Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Evaluating Patient Preferences of Maintenance Therapy for the Treatment of Chronic Obstructive Pulmonary Disease: A Discrete Choice Experiment in the UK, USA and Germany
Authors Lewis HB, Schroeder M , Gunsoy NB, Janssen EM, Llewellyn S, Doll HA , Jones PW , Ismaila AS
Received 4 July 2019
Accepted for publication 21 February 2020
Published 18 March 2020 Volume 2020:15 Pages 595—604
DOI https://doi.org/10.2147/COPD.S221980
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Hannah B Lewis,1 Melanie Schroeder,2 Necdet B Gunsoy,3 Ellen M Janssen,4 Samuel Llewellyn,5 Helen A Doll,1 Paul W Jones,6 Afisi S Ismaila7,8
1Patient Centred Outcomes, ICON plc., London, UK; 2Value Evidence and Outcomes, GlaxoSmithKline plc., Brentford, UK; 3Value Evidence and Outcomes, GlaxoSmithKline plc., Uxbridge, UK; 4Patient Centred Outcomes, ICON plc., Gaithersburg, MD, USA; 5Patient Centred Outcomes, ICON plc., Abingdon, UK; 6Respiratory Therapy Area, GlaxoSmithKline plc., Brentford, UK; 7Value Evidence and Outcomes, GlaxoSmithKline plc., Collegeville, PA, USA; 8Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, ON, Canada
Correspondence: Afisi S Ismaila
Value Evidence and Outcomes, GlaxoSmithKline plc., 1250 South Collegeville Road, Collegeville, PA 19426-0989, USA
Tel +1 919 932 0430
Email [email protected]
Introduction: With increasing availability of different treatments for chronic obstructive pulmonary disease (COPD), we sought to understand patient preferences for COPD treatment in the UK, USA, and Germany using a discrete choice experiment (DCE).
Methods: Qualitative research identified six attributes associated with COPD maintenance treatments: ease of inhaler use, exacerbation frequency, frequency of inhaler use, number of different inhalers used, side effect frequency, and out-of-pocket costs. A DCE using these attributes, with three levels each, was designed and tested through cognitive interviews and piloting. It comprised 18 choice sets, selected using a D-efficient experimental design. Demographics and disease history were collected and the final DCE survey was completed online by participants recruited from panels in the UK, USA and Germany. Responses were analyzed using mixed logit models, with results expressed as odds ratios (ORs).
Results: Overall, 450 participants (150 per country) completed the DCE; most (UK and Germany, 97.3%; USA, 98.0%) were included in the final analysis. Based on relative attribute importance, avoidance of side effects was found to be most important (UK: OR 11.65; USA: OR 7.17; Germany: OR 11.45; all p< 0.0001), followed by the likelihood of fewer exacerbations (UK: OR 2.22; USA: OR 1.63; Germany: OR 2.54; all p< 0.0001) and increased ease of use (UK: OR 1.84; USA: OR 1.84; Germany: OR 1.60; all p< 0.0001). Number of inhalers, out-of-pocket costs, and frequency of inhaler use were found to be less important. Preferences were relatively consistent across the three countries. All participants required a reduction in exacerbations to accept more frequent inhaler use or use of more inhalers.
Conclusion: When selecting COPD treatment, individuals assigned the highest value to the avoidance of side effects, experiencing fewer exacerbations, and ease of inhaler use. Ensuring that patients’ preferences are considered may encourage treatment compliance.
Keywords: discrete choice experiment, DCE, symptomatic chronic obstructive pulmonary disease, COPD, stated-preference methods
Plain Language Summary
Chronic obstructive pulmonary disease (COPD) treatments can differ widely based on a number of characteristics, for example the number of inhalers required, the number of times a patient needs to use their inhaler per day, associated side effects, and out-of-pocket costs.
Using an online treatment choice survey designed to assess preference for treatment characteristics, this study demonstrates that in patients with COPD in the United Kingdom (UK), United States of America (USA), and Germany, the importance of these characteristics varies. The strongest preference of these patients was for treatments associated with fewer side effects, followed by treatments that reduced the number of COPD exacerbations they were likely to experience per year. Patients also preferred treatments that were easy to use.
The findings from this study may help doctors to understand their patients’ preferences better and prescribe treatment regimens that patients may be more likely to follow. This may have a positive impact on treatment effectiveness.
Introduction
Chronic obstructive pulmonary disease (COPD) is associated with high morbidity and is a common cause of death globally.1 Depending on the severity of COPD, a combination of β2-agonists (which may be short-acting [SABA] or long-acting [LABA]), long-acting muscarinic antagonists (LAMA), and inhaled corticosteroids (ICS) has been shown to improve lung function, symptoms, and health status, and to reduce exacerbations vs ICS/LABA and LAMA monotherapy in patients with COPD and a history of exacerbations.2 While triple combination therapy with ICS, LAMA and LABA has demonstrated benefits over a combination of ICS/LABA or LAMA monotherapy alone,2 patients have, until recently, been required to use at least two inhalers to deliver the three molecules; these three drug classes are now available combined in single-inhaler triple therapy.3–5
On account of the wide variety of existing treatments for COPD, which can differ by dosing regimen, number of required inhalers, and potential side effects, an improved understanding of patient preferences, and what drives these preferences, may help to inform physician prescribing decisions. As part of a wider effort to understand patient preferences in both the United States of America (USA) and Europe, we sought to develop a discrete choice experiment (DCE) for the assessment of patient preferences for characteristics of COPD treatments in the UK, USA, and Germany. A DCE is a quantitative method that allows the relative strength of patient preferences for the attributes of a treatment or product to be compared with respect to each other, and shows how these attributes may impact patient choices.6 Here we present the methodology and results of the DCE.
Methods
Overall Study Design
The development and implementation of the DCE to investigate patient preferences for characteristics of treatment involved four key stages: a qualitative phase based around interviews with COPD patients; a DCE design phase; a pilot survey to test the DCE design; and the final DCE.
Institutional review board (IRB) approval (Salus IRB, Texas, USA) was obtained for research carried out in all three countries prior to commencing recruitment and electronic informed consent was obtained from all participants prior to study initiation.
Qualitative Phase
Initial qualitative research (see Supplementary material) identified six key attributes of COPD treatment for inclusion in the DCE: ease of inhaler use, frequency of exacerbations, frequency of inhaler use (number of times per day), number of different inhalers used, frequency of side effects, and extent of out-of-pocket costs (USA and Germany only) (Table 1). Cost was omitted as an attribute in the UK survey, since patients in the UK pay a standard per-prescription fee, regardless of treatment type;7 therefore, cost factors were not considered to play a role in determining treatment preference for participants in the UK. Three levels were used for each attribute; this was determined based on data derived from clinician and patient interviews, and patient focus groups, along with feedback from respiratory experts and relevant literature.
 |
Table 1 Discrete Choice Experiment (DCE) Attributes and Levels |
Study Population for the Pilot Survey and DCE
Individuals with a self-reported diagnosis of COPD were recruited between February 2017 and February 2018 by Global Perspectives, a patient recruitment agency, for both the initial pilot survey and subsequent DCE. Eligible participants were aged ≥40 years with a diagnosis of moderate-to-severe COPD (COPD Assessment Test [CAT] score ≥10 or modified Medical Research Council [mMRC] Dyspnea Scale score ≥2),8,9 and were prescribed either an ICS/LABA, LAMA/LABA, ICS/LAMA/LABA, or LAMA alone. Participants were residents of the UK, USA, or Germany, fluent in the language of their country of residence, and had access to the internet. Participants were recruited through established channels in each country: in Germany, the recruitment agency used their own patient database, social media, and patient key opinion leaders to recruit; consumer and medical recruiter networks were utilized in the UK, alongside support groups and nurses; in the USA, the recruiters’ proprietary patient database was used.
DCE Design
A D-efficient experimental design6 determining the exact choice sets in the pilot survey was constructed using Ngene software.10 This method was selected to maximize the statistical efficiency in measuring the main effects. One dominant choice question (in which one scenario is objectively better than the other) was included in each survey to check for logical responses. Only participants who answered the logical choice test correctly were included in the final model. Attribute levels were specified as ordinal to minimize the number of dominant choice sets.11 A consistency test was also included in the survey whereby the third or fourth choice set was repeated at the end of the survey to check for consistency in responses.12,13
DCE Testing
Draft versions of the DCE survey were first tested in participants with symptomatic COPD in each country (n=6 per country) by way of cognitive interviews to evaluate general readability of the country-specific DCE surveys and item comprehension. Review of the data from the cognitive interviews confirmed that no major changes to the DCE survey were required; however, a number of simple changes were made. Translations of the levels in each attribute, for example, were reviewed and adjusted to ensure they would not be construed as questions by participants completing the DCE survey in German. Similarly, minor adjustments were made to the wording of the DCE survey to ensure that participants would not expect to be asked qualitative questions regarding their experience of COPD and their medications, and to ensure that patients considered only maintenance therapy in their answers for the survey. Additionally, an example of a DCE choice set was included in the survey to prepare participants for the type of questions they would be asked. Finally, for the data analysis, the reference level for each attribute was changed from the “best” to the “worst” between the pilot and the final analysis, to allow for more intuitive interpretation in the main analysis.
Pilot Survey
To confirm the feasibility of the DCE and refine the underlying design, an initial pilot survey was carried out. A total of 54 participants was included in the pilot survey (UK, n=20; USA, n=14; Germany, n=20). Data collected included demographic characteristics and responses to the DCE choice sets. Full details of the analytical methods and results of the pilot survey are provided in the Supplementary material and Supplementary Tables S1–S3.
Final DCE
The final DCE contained 18 choice sets that each participant was required to complete (Supplementary Table S4), including one dominant task to test the logic of their choices. One additional repeated choice set was also included as a consistency check. While the 18 choice sets for the USA and Germany were identical (see Table 2 for an example choice set), the cost attribute was omitted prior to designing the UK survey. A D-efficient design was used to maximize the statistical efficiency in measuring the main effects, with attribute levels specified as ordinal to minimize the number of dominant choice sets (in which one scenario is objectively better than the other). The survey was administered online and included instructions for participants, an example question (choice set, see Supplementary Figure S1), and sociodemographic and medical history questions.
 |
Table 2 Example Discrete Choice Experiment (DCE) Choice Set |
Final DCE Data Analysis
Demographic data were summarized in SAS software, version 9.4, using descriptive statistics (mean, standard deviation [SD] or median, interquartile range [IQR] for continuous variables, and numbers and percentages for categorical variables). The analysis of the DCE was conducted using R statistical software,14 version 1.1.414, with estimates of the impact of each attribute (independent variable) on the participants’ choices (dependent variable) obtained from mixed logit models converted into odds ratios (ORs) and associated 95% confidence intervals (CIs). Exacerbations, frequency, number of inhalers, and out-of-pocket costs were coded as continuous variables. Ease of use and side effects were treated as categorical variables, with the “worst” level used as reference. As an example, greatest frequency of exacerbations was the reference level for the frequency of exacerbations attribute. A sample size of n=150 per country was targeted, based on the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Task Force publication,6 which demonstrated that the precision of parameter estimates increases rapidly at sample sizes less than 150, and flattens out at approximately 300 observations. With a sample size of n=150, separate analyses could be conducted in each of the three countries but formal statistical comparisons were not made between countries.
Additional analyses were conducted to explore the relative importance of different attributes in the decision-making process, the extent to which different individual characteristics (eg, age, gender, and severity of illness) influence the strength of these preferences, and the marginal rate of substitution (MRS), or how much of one attribute individuals are willing to give up for changes in another. The relative importance of each attribute was calculated by determining the difference between the minimum and maximum coefficients of each attribute.15 To estimate the relative attribute importance as a percentage, the difference was divided by the sum of the differences between all the coefficients of all the attributes. Using these data, a ranking was provided for the attributes. An exploratory analysis was also conducted to assess preference heterogeneity by incorporating additional interaction terms between attributes or individual characteristics and an attribute. The MRS for out-of-pocket costs and number of exacerbations was calculated (details provided in the Supplementary material) using the results from a multinomial logit model. The MRS was used to provide a measure of (1) the reduction in exacerbations required to accept a treatment with a higher number of required inhalers or a higher number of uses per day, and (2) the overall cost reduction required to accept a treatment with a higher number of required inhalers, a higher frequency of use per day or an increase in exacerbations.
Results
Overall, 450 participants took part in the DCE (UK, n=150; USA, n=150; Germany, n=150). Participants were split fairly evenly between males and females (56% vs 44%, respectively) and had a median age of 64 years, with a median time since diagnosis of approximately 6 years (Table 3; additional baseline characteristics and treatment history are presented in Supplementary Tables S5 and S6, respectively). All participants had been receiving maintenance treatment for approximately 3 years; in total, 21% of participants were not using rescue medication at the time of the survey.
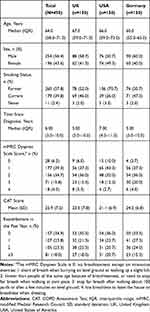 |
Table 3 Participant Characteristics |
Effect of Treatment Attributes on Choice
Results between the conditional logit and mixed logit model were consistent. Here we present the results of the mixed logit model, as per current standard practice, in order to account for unobserved preference heterogeneity. Across all attributes for the mixed logit model (random effects for each attribute), ORs were greater than 1, showing that for an improvement in the attribute level (eg, from “a lot” to “some” side effects) there was an increased likelihood of participants preferring the treatment (Figure 1 and Supplementary Table S7). Of the 150 UK participants, 146 (97%) answered the logical choice test correctly and were included in the final model. The strongest drivers of treatment choice, associated with the highest ORs, were an avoidance of side effects (OR 11.65 [p<0.0001] when participants considered no side effects vs a lot of side effects) and a reduction in the number of exacerbations (OR 2.22 [p<0.0001] for each unit reduction per year; Figure 1 and Supplementary Table S7). Of the 150 USA respondents, 147 (98%) answered the logical choice test correctly and were included in the final model. The strongest drivers of treatment choice were an avoidance of side effects (OR 7.17 [p<0.0001] when participants considered no side effects vs a lot of side effects) and ease of inhaler use (OR 1.84 [p<0.0001] when participants considered no mistakes vs a lot of mistakes; Figure 1 and Supplementary Table S7). Of the 150 German participants, 146 (97%) answered the logical choice test correctly and were included in the final model. The strongest drivers of treatment choice were an avoidance of side effects (OR 11.45 [p<0.0001] when participants considered no side effects vs a lot of side effects) and a reduction in exacerbations (OR 2.54 [p<0.0001] for each unit reduction per year; Figure 1 and Supplementary Table S7).
Assessment of preference heterogeneity within each country showed that the participants’ preferred treatment attributes were relatively similar (Supplementary Table S8). In none of the countries was there a significant interaction between participant age and the ease of inhaler use attribute, the time since diagnosis and the number of side effects experienced attribute, and the current number of inhalers and number of inhalers attribute (Supplementary Table S8). However, a significant interaction was noted in the UK (–0.1175, p=0.0002) and Germany (–0.0908, p=0.0043) between the participants’ exacerbation history and the number of exacerbations attribute, indicating that participants who had experienced more exacerbations in the past assigned less value to a decrease in exacerbations of <2 in the next year.
Attribute Relative Importance
Overall, avoidance of side effects whilst on treatment was ranked as the most important attribute across all three countries (relative importance: UK, 59%; USA, 51%; Germany, 55%), followed by reduction in exacerbations and ease of inhaler use (means of 18% and 14% across countries, respectively). The mean relative importance of number of inhalers, out-of-pocket costs, and frequency of use (number of times per day) was 7%, 5%, and 5%, respectively. While these trends were generally similar between countries, participants in the USA did assign more importance to ease of inhaler use (16%) vs those in the UK (13%) and Germany (11%).
Marginal Rates of Substitution
Participants in the USA and Germany (analysis not applicable to the UK) would require a reduction in out-of-pocket costs if they were required to tolerate additional exacerbations (a 12% and 21% cost reduction per exacerbation per year, respectively). A reduction in out-of-pocket costs would also be required if patients needed to use inhalers more frequently (6% and 3% cost reduction per additional use of inhaler per day, respectively), or if patients were required to use additional inhalers (10% and 5% cost reduction per additional inhaler, respectively; Figure 2).
Regarding the number of exacerbations, participants in the UK, USA, and Germany would also require a reduction in the number of exacerbations per year to accept a higher frequency of inhaler use (a 0.15, 0.50, and 0.15 reduction per additional use per day, respectively), or to accept using more inhalers (a 0.35, 0.81, and 0.26 reduction per additional inhaler, respectively; Figure 3).
 |
Figure 3 Marginal rate of substitution (MRS) for a reduction in exacerbations. Abbreviations: MRS, marginal rate of substitution; UK, United Kingdom; USA, United States of America. |
When presented with an alternative scenario of using a single inhaler once daily compared with the reference scenario of two inhalers twice daily, the probability of participants taking up the alternative scenario was consistent across countries: 59% in the UK and Germany, and 65% in the USA. Participants across all countries would also be willing to accept an increase in the frequency of exacerbations per year to accommodate this switch (UK, 0.46 per year; USA, 1.29 per year; Germany, 0.39 per year) and showed a willingness to pay for a reduction in the frequency and number of inhalers (Table 4).
 |
Table 4 Uptake Probability, Exacerbation Trade-Off and Willingness to Pay for a Switch from Two Inhalers Twice Daily to a Single Inhaler Once Daily |
Discussion
The results of the DCE suggest that, based on relative attribute importance, participants with COPD in the UK, USA, and Germany assign most value to the avoidance of side effects when choosing a treatment, followed by likelihood of fewer exacerbations and ease of inhaler use. While the number of inhalers, out-of-pocket costs, and frequency of inhaler use were also found to be important drivers of choice, they were less important overall. Overall, all attributes of treatment played a role in participants’ choices. The findings also suggest that participants would be willing to accept a cost increase for a regimen that necessitated only a single inhaler once daily, rather than two inhalers twice daily. With an increasing number of treatment options now available, including the recent approval of single-inhaler triple therapies for the treatment of COPD, understanding patient preferences for certain aspects of treatment could be used to inform physicians’ prescribing decisions. Additionally, improved understanding of patient preferences may improve long-term patient adherence to treatment, and therein may improve patient care. Further research to explore this postulation may be beneficial.
Preference heterogeneity, highlighting the variability in participant responses according to certain participant characteristics, was relatively low in each of the three countries. In the UK and Germany, participants who had experienced more exacerbations assigned less importance to treatments which offered fewer than two exacerbations per year compared with participants who had experienced fewer exacerbations. This might be because they have become used to experiencing exacerbations and are confident in how to handle them (or rather that participants who had fewer exacerbations had more concerns regarding their management), although this would not explain why a similar heterogeneity in preferences was not observed in patients in the USA. Little heterogeneity was identified for the interactions between age and the ease of inhaler use attribute, time since diagnosis and the side effects attribute, or current number of inhalers and the number of inhalers attribute in each of the three countries. This suggests that, for example, the age of the participant (younger or older) did not impact on preference for an inhaler that is easier to use. There was some heterogeneity in the importance of avoiding side effects, particularly for participants with different comorbidities since those with more severe comorbidities assigned less value to the avoidance of side effects when stating their treatment preferences. However, this relationship was not consistent for different comorbidities within or across countries. There was also some evidence of preference heterogeneity between current inhaler use and the ease of inhaler use attribute for all countries, although these relationships were also not consistent within or across countries. It is important to note that between-country comparisons are qualitative and exploratory only, and no statistical significance of these country differences was assessed.
The MRS calculations suggest that participants in the USA and Germany would require a reduction in cost if the treatment was associated with additional exacerbations, increased frequency of use, or the need for additional inhalers. Overall, participants in all three countries would be willing to accept an increase in exacerbations if they were required to use their inhaler(s) less often or use fewer devices overall.
The mean CAT score for study participants was 22.9, representing the high clinical impact of COPD on the individuals included.14 While this study has provided novel insights into the treatment preferences of individuals with symptomatic COPD, it does have some limitations regarding the methodology and its assumptions. The DCE did not contain all possible medication characteristics that may influence patient preferences, although our approach aligns with good practice16,17 in the field and captured the most important attributes according to patients. As specific guidance on what constituted a side effect of their treatment was not provided, individual participants may have considered different side effects (eg, sore throat, headache, dry mouth, or oral thrush) when interpreting the attribute “side effects”, with the potential for this to influence the level of preference they assigned to this attribute. Also, as is inherent with this type of analysis, the results of this study can only be interpreted within the context of the attribute levels selected and it is not possible to calculate the effect of additional levels (eg, level of participant preference for avoiding treatments requiring administration >3 times per day) or additional attributes. Participants were recruited through consumer and medical recruitment networks and may have previously volunteered or expressed interest in participating in research. This could create some selection bias, which may limit the generalizability of the findings. The DCE was only hosted online, which could have limited participation for some patients, particularly in the older age range. This is an important consideration, given that COPD is more prevalent in older populations.18,19 As elderly patients are typically underrepresented in clinical trials,20 extrapolation of these DCE findings to older patients with COPD in real-world clinical practice should be approached with caution.
Conclusions
This study suggests that individuals with COPD in the UK, USA, and Germany may place the highest value on the avoidance of side effects, followed by the likelihood of fewer exacerbations and receiving an inhaler which is easy to use, when selecting treatment. The benefit of having to use fewer inhalers and using an inhaler less frequently are also important considerations. Overall, the findings were generally consistent across countries, although participants in the USA appeared more sensitive to changes in treatment burden, including ease of inhaler use, than those in the UK and Germany; further exploration is required to confirm this. Inhaler attributes, as well as efficacy and safety, appear to be relevant for patients. Ensuring patient preferences are met when selecting COPD therapy is important since it may improve treatment compliance and thus effectiveness.
Abbreviations
CAT, COPD Assessment Test; CI, confidence interval, COPD, chronic obstructive pulmonary disease; DCE, discrete choice experiment; DPI, dry-powder inhaler; ICS, inhaled corticosteroid; IQR, interquartile range; IRB, institutional review board; ISPOR, International Society for Pharmacoeconomics and Outcomes Research; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; mMRC, modified Medical Research Council; MRS, marginal rate of substitution; N/A, not applicable; N/D, no data; OR, odds ratio; pMDI, pressurized metered dose inhaler; SABA, short-acting β2-agonist; SD, standard deviation; SMI, soft-mist inhaler; UK, United Kingdom; USA, United States of America.
Data Sharing Statement
Study documents can be requested from www.clinicalstudydatarequest.com.
Acknowledgments
The authors would like to thank the individuals who participated in the study, Global Perspectives (Oviedo, Spain) for participant recruitment, and Karissa Johnston (Broadstreet Data Solutions Inc.) for undertaking the data analysis.
Funding
The study was funded by GlaxoSmithKline plc. (clinicaltrials.gov NCT03046069; GlaxoSmithKline plc. study CTT206455). Editorial support (in the form of writing assistance, collating author comments, assembling tables/figures, grammatical editing, and referencing) was provided by Molly Macpherson, BSc, of Gardiner–Caldwell Communications (Macclesfield, UK), and was funded by GlaxoSmithKline plc. Trade marks are owned by or licensed to their respective owners (the GSK group of companies or AstraZeneca PLC).
Disclosure
The authors declare the following conflicts of interest during the last 3 years in relation to this article: MS, ASI, NBG, and PWJ are employees of, and hold shares in, GlaxoSmithKline plc. ASI is also unpaid faculty at McMaster University, Canada. HBL is an employee of ICON plc. EMJ, SL, and HAD were employees of ICON plc. when the study was conducted. ICON plc. received GlaxoSmithKline plc. funding to conduct this study, but was not paid for the development of this manuscript. The authors report no other conflicts of interest in this work.
References
1. World Health Organization (WHO). Chronic obstructive pulmonary disease (COPD); 2015. http://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd).
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2019 report); 2019. Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf.
3. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
4. Singh D, Corradi M, Spinola M, et al. Triple therapy in COPD: new evidence with the extrafine fixed combination of beclomethasone dipropionate, formoterol fumarate, and glycopyrronium bromide. Int J Chron Obstruct Pulmon Dis. 2017;12:2917–2928. doi:10.2147/COPD
5. Vanfleteren L, Fabbri LM, Papi A, Petruzzelli S, Celli B. Triple therapy (ICS/LABA/LAMA) in COPD: time for a reappraisal. Int J Chron Obstruct Pulmon Dis. 2018;13:3971–3981. doi:10.2147/COPD
6. Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13. doi:10.1016/j.jval.
7. Pharmaceutical Services Negotiating Committee. Dispensing and supply: what does the patient pay? 2019. Available from: https://psnc.org.uk/dispensing-supply/receiving-a-prescription/patient-charges/.
8. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi:10.1136/thx.54.7.581
9. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509
10. ChoiceMetrics. Ngene: user manual and reference guide; 2018. Available from: http://www.choice-metrics.com/NgeneManual120.pdf.
11. Tervonen T, Schmidt-Ott T, Marsh K, Bridges JFP, Quaife M, Janssen E. Assessing rationality in discrete choice experiments in health: an investigation into the use of dominance tests. Value Health. 2018;21(10):1192–1197. doi:10.1016/j.jval.
12. Janssen EM, Marshall DA, Hauber AB, Bridges JFP. Improving the quality of discrete-choice experiments in health: how can we assess validity and reliability? Expert Rev Pharmacoecon Outcomes Res. 2017;17(6):531–542. doi:10.1080/14737167.2017.1389648
13. Johnson FR, Yang J-C, Reed SD. The internal validity of discrete choice experiment data: a testing tool for quantitative assessments. Value Health. 2019;22(2):157–160. doi:10.1016/j.jval.2018.07.876
14. Ejiofor SI, Stolk J, Fernandez P, Stockley RA. Patterns and characterization of COPD exacerbations using real-time data collection. Int J Chron Obstruct Pulmon Dis. 2017;12:427–434. doi:10.2147/COPD.S126158
15. Hauber AB, Gonzalez JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19(4):300–315. doi:10.1016/j.jval.2016.04.004
16. Bridges J, Hauber A, Marshall D, et al. Conjoint analysis applications in health–a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413. doi:10.1016/j.jval.2010.11.013
17. Janssen EM, Segal JB, Bridges JF. A framework for instrument development of a choice experiment: an application to type 2 diabetes. Patient. 2016;9(5):465–479. doi:10.1007/s40271-016-0170-3
18. Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. doi:10.1183/09031936.06.00124605
19. Varmaghani M, Dehghani M, Heidari E, Sharifi F, Moghaddam SS, Farzadfar F. Global prevalence of chronic obstructive pulmonary disease: systematic review and meta-analysis. East Mediterr Health J. 2019;25(1):47–57.
20. Valente S, Pasciuto G, Bernabei R, Corbo GM. Do we need different treatments for very elderly COPD patients? Respiration. 2010;80(5):357–368. doi:10.1159/000320221
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


