Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Evaluating Opicapone as Add-on Treatment to Levodopa/DDCI in Patients with Parkinson’s Disease
Authors Jost WH
Received 11 May 2022
Accepted for publication 23 July 2022
Published 6 August 2022 Volume 2022:18 Pages 1603—1618
DOI https://doi.org/10.2147/NDT.S279362
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Roger Pinder
Wolfgang H Jost
Parkinson-Klinik Ortenau, Wolfach, 77709, Germany
Correspondence: Wolfgang H Jost, Parkinson-Klinik Ortenau, Kreuzbergstraße 12-16, Wolfach, 77709, Germany, Tel +49 7834 971-212, Fax +49 7834 971-340, Email [email protected]
Abstract: COMT (catechol-O-methyltransferase) inhibitors are key therapeutic agents in the management of motor fluctuations (MF) in patients with Parkinson’s disease (PD). As levodopa/DDCI add-on therapy, their main benefit lies in increasing ON-time and reducing OFF-time for PD patients in the middle stages of the disease. Two of the three available COMT inhibitors, tolcapone and entacapone, have been approved for over two decades.
Opicapone, a third-generation COMT inhibitor approved in 2016, was designed with the aim of overcoming specific challenges of the earlier generation compounds, specifically hepatotoxicity and short effect duration. This review aims at highlighting the specific properties and characteristics of opicapone, namely combining efficacy with good tolerability as demonstrated in the registration studies and since then confirmed under real-world conditions. Opicapone has been shown to be effective in patients with early, as well as late motor fluctuations. Whilst patients in the earlier Hoehn and Yahr stages benefit more than patients in later stages, the incidence of dyskinesia in patients with recent onset MF is around half that of patients with more established fluctuations. With the added advantage of a once-daily administration, this particular COMT inhibitor provides a simple, yet effective therapy for patients with Parkinson’s disease and MF.
Keywords: Parkinson’s disease, motor fluctuations, opicapone, entacapone, COMT inhibitor, therapy
Introduction
In drug therapy of Parkinson’s disease (PD), various substance classes and quite a few drugs have been introduced in the last five decades, most of which directly or indirectly improve dopaminergic stimulation. Since the causes of the degenerative process have not yet been definitively determined, symptomatic therapy continues to be used to date. Levodopa remains the lynchpin of drug therapy.2 In order to reduce decarboxylation, levodopa is always given in combination with a dopa decarboxylase inhibitor (DDCI).3
However, motor complications inevitably occur as the disease progresses, and the half-life and duration of levodopa/DDCI continue to converge. Inhibitors of catechol-O-methyltransferase (COMT) have been available as add-on therapy for over 20 years. They improve the availability of levodopa, as COMT becomes the main metabolizing enzyme of levodopa when only DDC is inhibited, catalysing its conversion to 3-O-methyldopa (3-OMD) in the brain and periphery.
The first approved COMT inhibitor was tolcapone. Tolcapone has shown very good efficacy data and is the only COMT inhibitor to date showing central inhibitory properties in addition to peripheral COMT inhibition. Due to its relatively short half-life, it must be taken three times daily. Tolcapone therapy is limited by its side effects, namely drug-induced liver damage, which on rare occasions has led to fulminant hepatitis. In addition, severe diarrhea has also been reported. Therefore, according to EMA approval, tolcapone may only be used in patients who do not respond to or are intolerant of other COMT inhibitors4 and is thus only recommended a “second-line” treatment after entacapone or opicapone.
Entacapone, the second COMT inhibitor to be approved,5 also has a relatively short half-life and therefore must be taken several times a day, usually simultaneously with levodopa. Severe diarrhea has also been observed during therapy with entacapone. In addition, a colour-intensive degradation product is formed during the catabolism of entacapone, with resulting urine discoloration. The latter is considered unpleasant and embarrassing by many patients and caregivers.
In 2016, opicapone, a third-generation COMT inhibitor, was approved as add-on therapy to levodopa/DDCI in adult patients with PD and motor “end-of-dose” fluctuations. It is currently available in Germany, Italy, Portugal, Spain, the UK, Republic of Ireland, Norway, Sweden, Finland, Denmark, Switzerland, Czech Republic, Japan, the USA and South Korea. The recommended dose is 50 mg daily, except for Japan, where 25 mg are recommended.
Opicapone combines several advantages: Its potency is similar to that of tolcapone,6 it only needs to be taken once daily and it provides long-lasting COMT-inhibition due to the high binding affinity and a slow dissociation rate of the opicapone-COMT-complex. After 24 h, COMT activity is only reduced by approx. 65%. It is this long duration of action which leads to a long-lasting stable COMT inhibition and allows once daily dosing.7 The typical COMT-induced diarrhea has not been observed for opicapone, and it does not cause discoloration of the urine. Most importantly, opicapone does not exhibit liver toxicity. Moreover, in studies, opicapone has shown to be more effective than entacapone with regards to parameters such as absolute ON-time gain, less OFF-time, more ON- and OFF-time responders, and clinician’s and patient’s global impression of change (CGI-C, PGI-C).8 A recent meta-analysis concluded that opicapone is superior to both entacapone and tolcapone in terms of efficacy and safety.6
Pharmacokinetics and Pharmacodynamics of Opicapone
Opicapone (2,5-dichloro-3-[5-(3,4-dihydroxy-5-nitro-phenyl]-1,2-4-oxadiazol-3-yl)-4,6-dimethylpyridine-1-oxide) has a pyridine N-oxide in position 1 (Figure 1), which leads to high COMT affinity and also prevents cell toxicity.9,10 At a dosage of 50 mg, the drug has a very high protein binding affinity in plasma (99.9%) and, as a consequence, a low complex dissociation rate constant and a long duration of action (>24 hours) in vivo.11–13 The strong binding of opicapone to plasma proteins remains unaffected even in the presence of warfarin, diazepam, digoxin or tolbutamide.14
 |
Figure 1 Structural formula of opicapone. |
In patients taking levodopa and a peripheral DDCI such as carbidopa or benserazide, opicapone increases plasma levodopa levels, thereby improving clinical response to levodopa. Compared with placebo, opicapone increases the bioavailability of levodopa by up to 55% following single oral administration of either 100/25 mg levodopa/carbidopa or 100/25 mg levodopa/benserazide, taken 12 hours after the opicapone dose. This results in a dose-dependent reduction in OFF-time.15
Pharmacodynamic Profile
Opicapone exhibits higher and longer-lasting efficacy compared to the other COMT inhibitors. Because of this effective, continuous COMT inhibition over 24 hours, the active ingredient only needs to be taken once a day.10,13 This makes COMT inhibition more predictable and has a constant influence on the pharmacokinetics of levodopa.
A comparison of the three COMT inhibitors in animal models showed COMT inhibition of 99% with opicapone versus 82% with tolcapone and 68% with entacapone 1 hour after administration.16 Nine hours after application, there was no COMT inhibition with entacapone and only minimal COMT inhibition of 16% with tolcapone, whereas opicapone continued to achieve a sustained COMT inhibition of 91%.
In healthy subjects, due the long-lasting and consistent COMT inhibition, opicapone (25 and 50 mg) showed higher levodopa bioavailability throughout the day compared with entacapone (Figure 2).13,15 This does not relate to maximum levels, which could increase the risk of dyskinesia, but to the area under the degradation curve (AUC) of levodopa.15
 |
Figure 2 Average profile of plasma levodopa concentration-time at day 12 with subsequent single daily oral administration of 50 mg opicapone (OPC) or placebo for 11 days (days 1 to 11) or concomitant administration of 200 mg entacapone (ENT) or placebo with each levodopa/carbidopa dose (n=16) (mod. from Rocha et al15). *Compared to ENT, OPC 50 mg showed a statistical difference in the increase in levodopa AUC. The comparison of ENT with placebo showed no statistical difference in the extent of exposure to levodopa (after estimation of the AUC). |
Elimination
In healthy volunteers, the elimination half-life (t1/2) of opicapone after repeated once-daily administration of up to 50 mg opicapone was 0.7 to 3.2 hours. The inactive metabolite opicapone sulfate has a long terminal phase with t1/2 of 94 to 122 hours.17
The major route of excretion of opicapone and its metabolites is the feces (70%) and urine (17%). In urine, the major metabolite is the glucuronide metabolite of opicapone, while concentrations of parent compound and other metabolites are generally below the limit of quantitation. Overall, it can be concluded that the kidney plays only a minor role in excretion.18
Pharmacokinetics in Special Patient Populations
No clinically relevant effects are present for opicapone exposure with respect to age or weight. In the range of 40 to 100 kg there is no correlation between opicapone exposure and body weight.19 Pharmacokinetics was studied in healthy volunteers and patients with moderate chronic liver dysfunction after a single dose of 50 mg. The bioavailability of opicapone was significantly higher in the latter. No safety problems were observed.18 Thus, no dose adjustment is generally necessary in moderate chronic hepatic impairment. However, since opicapone is to be given in addition to levodopa therapy, dose adjustments may be considered due to a potentially increased dopaminergic response to levodopa and corresponding tolerability.18 No clinical experience is available in patients with severe hepatic impairment (Child–Pugh class C), and therefore, opicapone is not recommended in this setting.19
The pharmacokinetics of opicapone has not been directly studied in patients with chronic renal impairment. However, an evaluation of kinetic data was performed for 50 mg opicapone in patients included in the pivotal studies who had moderately reduced renal elimination capacity. Plasma levels of the major metabolite of opicapone were unaffected in these patients; thus, no dose adjustment needs to be considered.
Cytotoxicity
Substance-induced liver toxicity is one of the most common reasons for drug non-approval or withdrawal from the market. Because tolcapone, the first COMT inhibitor, rarely has severe liver toxicity which limits the use of the drug, a risk assessment for the remaining COMT inhibitors is of great importance. In an in vitro comparison of hepatotoxic risk, opicapone and entacapone (up to 200 μM) did not affect HepaRG cell viability or caspase activity, nor did they deplete glutathione.9 In contrast, tolcapone (200 μM) significantly damaged cell health and decreased hepatic glutathione levels.
Clinical Studies with Opicapone
several clinical trials.14 These included 28 Phase I studies with more than 900 subjects, two Phase II studies and two Phase III studies.
The Pivotal Studies of Opicapone
Evidence of efficacy and safety and approval of opicapone are based on the two double-blind, placebo-controlled, randomized Phase III BIPARK-I and -II studies.8,20,21 They involved 1027 adult patients with PD treated with levodopa/DDCI (alone or in combination with other anti-parkinsonian drugs) who had end-of-dose motor fluctuations for up to 15 weeks. At screening, the mean age in all treatment groups was comparable in both studies and ranged from 61.5 to 65.3 years. Patients had disease stages I to III in the ON phase (modified Hoehn and Yahr staging), received three to eight levodopa/DDCI doses daily, and had an average daily OFF-time of at least 1.5 hours.
In the BIPARK-I study,8,20 opicapone was evaluated at three doses for efficacy and tolerability in patients with motor fluctuations compared with entacapone and placebo. The primary endpoint was the absolute reduction in OFF-time as assessed by patient diaries, and secondary endpoints were ON- and OFF-time responder rates, ie, the percentage of patients with at least 1 hour of ON or OFF-time change. Additional endpoints included quality of life (Parkinson’s Disease Questionnaire, PDQ-39), global assessment (CGI-C/PGI-C), assessment of non-motor symptoms (Non-Motor Symptoms Scale, NMSS), general course assessment (Unified Parkinson’s Disease Rating Scale, UPDRS), and sleep quality (Parkinson’s Disease Sleep Scale, PDSS).
The study enrolled 590 patients who were randomly allocated in equal proportions to five parallel treatment arms (placebo, entacapone, and 5 mg, 25 mg, or 50 mg opicapone). In total, 542 of the participants completed the study. The patients, aged 30 to 83 years, had had PD for at least 3 years, had been on levodopa for at least 1 year, and were in ON status on the Hoehn and Yahr scale at level 1 to 3. They were OFF for at least 1.5 hours per day, not including morning akinesia before the first levodopa administration. After a 2-week screening period, patients received either a daily single dose of opicapone, placebo, or 200 mg of entacapone with each levodopa dose in addition to their levodopa medication. If the patient received other medications, their dosage remained constant throughout. The actual treatment phase lasted 15 weeks, with the levodopa dosage being individually adjusted during the first 3 weeks of treatment. After these 15 weeks, a 2-week follow-up phase followed.
Opicapone at 50 mg significantly reduced daily OFF-time versus placebo by 1.1 hours (LS hours: least square means). The difference in change in LS mean in OFF-time between entacapone and placebo was −0.7 hours. The difference in change in LS mean in OFF-time between opicapone 50 mg and entacapone was −0.4 hours. Noninferiority of opicapone 50 mg versus entacapone 200 mg was demonstrated (95% confidence interval [95% CI]: −61.4–11.8). Unlike entacapone, both OFF-time reduction and ON-time prolongation were significant with opicapone 50 mg versus placebo. Compared with entacapone, opicapone resulted in a 20% increase in ON-time (in hours per day) (Figure 3).
 |
Figure 3 Effect of 15-week treatment with opicapone (OPC), entacapone (ENT), or placebo (PLC) on the duration of OFF or ON-time. *p<0.05; **p<0.001. Data from Ferreira et al.8 |
Treatment with opicapone also resulted in significant improvement in patients’ global assessments of both, CGI-C and PGI-C, in contrast to the use of entacapone or placebo.
The rate of adverse events in the BIPARK-I study was 50% with placebo and ranged from 52% to 55% with opicapone in a dose-dependent manner. The rate for entacapone was 57%. The most common adverse events experienced with opicapone compared with placebo were dyskinesia (16% vs 4%), insomnia (6% vs 1%), and drowsiness (5% vs 1%). Compared with entacapone, opicapone had a fundamentally similar side effect profile, with entacapone more often resulting in nausea (3% vs 7%). Dyskinesias occurred more frequently with opicapone than with entacapone. Diarrhea was not experienced by any of the patients treated with opicapone.
The BIPARK-II study was another multinational, multicenter, double-blind, placebo-controlled, phase III study of two doses of opicapone.21 Subjects were randomized into three study groups and treated for 14–15 weeks with placebo (n=135), opicapone 25 mg (n=125), or opicapone 50 mg (n=147). As in BIPARK I, the primary endpoint was the change in absolute OFF-time based on patient diary entries.
In both opicapone groups (25 and 50 mg), the mean reduction in absolute OFF-time was significantly greater (1.7 and 2.0 hours, respectively) than in the placebo group (1.1 hours). Despite the good placebo effect, the group receiving 50 mg opicapone showed a significant improvement in OFF-time compared to placebo (p<0.05). In both groups (25 or 50 mg opicapone), significantly more patients achieved the OFF-time responder endpoint compared to placebo (62.4%; p<0.05 and 66.0%, respectively; p<0.05 vs 50.4%), as well as a higher OFF-time reduction (11.0%; p<0.05 and 12.1%, respectively; p<0.05 vs 6.7%). In addition, in both opicapone groups (25 and 50 mg), the mean prolongation of absolute ON-times without or with non-disruptive dyskinesias was significantly greater (1.4 and 1.43 hours, respectively) than in the placebo group (0.8 hours).
A total of 758 patients (placebo n=255, opicapone 25 mg n=241, opicapone 50 mg n=262) were treated in both studies.22,23 Meta-analysis of the two pivotal trials showed that the additional administration of opicapone (25 and 50 mg, respectively) significantly reduced OFF-time (−35.1 and −58.1 minutes vs placebo, respectively; p<0.05 and p<0.0001, respectively) and prolonged ON-time without distressing dyskinesia (42.7 and 64.7 minutes vs placebo, respectively; p<0.05 and p<0.0001, respectively). In addition, a significantly higher proportion of patients on opicapone 25 mg (61.4%; p<0.05) or 50 mg (67.6%; p<0.0001) achieved OFF-time reductions of ≥1 hour compared to placebo (49%). Similar results were obtained for ON-time response rate.22
Open Extension Studies of the Registration Studies
The open-label (OL), 1-year extension phases (BIPARK-I-OL and BIPARK-II-OL), in which the treatment of the double-blind studies was continued, confirmed the maintenance of the effect of opicapone achieved in the double-blind study phases in 862 patients.24,25 In the open-label studies, treatment was initiated in all patients in the first week (7 days) with a dose of 25 mg opicapone, regardless of their pretreatment in the double-blind phase. If motor “end-of-dose” fluctuations could not be adequately controlled and tolerability permitted, the opicapone dose could be increased to 50 mg. If unacceptable adverse dopaminergic events occurred, the levodopa dose was to be adjusted.
The one-year BIPARK-I-OL open-label study enrolled 495 patients after completion of the double-blind (DB) phase.24 The primary end point was the change in absolute OFF-time based on patient diary entries. Statistical analysis was performed by a linear mixed-effects model of repeated measures. P-values were calculated post hoc.
In the DB phase, a reduction in OFF-time of more than 2 hours (−127 minutes) was achieved compared to baseline. At the end of the OL-phase, the mean OFF-time had decreased by a further 34 minutes compared to the start of the OL phase. Patients who had been switched from placebo and entacapone to opicapone showed a significant reduction in OFF-time (placebo −65 minutes, p<0.0001; entacapone −39 minutes, p<0.05) and a significant increase in ON-time without dyskinesia (placebo 43 minutes, p<0.05; entacapone 46 minutes, p<0.05). Patients who had previously received opicapone still showed improvements in OFF and ON-time, but without statistical significance (Figure 4).
 |
Figure 4 Significant reduction in OFF-time when patients switched from placebo or entacapone to opicapone in an open-label extension study of the BIPARK I trial. Data from Ferreira et al.24 |
The BIPARK-II-OL study used the NMSS to evaluate the efficacy of opicapone on non-motor symptoms.26 The mean NMSS scores at the beginning of the DB phase of the BIPARK-II trial were 36.7 in the opicapone 50 mg group and 38.2 in the opicapone 25 mg and placebo groups, respectively. At the end of the DB phase, the total NMSS score had improved slightly for all groups (opicapone: −2.02 [25 mg] and −4.9 [50 mg], respectively, −5.2 [placebo]). Differences between groups were not significant. Numerical differences in favor of opicapone were seen for the sleep/fatigue domain. At the end of the one-year OL period, the mean score on the NMSS total scale had improved by −4.2. No deterioration was observed in any domain.
Subgroup Analyses on Syndrome-Relevant Questions
Efficacy in Patients with Early Morning off
To investigate the impact of opicapone and entacapone on early morning OFF (EMO), which can affect up to 80% of patients, diary data from 235 patients stratified by hour of day were analysed in a post hoc analysis of the BIPARK I study.27–29 At baseline, less than 15% of these patients with end-of-dose motor fluctuations awoke in the ON state and the time to ON was more than 1 hour. EMO patterns were present between 6 and 8 am at this time.
Treatment with opicapone resulted in a greater increase in the proportion of patients who awoke in the ON state compared with treatment with entacapone. In addition, opicapone shortened the time to ON onset from the first-morning levodopa intake by 17.7%, whereas entacapone shortened it by only 1.9%. Because morning OFF-time was significantly reduced in patients treated with opicapone, no EMO patterns were detected, in contrast to entacapone.
Efficacy in Patients at Different Stages of Disease
The efficacy of opicapone was also investigated in the BIPARK-I and II trials stratified by disease stage.30,31 This showed that patients in early Hoehn and Yahr (H and Y) stages benefited more than patients in later stages. For example, the time in OFF under opicapone vs placebo was reduced by 82.1 minutes (p<0.0001) in patients in H and Y stages <2.5. Patients in H and Y stages ≥2.5 also benefited from opicapone. However, here the OFF-time reduction vs placebo was only 41.1 minutes (p<0.05).
Similar results were seen with respect to disease duration. Patients with disease duration <8 years achieved a reduction in OFF-time of 66.7 minutes (p<0.0001) versus placebo. For disease duration ≥8 years, the reduction in OFF-time versus placebo was lower at 47.4 minutes (p<0.05).
Efficacy with Various Doses of Levodopa
The efficacy of opicapone was evaluated in a post hoc analysis of the two pivotal trials in combination with different levodopa dosing regimens (300–400, 400–500, and 500–600 mg/day).31,32 Overall, this evaluation demonstrates the efficacy of opicapone 50 mg for all levodopa dosing regimens: the decrease in OFF-time was at least twice as high as with placebo in each case, regardless of the levodopa dosing regimen (Figure 5).
 |
Figure 5 OFF-time reduction under opicapone vs placebo at different levodopa dosing regimens (300–400, 400–500, and 500–600 mg/day). Data from LeWitt et al.32 |
Efficacy in Patients with Recent Motor Fluctuations (<1 Year)
According to another post hoc analysis of the BIPARK I and II trials, opicapone 50 mg achieved significant superiority over placebo in reducing OFF-time and prolonging ON-time in patients with both recent (<1 year) and prolonged (>1 year) motor fluctuations (Figure 6).31,33 At the same time, the incidence of dyskinesia in patients with recent motor fluctuations (RMF) was approximately half of that seen in patients with longer-standing motor fluctuations (LMF, Figure 7).
 |
Figure 6 Efficacy of opicapone vs placebo in patients with recent motor fluctuations (RMF, <1 year) and with long-standing motor fluctuations (LMF > 1 year). Data from Ebersbach et al.33 |
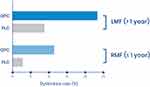 |
Figure 7 Rate of dyskinesia on opicapone vs placebo in patients with recent (<1 year) and with long-standing motor fluctuations (>1 year). Data from Ebersbach et al.33 |
Within the subgroup of patients with recent motor fluctuations, the efficacy of opicapone 50 mg versus entacapone was also evaluated in a group of patients who were not pretreated with a COMT inhibitor.34 This post hoc analysis included 97 COMT inhibitor-naive patients randomized to opicapone 50 mg (n=50) or entacapone (n=47). Opicapone 50 mg was found to significantly increase absolute ON-time by 64 minutes more than entacapone in this patient subgroup (124.0 vs 60.0 minutes, p<0.05) (Figure 8).
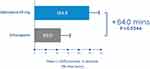 |
Figure 8 Change in absolute ON-time in patients with recently diagnosed (<1 year) motor fluctuations treated with opicapone 50 mg and entacapone from baseline to endpoint. Data from Ferreira et al.34 |
Efficacy in Combination with DA or MAO-B Inhibitors
Another post hoc analysis of the BIPARK I and II trials investigated the efficacy of opicapone in the subgroup of patients who had levodopa/DDCI and in addition a dopamine agonist (DA) or a monoamine oxidase B (MAO-B) inhibitor as baseline treatment.31,35
Of the total group, 521 patients (69%) had received an additional DA (placebo n=185, 25 mg opicapone n=158, 50 mg opicapone n=178) and 151 patients (20%) had received an additional MAO-B inhibitor (placebo n=49, 25 mg opicapone n=46, 50 mg opicapone n=56). Pramipexole was the most common used DA (34.5%), followed by ropinirole (26.8%). Of the MAO-B inhibitors, rasagiline was the most common used (12.5%), followed by selegiline (7.7%).
In patients receiving 50 mg opicapone in combination with a DA/levodopa/DDCI as baseline treatment, OFF-time was reduced by 54.6 minutes (p<0.05) compared to baseline. In patients with additional 50 mg opicapone to a MAO-B inhibitor/levodopa/DDCI as baseline treatment, the OFF-time reduction was −63.7 minutes (p<0.05). The incidence of adverse events with opicapone plus DA/levodopa/DDCI (59.6%) or opicapone plus MAO-B inhibitor/levodopa/DDCI (73.3%) was comparable to the results for the entire patient population in the Phase III trials.
Opicapone in Elderly Patients
PD mainly affects older people and the incidence increases with age, making it the second most common neurodegenerative disease in Europe. Therefore, the combined data of the BIPARK I and II studies were also analysed in relation to the age of the participants (<70 and >70 years).36 In total, data from 221 patients older than 70 years were analysed (n=69, 66 and 86 for placebo, 25 mg, and 50 mg opicapone, respectively).
The mean OFF-time reduction from baseline for patients aged 70 years and older was −1.41 hours in the placebo group, −1.77 hours in the 25 mg opicapone group (p<0.05 vs placebo) and −2.26 hours in the 50 mg opicapone group (p<0.05 vs placebo). Patients treated with 25 mg and 50 mg opicapone also achieved OFF- and ON-time response (improvement of at least 1 hour) more frequently than patients on placebo (p<0.05 for 50 mg OPC).
Studies After Market Approval
OPTIPARK Study
Approval studies always represent only part of reality. Therefore, it makes sense to investigate the efficacy and tolerability under real conditions after approval. With the OPTIPARK study, this was tested under real-life conditions for opicapone.37 Primary endpoint was the assessment of the treating physicians in the CGI-C. Secondary endpoints included treating patients’ assessment in the PGI-C, the UPDRS, patients’ quality of life in the PDQ-8, and non-motor symptoms in the NMSS.
The study enrolled 502 patients, of whom 386 completed the study. A total of 109 patients (21.5%) left the study. Eighty-four (17.0%) patients left the study because of a TEAE. Of these, 66 (13.3%) had at least one TEAE related to opicapone therapy. A total of 332 patients (69.9%) continued therapy with opicapone after the study.
After 3 months of treatment with opicapone, the majority of patients (71.3%) showed clinical improvement according to the treating physicians. In 43% of the patients, the physicians reported that the clinical impression was very much or much improved (Figure 9). The patients’ assessment was comparable to that of the treating physicians. And, 76.9% of patients noted improvement as a result of treatment. Of these, 48.1% rated the clinical impression as very much or much improved (Figure 9).
 |
Figure 9 Attending physicians’ (CGI-C) and treated patients’ (PGI-C) assessment of treatment success under opicapone in the OPTIPARK study. Data from Reichmann et al.37 |
Opicapone also resulted in improvements in patients’ quality of life and non-motor symptoms under everyday conditions. The mean improvements (± standard deviation) of −3.4 ± 12.8 points in PDQ-8 and −6.8 ± 19.7 points in NMSS were statistically significant (p<0.0001 each). In the NMSS, most subscores remained stable or improved from baseline. For example, there were mean improvements in sleep/fatigue by −1.3 ± 6.3 points (p<0.0001) and in mood/cognition by −1.5 ± 6.82 points (p<0.0001).
Real World Evidence Data
Under “real-world conditions”, 57 patients were followed for 3 months and 20 patients for 12 months after adjustment on opicapone. The results of therapy on the different aspects of PD were measured with the UPDRS and the Wearing-OFF -Questionnaire (WOQ-9).38 The WOQ-9 can be used to assess both motor and non-motor symptoms of Wearing-OFF.
After 3 months of therapy, the median UPDRS total score improved from 44 to 33, followed by a further decrease to 31 after 12 months. In detail, the majority of UPDRS subscores showed significant improvement at both three and 12 months (p<0.05) (Table 1). Wearing-OFF in the WOQ-9 total score improved in 75% of the patients at both three and 12 months (Figure 10), with significant improvement at 3 months in both the total score and the two subscores for motor and non-motor symptoms (p<0.05 each).
 |
Table 1 Improvement of UPDRS Subscores Under Therapy with Opicapone in Day-to-Day Treatment |
 |
Figure 10 (A) The nine domains of the Wearing-OFF Questionnaire (WOQ-9). (B) Improvement in WOQ-9 during therapy with opicapone in day-to-day treatment. Data from Oehlwein et al.38 |
Effect on the Microbiome
Parkinson’s also leads to changes in the composition of the gut microbiota. Not only PD but also drug therapy have an influence on the gut microbiota. Therefore, the influence of medication on the microbiota in patients with PD is becoming increasingly important.39–42
In a previous study, Faecalibacterium prausnitzii was significantly reduced in diseased individuals.43 Faecalibacterium prausnitzii belongs to the butyrate-producing bacteria.44–46 There is evidence that butyrate has anti-inflammatory and other positive properties on the intestinal epithelium.47
Furthermore, it has been shown that COMT inhibitors may also be involved in altering the gut microbiota. Thus, a significant negative correlation between treatment with entacapone and the frequency of Faecalibacterium prausnitzii and butyrate in stool samples was found in Parkinson’s disease patients.43
Therefore, the influence of the three available COMT inhibitors (entacapone, opicapone, tolcapone) on the gut microbiota was investigated.48 Here, it was shown that opicapone and tolcapone – in contrast to entacapone – did not reduce the number of Faecalibacterium prausnitzii (Figure 11) and also did not reduce the concentrations of its putative biologically active metabolite butyrate.
 |
Figure 11 Effect of COMT inhibitors on Faecalibacterium prausnitzii in the gut microbiota. Data from Grün et al.48 |
Safety and Compatibility
The most frequently reported side effects of opicapone in the pivotal studies were nervous system disorders. In the case of dyskinesias and PD symptoms, it should be noted that the usual adjustment of the levodopa dose in this situation was not allowed in the study design.
In a pooled analysis of data from the BIPARK I and II pivotal trials, the safety profiles of 750 patients (n=257 for placebo, n=244 and n=265 for 25 mg and 50 mg opicapone, respectively) were evaluated.22 The most common treatment-emergent adverse events (TEAEs) were directly attributable to dopamine action and other parkinsonian symptoms. Dyskinesias were the most common TEAEs (18.3% opicapone vs 6.2% placebo), but the incidence of severe cases was low (1.2% opicapone vs 0.8% placebo). Other TEAEs included constipation (5.7% vs 1.9%), insomnia (5.1% vs 1.6%), and dry mouth (4.7% vs 1.2%). No dose–response relationship was available for most TEAEs. Serious adverse events were observed in 4.9% of the patients on placebo and in 2% of the patients on opicapone.
No severe diarrhea, myocardial infarction, prostate cancer, melanoma, or liver dysfunction occurred in the opicapone group. Impulse control disorders were reported by <1% of the opicapone-treated patients. Laboratory parameters, vital signs, and ECG values were comparable in both groups.
Adverse events that occurred more frequently with opicapone than with placebo in elderly patients (>70 years) were hallucinations (4.6% vs 0.1%), visual hallucinations (3.8% vs 0.1%), and weight loss (4.6% vs 1.1%). There were fewer serious adverse events in patients on opicapone than in patients on placebo.36
Concomitant medication with opicapone was generally safe and well tolerated. There is no apparent association with any known risks associated with other COMT inhibitors or anti-parkinsonian drugs.
Opicapone has been on the market for several years. No other adverse reactions or risks have occurred during this time. This is also reflected in the listing of the frequency of adverse reactions (MedDRA) in placebo-controlled Phase 3 studies in the Summary of Product Characteristics (Table 2).19
 |
Table 2 Frequency of Adverse Events (MedDRA) in Placebo-Controlled Phase 3 Trials |
Interactions with Other Medicinal Products and Other Warnings
The combination of opicapone with MAO inhibitors could lead to the inhibition of most of the metabolic pathways responsible for the metabolism of catecholamines. Therefore, the concomitant use of opicapone and MAO inhibitors (eg, phenelzine, tranylcypromine, and moclobemide), with the exception of MAO inhibitors used in PD, is contraindicated19 Concomitant use of opicapone and MAO-B inhibitors for the treatment of PD is permitted, eg, opicapone and rasagiline (up to 1 mg/day) or selegiline (up to 10 mg/day in an oral dosage form or 1.25 mg/day in a buccal absorption dosage form). No study data are available on the concomitant use of opicapone with the MAO-B inhibitor safinamide. My personal, clinical experience indicates that no interactions have occurred to date.
Dosage and Method of Use
The recommended dose of opicapone is 50 mg, taken once daily at bedtime, at least 1 hour before or after taking the levodopa/DDCI preparation. If a dose is missed, the next dose should be taken at the scheduled time. The patient should not take a double dose if the previous dose has been forgotten.
In a patient who has wearing-OFF several times a day on levodopa/DDCI, additional administration of opicapone may be considered. If dyskinesias subsequently occur, these can be seen as a sign of the efficacy of the COMT inhibitor, as opicapone potentiates the effect of levodopa. In this case, it may be necessary to adjust the levodopa dosage in the first few days to first few weeks after starting treatment with opicapone.19 The levodopa dose should be reduced in this case.49
In a subgroup of 41 patients in the BIPARK I and II trials, levodopa dose was reduced either because of dopaminergic adverse events (n=30 [73.2%]) or proactively (n=11 [26.8%]).50 The reduction in dose averaged 192 mg/day (23.4%), from 842 mg/day at baseline to 650 mg/day at the end of the adjustment period during the study.
In this subgroup of patients on reduced levodopa dose, mean OFF-time improved by −131.2 minutes and mean ON-time improved by 125.4 minutes versus baseline. The mean changes in UPDRS subscores II and III versus baseline were −3.3 points and −1.7 points, respectively. Quality of life, as measured by the PDQ-39 questionnaire, improved by −2.8 points versus baseline.
Discussion
What are the differences between the two first-line COMT inhibitors entacapone and opicapone?
What is immediately noticeable are the differences in peripheral COMT inhibition. While opicapone shows a COMT inhibition of 99% directly after intake, entacapone inhibits the COMT by 68%.12,13 The increase in peripheral levodopa levels is analogous.51 Entacapone has a short half-life and must therefore be taken with each dose of levodopa.52 Although a combination tablet is available, which makes it easier for the patient to take, peripheral COMT inhibition varies greatly between doses and thus several times a day, whereas the COMT inhibition with opicapone is almost constant throughout the day.51
Of course, this also has its price. Due to the stronger and longer duration of action, dopaminergic stimulation in the upper therapeutic range can also result in dyskinesia and other adverse effects. In the BIPARK studies, the proportion of patients with dyskinesia was higher with opicapone than with entacapone. With RMF, the proportion of patients with dyskinesia was lower than with LMF, due to the larger therapeutic window. This argues for an early use of opicapone.
More advanced patients or those with dementia and high risk of delirium can also be treated with opicapone. However, these patients should be treated with caution to avoid dopaminergic overstimulation. It is advisable to lower the levodopa dose first and then carefully adjust it if necessary, before adjusting to opicapone.
Opicapone has been on the market for 6 years and thus there are many patients treated with opicapone. Opicapone is well tolerated. Most adverse effects can be attributed to the increase in peripheral levodopa levels. Of course, it cannot be ruled out, but it is not to be expected that other, yet unknown, adverse effects will occur.
Conclusion and Outlook
COMT inhibitors are invaluable in the management of MF in patients with PD.
The first ever approved COMT inhibitor, tolcapone, was already so effective that further development of this drug-group would not have been required, had it not been for the very rare cases of drug induced fulminant hepatitis observed shortly after its marketing authorization.
Whilst entacapone has an improved safety profile with regards to liver toxicity, the drawback is reduced COMT-inhibition and efficacy. In addition, sometimes troublesome diarrhea or urine discoloration are treatment-limiting.
In contrast, opicapone combines potent COMT inhibition, in par with tolcapone, with good tolerability. It does not cause diarrhea, does not discolor urine, and has no negative effect on liver function. In addition to the improvement of motor and non-motor symptoms, positive effects on quality of life were shown. Long-lasting COMT-inhibition has the added advantage of once-daily administration, allowing for tailored levodopa therapy. Furthermore, as the single daily dose is taken in the evening, sleep architecture is improved through increased mobility during sleep.
In the pivotal trials, opicapone reduced OFF-time and increased ON-time compared to placebo and entacapone. Furthermore, opicapone also showed positive effects on early-morning-Off (EMO), which affects up to 80% of the patients with PD and can be very distressing.
Since first gaining market authorization, the efficacy of opicapone was further investigated in post hoc analyses of the BIPARK studies with particular focus on relevant subgroups. Here, patients in an early H and Y stage or with a shorter disease duration were shown to benefited more from treatment with opicapone, than patients in a later H and Y stage or with a longer disease duration. Superiority of opicapone over placebo was also observed in patients receiving low doses of levodopa (<300-400 mg/day): Those with recent and those with longer-standing fluctuations. For COMT naïve patients recently diagnosed with MF, On-time was significantly increased in those receiving opicapone compared to those receiving entacapone.
Once levodopa has been introduced, the goal of any therapy adjustment is to increase dopamine bioavailability, smoothen dopamine levels, and restore continuous dopaminergic stimulation to achieve the best possible quality of life, ideally without inducing dyskinesias. The therapeutic window for levodopa is wider during the earlier stages of the disease and conversely, the incidence of dykinesias is reduced. It is therefore perhaps unsurprising that the incidence of dyskinesia in patients receiving opicapone or placebo, was about twice as high in patients with longstanding MF compared to those with recent MF.
Bearing in mind the above, commencing opicapone at the onset of MF is a realistic and reasonable choice with clear advantages for both patient and practitioner. Patients have now entered the middle stages of the disease and typically receive 3–4 levodopa intakes per day. By adding opicapone, the established treatment regimen can be maintained over a long period of time, and only the levodopa dose needs to be adjusted as the disease progresses further. This provides patients with a simple and very effective therapy in the middle stages of Parkinson’s disease, when putative becomes difficult and complicated.
Whilst opicapone has been shown to increase the bioavailability of levodopa as well as to smoothe levodopa serum levels,53 Bial is currently conducting a phase II pharmacokinetics study (“203 study”)54 to investigate this further in the context of different levodopa/carbidopa-sparing treatment regimens.
Furthermore, to demonstrate the benefit of early use of opicapone in patients with recent motor fluctuations, the ADOPTION study is currently underway. This is a randomized, prospective, open-label, Phase IV pilot study. Patients with 3–4 daily oral doses of levodopa and evidence of Wearing-Off (<2 years) will be included and receive either opicapone or 100 mg levodopa. Primary endpoint is change in Off-time from baseline.55,56
Pulsatile stimulation is considered to be a factor contributing to the damage of dopaminergic neurons. Following their demise, the therapeutic window for levodopa becomes smaller and smaller with time. In both BIPARK studies, the fluctuation index was decreased in patients treated with opicapone.53 Thus, opicapone may have neuroprotective properties by directly reducing pulsatile stimulation of dopaminergic neurons. To investigate this aspect further in patients in the early stages of PD, the EPSILON study is currently underway. Patients with idiopathic PD and 3–4 daily oral doses of levodopa – but without motor complications – are included. In this two-arm, double-blind, randomized, Phase III study, patients receive either opicapone or placebo. The primary endpoint is the change in MDS-UPDRS Part III from baseline.57,58
In summary, opicapone combines excellent efficacy with good tolerability and has contributed to a substantial improvement in the treatment of PD with regards to motor as well as non-motor symptoms. Patients treated with opicapone achieved clinical improvement in symptoms and showed a significant increase in quality of life.
Acknowledgement
The author would like to thank Ms. Eiden and Drs. Rückert and Kemmer, who helped with the literature research, the creation of the figures and the translation.
Disclosure
The author occasionally acts as a consultant and speaker for Bial. The author reports no other conflicts of interest in this work.
References
1. S3 guideline idiopathic Parkinson’s disease. AWMF-Register-Nummer: 030–010; 2016. https://www.awmf.org/uploads/tx_szleitlinien/030-010k_S3_Parkinson_Syndrome_Idiopathisch_2016-06-abgelaufen.pdf. Accessed July 19, 2022.
2. Oertel WH, Berardelli A, Bloem BR, et al. Early (uncomplicated) Parkinson’s disease. Eur Handb Neurol Manag. 2011;1:217–236.
3. Tambasco N, Romoli R, Calabresi P. Levodopa in Parkinson’s disease: current status and future developments. Curr Neuropharmacol. 2018;16(8):1239–1252. doi:10.2174/1570159X15666170510143821
4. SmPC Tolcapone. Available from: https://www.ema.europa.eu/en/documents/product-information/tasmar-epar-product-information_en.pdf.
5. SmPC Entacapone. Available from: https://www.ema.europa.eu/en/documents/product-information/entacapone-orion-epar-product-information_en.pdf.
6. Song Z, Zhang J, Xue T, et al. Different catechol-O-methyl transferase inhibitors in Parkinson’s disease: a Bayesian network meta-analysis. Front Neurol. 2021;12. doi:10.3389/fneur.2021.707723
7. Rocha F, Almeida L, Falcão A, et al. Opicapone: a short lived and very long-acting novel catechol- O-methyltransferase inhibitor following multiple dose administration in healthy subjects. Br J Clin Pharmacol. 2013;76(5):763–775. doi:10.1111/bcp.12081
8. Ferreira JJ, Lees A, Rocha JF, et al. Opicapone as an adjunct to levodopa in patients with Parkinson’s disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial. Lancet Neurol. 2016;15(2):154–165. doi:10.1016/S1474-4422(15)00336-1
9. Bonifacio MJ, Sousa F, Loureiro A, et al. Evaluation of potential mechanisms of cellular toxicity by nitrocatechol COMT inhibitors: opicapone, entacapone and tolcapone
10. Kiss LE, Ferreira HS, Torrao L, et al. Discovery of a long-acting, peripherally selective inhibitor of catechol-O-methyltransferase. J Med Chem. 2010;53(8):3396–3411. doi:10.1021/jm1001524
11. Palma PN, Bonifacio MJ, Loureiro AI, et al. Computation of the binding affinities of catechol-O-methyltransferase inhibitors: multisubstrate relative free energy calculations. J Comput Chem. 2012;33(9):970–986. doi:10.1002/jcc.22926
12. Almeida L, Rocha JF, Falcao A, et al. Pharmacokinetics, pharmacodynamics and tolerability of opicapone, a novel catechol-O-methyltransferase inhibitor, in healthy subjects: prediction of slow enzyme-inhibitor complex dissociation of a short-living and very long-acting inhibitor. Clin Pharmacokinet. 2013;52(2):139–151. doi:10.1007/s40262-012-0024-7
13. Rocha JF, Almeida L, Falcao A, et al. Opicapone: a short lived and very long-acting novel catechol-O-methyltransferase inhibitor following multiple dose administration in healthy subjects. Br J Clin Pharmacol. 2013;76(5):763–775.
14. ClinicalTrails.gov, NCT02169440. Effect of BIA 9–1067 on the pharmacokinetics and pharmacodynamics of Warfarin. Available from: https://clinicaltrials.gov/ct2/show/NCT02169440. Accessed July 19, 2022.
15. Rocha JF, Falcao A, Santos A, et al. Effect of opicapone and entacapone upon levodopa pharmacokinetics during three daily levodopa administrations. Eur J Clin Pharmacol. 2014;70(9):1059–1071. doi:10.1007/s00228-014-1701-2
16. Bonifacio MJ, Torrao L, Loureiro AI, Wright LC, Soares-da-Silva P. Opicapone: characterization of a novel peripheral long acting catechol-O-methyltransferase inhibitor. Parkinsonism Relat Disord. 2012;18(suppl2):S125.
17. Falcao A, Rocha JF, Santos A, et al. Opicapone pharmacokinetics and pharmacodynamics comparison between healthy Japanese and matched white subjects. Clin Pharmacol Drug Dev. 2016;5(2):150–161. doi:10.1002/cpdd.213
18. Rocha JF, Santos A, Falcao A, et al. Effect of moderate liver impairment on the pharmacokinetics of opicapone. Eur J Clin Pharmacol. 2014;70(3):279–286. doi:10.1007/s00228-013-1602-9
19. SmPC Opicapon. https://www.ema.europa.eu/en/documents/product-information/ongentys-epar-product-information_en.pdf.
20. Ferreira J, Lees A, Rocha JF, et al. Opicapone ́s efficacy in Parkinson ́s disease patients with motor fluctuations: a Phase III, randomised, double- blind, placebo and active-controlled study - BIPARK I study.
21. Lees A, Ferreira JJ, Costa R, et al. Efficacy and safety of opicapone, a new COMT inhibitor, for the treatment of motor fluctuations in Parkinson’s disease patients: BIPARK-II study [abstract no. 1038]. J Neurol Sci. 2013;333(suppl 1):e116. doi:10.1016/j.jns.2013.07.391
22. Ferreira JJ, Lees A, Santos A, et al. Pooled efficacy analysis of opicapone as adjunctive therapy to levodopa in patients with Parkinson’s disease and motor fluctuations.
23. Ferreira JJ, Lees A, Rocha JF, et al. Long-term efficacy of opicapone in fluctuating Parkinson’s disease patients: a pooled-analysis of data from two phase 3 clinical trials and their open-label extensions. Eur J Neurol. 2019;3:1331–1468.
24. Ferreira JJ, Lees A, Tolosa E, et al. Switching double-blind opicapone, entacapone or placebo to open-label opicapone: efficacy results of the 1-year extension of study BIPARK I. Mov Disord. 2016;31(suppl 2):S633.
25. Costa R, Oliveira C, Pinto R, et al. One-year open-label efficacy and safety of opicapone in Parkinson’s disease BIPARK-II study. Mov Disord. 2014;29(suppl1):S233.
26. Oliveira C, Lees A, Ferreira J, et al. Opicapone and non-motor symptoms in Parkinson ́s disease: results from double-blind, randomized, placebo-controlled study and open-label extension. Mov Disord. 2015;30(Suppl1):p173.
27. Lees A, Rascol O, Ferreira JJ, et al. Effect of opicapone and entacapone on daily pattern of motor fluctuations in Parkinson’s disease patients. Mov Disord. 2020;35(suppl 1):
28. Videnovic A, Poewe W, Lees A, et al. Effect of opicapone and entacapone on early morning-OFF pattern in Parkinson’s disease patients with motor fluctuations. Mov Disord. 2020;35(suppl1):
29. Katzenschlager R, Ferreira JJ, Chaudhuri KR, et al. What to do when the honeymoon wears OFF. CNS. 2022;7(2):1–32.
30. Lopes N, Ferreira J, Lees A, et al. Efficacy of opicapone in Parkinson’s disease patients with motor fluctuations at different stages of symptom progression. Eur J Neul. 2016;23(suppl 1):601–879.
31. Rocha F, Ebersbach G, Lees A, et al. The added benefit of opicapone when used early in parkinson’s disease patients with levodopa-induced motor fluctuations: a post-hoc analysis of BIPARK-I and -II. Front Neurol. 2021;12(Article):754016. doi:10.3389/fneur.2021.754016
32. LeWitt P, Stocchi F, Ferreira JJ, et al. Efficacy of opicapone at different levodopa regimens up to a threshold of 600 mg/day levodopa in Parkinson’s Disease patients with motor fluctuations. Neurology. 2020;88(suppl 25):
33. Ebersbach G, Rascol O, Ferreira JJ, et al. Efficacy and safety of opicapone in Parkinson’s disease patients according to duration of motor fluctuations: post-hoc analysis of BIPARK-I and II. Mov Disord. 2020;35(suppl 1):
34. Ferreira F, Lees A, Ebersbach G, et al. Efficacy of opicapone compared to entacapone in catechol-O-methyltransferase inhibitor-naïve Parkinson’s disease patients recently diagnosed with motor fluctuations: a post-hoc conservative analysis. Mov Disord. 2020;35(suppl 1):
35. Antonini A, Ebersbach G, Rascol O, et al. Efficacy of opicapone in different treatment regimens in Parkinson’s disease patients with motor fluctuations. Park Relat Disord. 2020;79:e64–e65. doi:10.1016/j.parkreldis.2020.06.239
36. Lees A, Ferreira J, Lopes N, et al. Efficacy and safety of opicapone in patients over 70 years with Parkinson’s disease and motor fluctuations. Mov Disord. 2015;30(suppl 1):99.
37. Reichmann H, Rocha R, Lees A, et al. Effectiveness and safety of opicapone in Parkinson’s disease patients with motor fluctuations: the OPTIPARK open-label study. Transl Neurodegener. 2020;9:9. doi:10.1186/s40035-020-00187-1
38. Oehlwein C, Mittmann K, Witt K, et al. Opicapone improves motor and non-motor wearing OFF symptoms in Parkinson’s disease: an observational real-world study.
39. Braak H, Rüb U, Gai WP, et al. Diopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110(5):517–536. doi:10.1007/s00702-002-0808-2
40. Braak H, Tredici KD, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi:10.1016/S0197-4580(02)00065-9
41. Svensson E, Horváth-Puhó E, Thomsen RW, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78(4):522–529; PMID: 26031848. doi: 10.1002/ana.24448
42. Rekdal V, Bess EN, Bisanz JE, et al. Discovery and inhibition of an interspecies gut bacterial pathway for levodopa metabolism. Science. 2019;364(6445):eaau6323. doi:10.1126/science.aau6323
43. Unger MM, Spiegel J, Dillmann KU, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. PMID: 27591074. doi:10.1016/j.parkreldis.2016.08.019
44. Laval L, Martin R, Natividad JN, et al. Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microbes. 2015;6(1):1–9; PMID: 25517879; PMCID: PMC4615674. doi: 10.4161/19490976.2014.990784
45. Martín R, Miquel S, Chain F, et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 2015;15(1):67. PMID: 25888448; PMCID: PMC4391109. doi:10.1186/s12866-015-0400-1
46. Keshavarzian A, Green SJ, Engen PA, et al. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30(10):1351–1360; PMID: 26179554. doi: 10.1002/mds.26307
47. Cantu-Jungles TM, Rasmussen HE, Hamaker BR. Potential of prebiotic butyrogenic fibers in Parkinson’s disease. Front Neurol. 2019;10:663. PMID: 31281287; PMCID: PMC6595503. doi:10.3389/fneur.2019.00663
48. Grün D, Zimmer VC, Kauffmann J, et al. Impact of oral COMT-inhibitors on gut microbiota and short chain fatty acids in Parkinson’s disease. Parkinsonism Relat Disord. 2020;70:20–22. PMID: 31790924. doi:10.1016/j.parkreldis.2019.11.020
49. Müller T New developments in Parkinson’s disease therapy with COMT inhibitors.
50. Rascol O, Poewe W, Ferreira JJ, et al. Efficacy of opicapone in patients with Parkinson’s disease with levodopa dose reduction: a pooled post-hoc analysis of BIPARK-I and II. Eur J Neurol. 2020;27(suppl1):
51. Rocha JF, Falcão A, Santos A, et al. Effect of opicapone and entacapone upon levodopa pharmacokinetics during three daily levodopa administrations. Eur J Clin Pharmacol. 2014;70(9):1059–1071. doi:10.1007/s00228-014-1701-2
52. Najib J. Entacapone: a catechol-O-methyltransferase inhibitor for the adjunctive treatment of Parkinson’s disease. Clin Ther. 2001;23(6):802–832. doi:10.1016/S0149-2918(01)80071-0
53. Loewen G, Vijan A, Olson K, et al. Effects of once-daily opicapone 50 mg on the pharmacokinetics of levodopa administered as carbidopa/levodopa extended-release capsules: an open-Label Phase 1 study. Mov Disorders. 2021;36(suppl. 1):1.
54. Ferreira J, Poewe W, Rascol O, et al. Study-design to assess the effect of opicapone on levodopa PK at different levodopa-optimized treatment regimens. Eur J NeuroL. 2021;28(suppl.1):558–752. doi:10.1111/ene.14557
55. Ferreira J, Poewe W, Rascol O, et al. The ADOPTION (eArly levoDopa with Opicapone in Parkinson’s paTients wIth motOr fluctuatioNs) study in Parkinson’s disease: design and rationale of a randomized prospective, open-label exploratory trial. Mov Disorders. 2021;36(suppl.1):36.
56. Clinicaltrails.gov, NCT04990284. eArly levoDopa with opicapone in Parkinson’s paTients wIth motOr fluctuatioNs. (ADOPTION). Available from: https://clinicaltrials.gov/ct2/show/NCT04990284. Accessed July 19, 2022.
57. Ferreira J, Poewe W, Rascol O, et al. The EPSILON (Early Parkinson’s with L-dopa/DDCi and OpicapoNe) study in early Parkinson’s disease: design and rationale of a phase III, double-blind, randomized, placebo-controlled study. Mov Disorders. 2021;36(suppl.1):2.
58. Clinicaltrails.gov, NCT04978597. Early Parkinson wIth L-DOPA/DDCI and OpicapoNe (EPSILON Study) (EPSILON). Available from: https://clinicaltrials.gov/ct2/show/NCT04978597. Accessed July 19, 2022.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
