Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Evacuation of chronic and subacute subdural hematoma via transcranial neuroendoscopic approach
Authors Cai Q, Guo Q, Zhang F, Sun D, Zhang W, Ji B, Chen Z, Mao S
Received 6 November 2018
Accepted for publication 28 December 2018
Published 30 January 2019 Volume 2019:15 Pages 385—390
DOI https://doi.org/10.2147/NDT.S193548
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Qiang Cai,1 Qiao Guo,1 Fan Zhang,2 Daofa Sun,2 Wenfei Zhang,1 Baowei Ji,1 Zhibiao Chen,1 Shanping Mao3
1Department of Neurosurgery, Renmin Hospital of Wuhan University, Wuhan, Hubei Province 430060, China; 2Department of Neurosurgery, Xiantao First People’s Hospital, Xiantao, Hubei Province 433000, China; 3Department of Neurology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province 430060, China
Objective: To evaluate the effectiveness and safety of neuroendoscopic surgery for chronic or subacute subdural hematoma.
Patients and methods: Between September 2016 and September 2018, neuroendoscopic surgery was performed on 25 patients with chronic and subacute subdural hematoma. Hematoma evacuation was performed with a 0°, 4 mm diameter rigid neuroendoscope via a transcranial neuroendoscopic approach.
Results: All patients successfully underwent neuroendoscopic surgery, and no surgical complications or rebleeding was observed. Postoperative computed tomography scans showed that the hematoma was successfully evacuated. All patients had recovered well at discharge, the observed 30-day mortality rate was 0%, and no patients suffered recurrence for 2–26 months after surgery.
Conclusion: Neuroendoscopic surgery was a safe and effective approach for the treatment of chronic and subacute subdural hematoma. This approach has the advantages of decent visualization and minimal invasiveness and could reduce recurrence and the mortality rate.
Keywords: subacute subdural hematoma, chronic subdural hematoma, transcranial neuroendoscopic approach
Introduction
Chronic subdural hematoma (CSDH) and subacute subdural hematoma (SASDH) are the two most common neurosurgical diseases; the incidence of these diseases has been increasing, partly due to an increase in the elderly population and the use of oral antiplatelet and anticoagulation agents.1,2 Patients often live with these two conditions in the community and do not seek medical attention until they become symptomatic. Indeed, SASDH is rare, and the management of this condition is often similar to CSDH,3 which includes twist-drill and burr-hole evacuation and craniotomy.4 The first two approaches are less invasive, but the recurrence rate is higher due to their limited exposure and insufficient drainage.4 Although craniotomy has been used to clear the membranes to reduce potential recurrence, it also carries the risk of damage and significant morbidity and mortality rates due to the invasive approach.
It was estimated that the recurrence rate was ~5%–33% in CSDH, and some patients suffered repeated recurrences, which was troublesome for both neurosurgeons and patients.5 Additionally, the overall outcome of CSDH has not changed substantially during the past three decades,1,6 which has prompted neurosurgeons to look for new approaches.
Recently, minimally invasive surgery with a neuroendoscope for CSDH and SASDH has been successfully demonstrated.4,7,8 This technique may provide broader visualization and the chance to separate the membrane of the intra-hematoma cavity to reduce the recurrence rate,9 which makes it a promising method for the treatment of CSDH and SASDH. To explore the safety and efficacy of this approach, we treated 25 patients with CSDH and SASDH by neuroendoscopy and reported their treatment outcomes herein.
Patients and methods
Clinical-radiological features
Between September 2016 and September 2018, 25 patients (17 male, 8 female) affected with CSDH and SASDH were treated by neuroendoscopy. Patient age ranged from 42 years to 96 years (average 65.8 years). All patients in this study had undergone a computed tomography (CT) scan and/or magnetic resonance imaging before the operation, and findings consistent with CSDH were apparent in 20 cases and SASDH was detected in five cases. Hematoma occurred on left side in 14 cases, on right side in ten cases, and on both sides in one case (Table 1). Among these patients, there were four recurrence cases, which had been treated with burr-hole surgery for the first time.
The presenting symptoms included headaches, mental status changes, weakness, seizure, gait disturbance, nausea, and vomiting. Two patients presented as comatose, and severe impairment with brain herniation occurred in one case. Among these patients, five were also on anticoagulation or antiplatelet therapy.
Surgical approaches and methods
A neuroendoscopic approach was used for these patients, and all procedures were approved by the ethics committee of Renmin Hospital of Wuhan University. All patients provided written informed consent, and this procedure was conducted in accordance with the Declaration of Helsinki.
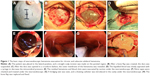
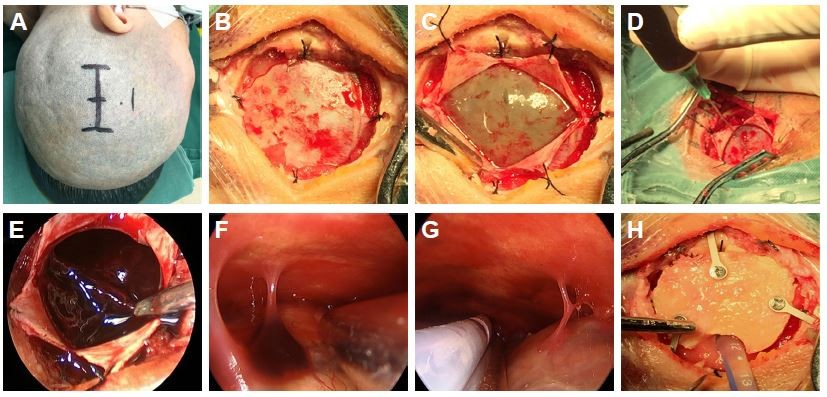
After receiving general anesthesia, the patient was placed in the lateral position, and a straight or curvilinear scalp incision was made on the parietal region. Then, a bone flap ~2.5–3 cm in diameter was created over the lesion. After the dura was suspended, it was opened in cruciform fashion. When the dura was retracted and retained with sutures, the outer membrane of hematoma was revealed. The liquefied blood was slowly aspirated with a syringe to decrease the intracerebral pressure. Then, the outer membrane was opened, and a 0°, 4 mm diameter rigid endoscope (Aesculap, Tutlingen, Germany) was inserted into the cavity; suction was used to remove the residual hematoma and blood clots. Under neuroendoscope control, the fibrin septa were cut, and the neovessels were coagulated. After the evacuation was complete, a draining catheter was introduced into the cavity, and irrigation was performed with normal saline at body temperature. Before closing the dura, the hematoma cavity was carefully inspected for bleeding. Finally, the bone flap was replaced and fixed, and then the scalp was closed (Figure 1A–H).
A postoperative CT scan was performed 24 hours after surgery, and the draining catheter was removed in 3–5 days based on the results of the CT scan. All patients were monitored for 2–26 months after the operation, and recurrence was defined as hematoma reaccumulation that required reoperation.
Results
All patients underwent neuroendoscopic surgery successfully. The average operative time was 58 minutes (range 43–75 minutes); the duration of the procedure increased by 25 minutes on average (16–33 minutes) because of the creation of the bone flap and the use of the neuroendoscope. The average maximum hematoma thickness was 24.3±6 mm, and the average shift of the third ventricle from the midline was 10.3±4 mm. Trabeculae and fibrin septa were found intraoperatively in 16 cases (64%), and all septa could be cut under the neuroendoscope (Figure 1F). After the hematoma was evacuated, the subdural space and hematoma cavity were inspected in all cases, and the bridge veins could be found in most of the patients (Figure 1G). No surgical complications were encountered, and no rebleeding after endoscopic surgery was observed in these patients.
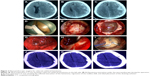
The symptoms improved in all patients after surgery, and postoperative CT scans showed the subtotal evacuation of the hematoma and the regression of the midline shift and revealed that the draining catheter had been placed in an ideal location (Figure 2A–L). All 25 patients recovered well; the modified Rankin Scale score was 0 in 19 patients, 1 in 4 patients, and 2 in two patients at discharge. No patient had died at discharge, the observed 30-day mortality was 0%, and no patients suffered recurrence for 2–26 months after the surgery (Table 1).
Discussion
An increasing trend in CSDH incidence has been observed and has primarily been attributed to an increase in the overall aging population resulting from an increase in life expectancy. In 1975, the incidence of CSDH was 1.7/100,000/year, it increased to 13.1/100,000/year in 1992, and in 2011, the incidence was 20.6/100,000/year. It has also been observed that the incidence of CSDH has increased significantly more in the elderly. The incidence in people over 65 years was 80.1/100,000/year in Karibe’s study, and it increased to 127.1/100,000/year in individuals over 80 years of age. The peak age of onset of CSDH also increased from 50 years in the 1970s to 80 years more recently.9
Surgical evacuation was the gold standard for treating symptomatic CSDH, and the traditional treatment options included twist-drill and burr-hole evacuation with or without drains and craniotomy.10 Twist-drill evacuation was the least invasive procedure; it could create a single cranial opening <1 cm in size and was often performed at bedside under local anesthesia. The use of a burr-hole with drainage under local anesthesia was the first-line treatment in most of the departments, even in elderly patients, due to its low morbidity and mortality indices. Although these two minimal treatments have been shown to be effective in the management of CSDH, recurrence still occurred in a small portion of patients. In contrast, craniotomy was used for cases with greater exposure, which enabled resection of the outer capsule and neomembranes and resulted in the lowest recurrence rate. However, due to its invasive approach, craniotomy was associated with a higher morbidity rate.4,11 Weigel et al found that the recurrence rate was 33% in the twist-drill group and 12% in the burr-hole group, while in the craniotomy group, the recurrence rate was only 10.8%. They also found that twist-drill and burr-hole evacuation and craniotomy had similar cure and mortality rates, but morbidity was higher in patients who underwent a craniotomy (12.3%), while morbidity was only 3% for the twist-drill procedure and 3.8% for the burr-hole procedure. This finding led to their support for the burr-hole procedure as the first-line approach in light of the cure-to-complication ratio.11 A recent meta-analysis by Ducruet et al also found that the twist-drill procedure had the highest rates of recurrence (28.1%) vs burr-hole evacuation (11.7%) and craniotomy (19.4%). However, they also found that the burr-hole procedure carried significantly higher complication rates (9.3%) compared with twist-drill evacuation (2.5%) and craniotomy (3.9%).12 Therefore, all these traditional treatments had advantages and shortcomings.
The technique of neuroendoscopic hematoma removal had been discussed extensively in the literature and was first introduced to SCSDH in 1988.4 It was found that endoscope-assisted techniques did not increase morbidity, and with improved intraoperative visualization, it could help identify and destruct neomembranes, septums, and solid clots and coagulate the source of bleeding, which made this approach more safe and effective for the treatment of CSDH.7 Májovský et al found that a flexible endoscope-assisted evacuation for CSDH permitted decent visualization of the hematoma cavity and retained the advantages of a minimally invasive approach. The main advantages were the correct positioning of the catheter under visual control, identification of septations, and early detection of cortex or vessel injury during surgery.4 Yokosuka et al performed endoscopic surgery using a rigid endoscope in eleven patients with acute and subacute subdural hematoma and obtained good outcomes.8
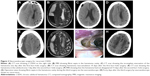
A significant problem with CSDH surgery was recurrence, and it was estimated that 8%–33% of all patients needed reoperation.12 Recurrence risk factors often included older age, male sex, brain atrophy, use of anticoagulant or antiplatelet drugs, and the presence of a mixed- or high-density hematoma on CT.5 A septated chronic subdural hematoma (SCSDH) is a special type that features hematoma cavities divided into different portions by fibrin septa. It was an independent risk factor for recurrence; a burr-hole drainage approach could only remove the fluid but not the solid blood clot, particularly for a thick, widely distributed solid hematoma.13 Craniotomy was considered for this situation, but it was associated with complications due to the invasive surgery itself. Neuroendoscopic surgery might be a suitable treatment for SCSDH, and with the help of a neuroendoscope, surgeons could view the hematoma cavity well and tear the neomembranes by using microscissors or microforceps, which could reduce the recurrence rate. Many studies concluded that this surgery was superior to other surgeries in treating SCSDH.13 In Yan et al’s study, 28 cases of severe SCSDH were successfully treated with endoscopic surgery, and there was no recurrence at 6 months to 2 years post-discharge.14 In our study, we also found fibrin septa in 16 cases, and four patients suffered from recurrence after the first surgical approach by burr-hole evacuation. After the neuroendoscopic technique was used to clear up the fibrin septa, all patients recovered well, and no recurrence occurred (Figure 3A–H).
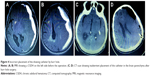
Catheter placement was another method that was used to reduce the recurrence rate. It was concluded that placement of the drain after burr-hole evacuation could reduce the risk of recurrence by nearly 15%.15 However, this procedure also had a risk of some potentially life-threatening complications such as inadvertent placement of the catheter into the brain parenchyma (Figure 4A–D) and parenchymal hematomas due to blindly inserted catheters.15,16 As an immediate verification approach, neuroendoscopic surgery could reveal the misplacement of 19% of catheters, and they could be adjusted to a desired location.4 In our study, all catheters were placed in an accurate location, and there were no complications due to draining catheters.
Conclusion
Based on the analysis of our data and findings of the related references, we could conclude that transcranial neuroendoscopic surgery was a safe and effective approach for the treatment of chronic and subacute subdural hematoma. This approach has the advantages of decent visualization and minimal invasiveness and could reduce recurrence and mortality rate.
Acknowledgments
This work was supported by National Natural Science Foundation of China (81671306) and the foundation of China Scholarship Council. Qiang Cai, Qiao Guo, Fan Zhang and Daofa Sun are co-first authors for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Kolias AG, Chari A, Santarius T, Hutchinson PJ. Chronic subdural haematoma: modern management and emerging therapies. Nat Rev Neurol. 2014;10(10):570–578. | ||
Rust T, Kiemer N, Erasmus A. Chronic subdural haematomas and anticoagulation or anti-thrombotic therapy. J Clin Neurosci. 2006;13(8):823–827. | ||
Tripathy S, Swarnakar P, Mishra S, et al. A review of sub acute subdural hematoma (SASDH) with our institutional experience and its management by double barrel technique (DBT): a novel technique. Surg Neurol Int. 2016;7(29):S767–S774. | ||
Májovský M, Masopust V, Netuka D, Beneš V. Flexible endoscope-assisted evacuation of chronic subdural hematomas. Acta Neurochir. 2016;158(10):1987–1992. | ||
Matsumoto H, Hanayama H, Okada T, et al. Clinical investigation of refractory chronic subdural hematoma: a comparison of clinical factors between single and repeated recurrences. World Neurosurg. 2017;107:706–715. | ||
Gelabert-González M, Iglesias-Pais M, García-Allut A, Martínez-Rumbo R. Chronic subdural haematoma: surgical treatment and outcome in 1,000 cases. Clin Neurol Neurosurg. 2005;107(3):223–229. | ||
Boyaci S, Gumustas OG, Korkmaz S, Aksoy K. Endoscopic evacuation of subdural collections. Turk Neurosurg. 2016;26(6):871–877. | ||
Yokosuka K, Uno M, Matsumura K, et al. Endoscopic hematoma evacuation for acute and subacute subdural hematoma in elderly patients. J Neurosurg. 2015;123(4):1065–1069. | ||
Uno M, Toi H, Hirai S. Chronic subdural hematoma in elderly patients: is this disease benign? Neurol Med Chir. 2017;57(8):402–409. | ||
Buchanan IA, Mack WJ. Minimally invasive surgical approaches for chronic subdural hematomas. Neurosurg Clin N Am. 2017;28(2):219–227. | ||
Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. 2003;74(7):937–943. | ||
Ducruet AF, Grobelny BT, Zacharia BE, et al. The surgical management of chronic subdural hematoma. Neurosurg Rev. 2012;35(2):155–169. | ||
Yan K, Gao H, Zhou X, et al. A retrospective analysis of postoperative recurrence of septated chronic subdural haematoma: endoscopic surgery versus Burr hole craniotomy. Neurol Res. 2017;39(9):803–812. | ||
Yan K, Gao H, Wang Q, et al. Endoscopic surgery to chronic subdural hematoma with neovessel septation: technical notes and literature review. Neurol Res. 2016;38(5):467–476. | ||
Sivaraju L, Moorthy RK, Jeyaseelan V, Rajshekhar V. Routine placement of subdural drain after Burr hole evacuation of chronic and subacute subdural hematoma: a contrarian evidence based approach. Neurosurg Rev. 2018;41(1):165–171. | ||
Kaliaperumal C, Khalil A, Fenton E, et al. A prospective randomised study to compare the utility and outcomes of subdural and subperiosteal drains for the treatment of chronic subdural haematoma. Acta Neurochir. 2012;154(11):2083–2089. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.