of a new nutraceutical formulation (berberine, red yeast rice and chitosan) on non-HDL cholesterol levels in individuals with dyslipidemia: results from a randomized, double blind, placebo-controlled study. Int J Mol Sci. 2017;18(7):7. doi:10.3390/ijms18071498
32. Marazzi G, Cacciotti L, Pelliccia F, et al. Long-term effects of nutraceuticals (berberine, red yeast rice, policosanol) in elderly hypercholesterolemic patients. Adv Ther. 2011;28(12):1105–1113. doi:10.1007/s12325-011-0082-5
33. Tshongo Muhindo C, Ahn SA, Rousseau MF, et al. Efficacy and safety of a combination of red yeast rice and olive extract in hypercholesterolemic patients with and without statin-associated myalgia. Complement Ther Med. 2017;35:140–144. doi:10.1016/j.ctim.2017.10.014
34. Cicero AFG, Fogacci F, Rosticci M, et al. Effect of a short-term dietary supplementation with phytosterols, red yeast rice or both on lipid pattern in moderately hypercholesterolemic subjects: a three-arm, double-blind, randomized clinical trial. Nutr Metab. 2017;14:61. doi:10.1186/s12986-017-0214-2
35. Becker DJ, French B, Morris PB, et al. Phytosterols, red yeast rice, and lifestyle changes instead of statins: a randomized, double-blinded, placebo-controlled trial. Am Heart J. 2013;166(1):187–196. doi:10.1016/j.ahj.2013.03.019
36. Gordon RY, Cooperman T, Obermeyer W, et al. Marked variability of monacolin levels in commercial red yeast rice products: buyer beware. Arch Intern Med. 2010;170(19):1722–1727. doi:10.1001/archinternmed.2010.382
37. Dujovne CA. Red yeast rice preparations: are they suitable substitutions for statins? Am J Med. 2017;130(10):1148–1150. doi:10.1016/j.amjmed.2017.05.013
38. Cohen PA, Avula B, Khan IA. Variability in strength of red yeast rice supplements purchased from mainstream retailers. Eur J Prev Cardiol. 2017;24(13):1431–1434. doi:10.1177/2047487317715714
39. Liao CC, Lin JG, Tsai CC, et al. An investigation of the use of traditional chinese medicine in stroke patients in taiwan. Evid Based Complement Alternat Med. 2012;2012:387164. doi:10.1155/2012/387164
40. Fujimoto M, Tsuneyama K, Chen SY, et al. Study of the effects of monacolin k and other constituents of red yeast rice on obesity, insulin-resistance, hyperlipidemia, and nonalcoholic steatohepatitis using a mouse model of metabolic syndrome. Evid Based Complement Alternat Med. 2012;2012:892697. doi:10.1155/2012/892697
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
