Back to Journals » Journal of Pain Research » Volume 12
Ethical justification of single-blind and double-blind placebo-controlled response tests in neuropathic pain and N-of-1 treatment paradigm in clinical settings
Authors Keppel Hesselink JM, Kopsky DJ , Bhaskar AK
Received 19 July 2018
Accepted for publication 6 December 2018
Published 14 January 2019 Volume 2019:12 Pages 345—352
DOI https://doi.org/10.2147/JPR.S180792
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Jan M Keppel Hesselink,1 David J Kopsky,2 Arun K Bhaskar3
1Institute for Neuropathic Pain, Bosch en Duin, The Netherlands; 2Institute for Neuropathic Pain, Amsterdam, The Netherlands; 3Pain Management Centre, Charing Cross Hospital Imperial Healthcare NHS Trust, London, UK
Abstract: At our center in the Netherlands, patients, who very often are treatment resistant to the analgesics recommended in the guidelines, suffering from symmetrical peripheral neuropathic pain are treated exclusively. We have developed a number of compounded topical formulations containing classical co-analgesics such as ketamine, baclofen, amitriptyline, and phenytoin for the treatment of neuropathic pain in treatment-resistant patients. In order to identify putative responders and exclude an (initial) placebo-response, we developed single-blind and double-blind placebo-controlled response tests. The test can be performed when the patient has a symmetrical polyneuropathy with a pain score difference of not more than 1 point on the 11-point numerical rating scale (NRS) between bilateral pain areas. On one area (eg, left foot) the placebo cream and on the other area (eg, right foot) the active cream will be applied. Within a time frame of 30 minutes, patients are considered responders if they rate a pain difference of at least 2 points on the NRS between the bilateral areas on which the active cream and placebo cream are applied. Response tests can be easily conducted during the first consultation. In this paper, we explore the ethical context of using a placebo in clinical practice in a single-blind and double-blind fashion to improve and individualize treatment of neuropathic pain outside a context of a formal clinical trial.
Keywords: ethics, trial, topical, treatment, enrichment
Introduction
Neuropathic pain has an estimated prevalence of 7%–10% in general.1 Patients with neuropathic pain constitute about one-third of the patients seen in pain clinics.2 Around one-third of the patients with diabetes develop painful diabetic neuropathy (PDN).3,4 The authors are affiliated to the Institute for Neuropath Pain, the Netherlands which is devoted exclusively in the assessment and management of patients suffering from neuropathic pain. More often than not they have consulted neurologists, anesthesiologists, and other pain specialists, without any effective treatment being successfully identified. Patients reported that either the treatment was not sufficiently effective in producing meaningful analgesia, or the patients suffered from undesirable adverse events, leading to treatment discontinuation.
The authors have been instrumental in successfully developing a number of compounded topical creams containing classical co-analgesics such as ketamine, baclofen, amitriptyline, and phenytoin for the treatment of pain in refractory patients and those who could not tolerate the systemic medications. The prescription of these formulations can be classified as off-label use. Off-label use refers to the practice of prescribing or ordering a medication for a use that is not recognized as an official indication by a national licensing authority.5 In the Netherlands, prescribing off-label use medication happens relatively often particularly in the field of pain management; over five million pharmacy compounded products were dispensed in 2015.6
Patients suffering from peripheral neuropathic pain frequently complained of severe pain in both feet and/or lower legs. Most patients pointed out that there was a perceptible reduction in pain within 30 minutes after the application of a topical analgesic. This led us to initially develop an open response test, comparing active cream applied on one foot to application of no cream on the other foot. Subsequently, in order to identify putative responders and exclude an (initial) placebo-response, we developed a single-blind placebo-controlled response test.7 This test can be performed when the patient has a symmetrical polyneuropathy with a pain score difference of not >1 point on the numerical rating scale (NRS) between the bilateral pain areas. On one area (eg, left foot) the placebo cream will be applied, and on the other area (eg, right foot) the active cream. Within a time frame of 30 minutes, patients are considered responders if they rate a pain difference of at least 2 points on the NRS between the bilateral areas on which the active cream and placebo cream are applied (Figure 1).
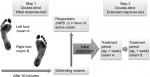  | Figure 1 Initial and extended double-blind response test. Abbreviation: NRS, numerical rating scale. |
We are currently evaluating the use of a double-blind placebo-controlled response test that could also be used in routine clinical practice when considering and evaluating various topical analgesic options. We are postulating that the essence of this new approach is to maximize the chance of finding the best therapy for the patient, while minimizing the risk of a placebo-response. We are presenting our approach, followed by a discussion on the clinical and ethical context of using these placebo-controlled blind response tests, and the treatment paradigm designed to distinguish between responders on a placebo or active cream in the light of individualized pain therapy.
Tests using active creams and placebo to evaluate responders
After developing topical creams containing established co-analgesics for off-label use in peripheral neuropathic pain, our initial approach was to start by prescribing one of those creams to patients; for instance, amitriptyline 10% cream for the treatment of PDN. One to 2 weeks later a follow-up consultation was arranged and patients reported one of the following – a considerable reduction in pain, sometimes just a slight reduction in pain, or no response at all. We also checked for and documented any local or systemic adverse events.
In order to avoid effective treatment delay and to screen for local adverse events, we subsequently started testing the effect of the analgesic creams during the consultation by applying one analgesic cream on one foot, and a different analgesic cream or no cream on the other foot. This was logical as most patients suffered from painful polyneuropathy with often comparable pain intensity in both feet and/or lower limbs; thus, the comparison between left and right was made feasible as compared to using only a single topical analgesic on one foot. Patients who responded to the topical analgesics reported to us that there was a reduction in pain within a 5–30 minutes period. Our observation was that when patients reported a clear analgesic effect in this short time period, they often expressed their confidence in the treatment and this was especially high in those who had various other treatments before but without good results. However, a number of patients at the next consultation noted that the analgesic effect was reduced after using the cream for some days or weeks; we concluded that this could be a reduction of the initial placebo response. This provoked us to develop a single-blind placebo-controlled response test, testing with an active and placebo creams. The essence of this new approach, including its follow-up based on a double-blind response test, is to maximize the chance of finding the best-suited therapy for the patient during the first visit, while reducing the incidence of a true placebo response and treatment delay. The use of placebos in clinical practice outside of a clinical trial context is not commonly accepted; hence, in this paper, we explored whether there is sufficient justification to do so.
The single-blind placebo-controlled response test can formally qualify as possibly the simplest variety of an N-of-1 treatment paradigm, because at least a blind assessment of outcome by the patient is possible. The single-blind response test can be performed when a patient has two similar, mostly bilateral, anatomical locations with similar pain intensity. In patients with peripheral neuropathic pain this condition is nearly always present in both the front of the feet, the entire foot area, or in a more progressive state in both feet and the lower legs. The reason for the equal distribution is that in peripheral neuropathy, the damage is in the longest nerves reaching the front of the feet due to toxicity (eg, hyperglycaemia, high alcohol intake, chemotherapy, and high vitamin B6 intake) or deprivation (vitamin B12 deficiency and hypothyroidism).8 In sustained suboptimal conditions, the peripheral neuropathy gradually spreads proximally up the lower limb. The single-blind response test can be performed when the maximum difference is 1 point on the NRS in pain intensity between two anatomically similar areas (eg, left and right foot). The physician “randomizes” the active and placebo creams and explains to the patients that two different creams will be applied on each foot. The test period is maximally 30 minutes and after the response test, the physician unblinds the treatment and the results, following which the implications of the test are directly discussed with the patient. The patient is regarded as an initial responder when the pain difference is 2 points or more on the NRS between the two areas after application of the active and placebo creams. This is based on the recommendation of the European Medicines Agency, in which a responder is defined as 2-point reduction on the NRS in favor of the active treatment.9
A responder will be prescribed the active cream to commence the treatment. Subsequently, we observed that responders rarely complained of a reduction of analgesic effects during the first 2 weeks of treatment. So it seems that responders to the test remain as responder even when they use the analgesic cream repeatedly. As a further development in objectifying the therapeutic effects of the compounded creams, we are currently in the process of developing and evaluating a double-blind placebo controlled test design. The aim of this test is to make an objective assessment of the pharmacological effect of the compound and to minimize the effect of the patient’s expectations and that of the treating clinicians. This would enable to create a greater chance of selecting the best initial treatment for the patient. Performing the double-blind response test, neither the physician nor the patient knows which tube contains the active or placebo cream, for the sake of simplicity we call these cream A and cream B. Cream A is applied on one foot and cream B on the other. After 30 minutes the tubes are unblinded and the results and consequences toward future treatment are discussed (Figure 1).
The second step is to observe the long-term effects in initial responders to the analgesic cream, using an extended response test during one to several weeks, in line with a more extended N-of-1 treatment paradigm. For instance, in such an extended N-of-1 treatment paradigm patients can receive two randomized blinded creams (A and B), in which one of the creams is an active and other a placebo cream. Cream A will be applied in the first treatment period (eg, week 1) and cream B will be applied in the second treatment period (eg, week 2). In case the person experiences not sufficient analgesia during the treatment periods, cream C can be applied. Cream C contains the active cream evaluated during the initial response test. After the extended testing period (eg, 2 weeks) the patient will report back to the physician which cream he/she finds to have the best analgesic effect, using for instance the Patient Global Impression of Change.10
After the 2 weeks period of our test, creams A and B can be unblinded by the physician and the results and its consequences can be discussed with the patient.
In all cases, for the use of a placebo in the clinic in the way we described above, a special context is needed based on the full informed consent and mutual trust that this step is included for reducing bias for the benefit of the patient. The ethical context is discussed herewith.
Placebo use in the clinical practice
Touwen and Engberts introduced an ethical debate on the use of placebos, both in the clinical as well in the research setting.11 They presented an operational definition of a placebo intervention, “as an intervention that the physician believes has no specific pharmacological, biochemical or physical mechanism of action according to the current standard of knowledge, upon the condition being treated”. The authors focused on two ethical problems in describing a placebo in the clinical practice. First, the prescription of a placebo in the clinical setting is deceptive, as the patient is not informed that a nonefficacious therapy is being given, and this results in harm as the trust in the physician can be breached and the autonomy of the patient is violated. Second, the prescription of the placebo enhances the risk of under treatment. Based on the results of modern research in the field of placebos, they further pointed out that patients are not able to clearly distinguish between proven effective therapies and treatment of which the mechanisms of action are unclear.12 According to the authors, this implies, “that patients are more tolerant towards being treated with unproven therapies, as long as they can trust their doctor to have their interests at heart”. They therefore feel there is limited room for prescribing a placebo in a clinical setting.11 If used, it would be mandatory to discuss it openly with the patient, explaining the unknown mechanism and the experience that it sometimes works, and suggesting to try it out to see whether this would apply to the said patient. The key issue for Touwen and Engberts, supported by more literature using placebos in a clinical setting, is not to mislead a patient, and to be transparent, trustworthy, and obtain informed consent.12–14
Placebo use in clinical practice as part of an N-of-1 treatment paradigm
Although there is a great deal of literature on placebo use in clinical trials, there is paucity of evidence in discussing the value and the ethical justification of placebos in the clinical practice. It seems important to understand that placebo and nocebo effects are always present in routine clinical care, even when a placebo is not given.15 Papers discussing the practical aspects of placebo use clinical practice are rare. It is of use to discuss in detail the clinical approach of the group of Guyatt et al from the McMaster University, Ontario, Canada. In 1988, they published an important paper on the use of placebos in the context of the N-of-1 randomized clinical trials (RCTs): “A clinician’s guide for conducting randomized trials in individual patients”.16 This stimulated clinicians to plan and execute their own N-of-1 RCTs directly in their own practice, and is recognized as a milestone paper and as the first practical approach encouraging clinicians to conduct N-of-1 trials.17 Partly based on their work, N-of-1 trials are now referred to as “a promising way to advance individualized medicine and a method for gaining insights into comparative treatment effectiveness among a wide variety of patients”.18
It is the above paper from McMaster University containing clear recommendations that supported us to develop initially a single-blind approach and more recently a double-blind test paradigm for neuropathic pain patients. Some years later the authors tested their approach in repositioning the use of amitriptyline for a new off-label indication in a chronic pain condition, diagnosed at that time as fibrositis (fibromyalgia), and reported the findings.19 In that paper, they stipulated the great value the N-of-1 RCT can have during the early phases of the development of fast-acting drugs designed to produce symptomatic benefit for chronic illness of which the biologic action ends soon after withdrawal, in line with their previous recommendations.
This guideline (1988) defined a number of questions to pave the way into such a RCT, and those key questions are described in Table 1.
  | Table 1 Key questions for N-of-1 RCT Abbreviation: RCT, randomized clinical trial. |
The N-of-1 treatment paradigm is still underused in clinical practice and not widely described and practiced. However, such an approach has proven to be quite relevant by answering many practical questions, for instance in the field of chronic pain and attention deficit hyperactivity disorder, and including in relation to putative side-effects.20,21 The N-of-1 clinical approach is seen as compatible with the ultimate end point of clinical practice – the care of individual patients using tailored treatments.18 We will discuss our single-blind and double-blind test approach in the treatment of peripheral neuropathic pain, as well as a potential N-of-1 study, step-by-step following the leading questions of the McMaster group.
Indication and feasibility of a single-blind and double-blind controlled N-of-1 treatment paradigm of active vs placebo creams
There are two steps possible in the evaluation of responders to the analgesic creams. The first step is to identify initial responders with the initial single or double-blind response test. This takes 30 minutes at most. A second step could be to offer responders to the initial response test an extended response test of 1 week or longer.
Our two initial placebo-controlled response tests can be regarded as based on the simplest design of an N-of-1 treatment paradigm.
In practice the initial response tests and the extended response test have shown to be feasible.
The ethical question whether such placebo-controlled test paradigms are justified toward our patients without the approval of the institutional review board (IRB), can be answered based on a number of specified questions, as designed by the McMasters group.16 While the first three questions (1a–1c) relate to the general question of accepting a placebo as part of an intervention in everyday practice, the six follow-up questions (2a–2f) relate to the individual patient level. Each question is followed by the answer related to our test modality. The last set off questions (3a–3c) analyze whether the “trial” is feasible in a clinical setting.
1a. Is the effectiveness of the treatment really in doubt?
Topically administered phenytoin to treat neuropathic pain has never been previously tested in clinical RCTs. In the Netherlands, it is prescribed as a compounded cream for off-label use in peripheral neuropathic pain. Thus, the efficacy of the treatment is unproven. The same holds true for other co-analgesics used in compounded topical formulations.
1b. Will the treatment, if effective, be long-term?
To date, the authors have gained experience in treating patents and reporting the therapeutic effects. Though there are nonresponders, our observation is that around 30%–40% of patients suffering from peripheral neuropathic pain are responders, and most of the responders use the cream in the long-term; as of now we have documented the long-lasting effects in a patient using phenytoin 10% cream for over 3 years.7
1c. Is the patient eager to collaborate in designing and carrying out an N-of-1 RCT?
Up to now all patients in our center expressed their willingness in carrying out an N-of-1 single-blind RCT; we are currently exploring the willingness of patients to participate in a double-blind N-of-1 RCT.
2a. Does the treatment have a rapid onset?
Most responders report that they feel a clear and clinical effective reduction in pain within 20–30 minutes, often earlier.
2b. Does the treatment stop acting soon after it is discontinued?
As soon as patients stop using cream the pain reemerges 3–72 hours later as per our observations.
2c. Is an optimal duration of treatment feasible?
Peripheral neuropathic pain is a chronic disorder and is used as an example by Guyatt et al.19 The authors mention the necessity of a treatment duration of at least 10 days; in our case, the duration of treatment is much longer, and we can repeatedly evaluate the effects on an ongoing basis. In the case of double-blind N-of-1 studies, it is possible to have at least two pairs of treatment periods before discontinuing the trial.
2d. Can clinically relevant targets be measured?
There is consensus about the best way to measure pain, based on the patient scoring pain on the NRS.22
2e. Can sensible criteria for stopping the trial be established?
Clear criteria for stopping the trial can be established; as soon as the clinical reduction of pain is <30% or <2 points on the NRS from baseline, we let the patient decide whether the pain reduction is still relevant, or if stopping and trying a new cream is a better option. As we have developed a number of analgesic creams, we can offer potential alternatives or if indicated other systemic options could be explored.
2f. Is an unblinded run-in period necessary?
The authors feel an open run-in period may be used to determine the adverse events and the optimal concentration of the topical analgesic. Tolerability is usually not an issue.7 In case of a suboptimal dose-response on phenytoin 10% cream, we can in the same session evaluate a higher dose, eg, 30%.23
3a. Is there a pharmacist who can help me?
In our case, we work alongside a compounding pharmacist, who is involved in the process.
3b. Are strategies for interpreting the data in place?
The authors of the 1988 paper suggested a number of simple approaches to analyze the data, and furthermore pointed out that the use of N-of-1 RCTs to improve patient care does not depend on the statistical analysis of the results. Strategies of randomization, double-blinding, replication, and quantitation of outcomes will still allow a better analysis of effect that is normally difficult in the clinic.
3c. Is the trial ethical?
This question is related to the question of whether an N-of-1 RCT is a clinical or a research undertaking. According to McMasters group, such undertaking can be both, and they argue that an N-of-1 RCT can and should be a part of routine clinical practice (underlined by us). If patients are informed about the undertaking, if there is no element of deception and patients have the freedom to stop the trial at any point of time, the approach is considered to be ethically correct. We will elaborate a bit more as this element of consent is quite crucial and relevant.
Information of patients during informed consent
In 2008, a report of the American Medical Association Council on Ethical and Judicial Affairs on Placebo Use in Clinical Practice was published.24 “Physicians may use placebos for diagnosis or treatment only if the patient is informed of and agrees to its use”. This is clearly in line with the above-described literature, where transparency, trust, and informed consent are needed to avoid any deceit. In order to achieve such transparency, Lichtenberg et al suggested the following wording for the use of a placebo during the informed consent procedure14:“I would like to offer you a pill which I believe can help lessen your suffering. I do not know exactly how it works”. For the active comparator Lichtenberg used the following wording: “I have other pills to offer whose mechanism is clearer, but I am not sure that they will work better for you, and they may also entail more serious side effects”. One additional advantage of this way of explaining is that the word “placebo” with its many definitions, associations and misunderstandings is avoided.
In our center, we have implemented the ethical approach of Lichtenberg, introducing our placebo-controlled response test to the patient, as follows:
I would like to offer you the choice between two creams which I believe can help lessen your pain. I do not know exactly how one of these creams work. And I have included one other cream, whose mechanism is clearer, but I am not sure which cream will work better for you. Therefore we will apply both creams, one on your left foot, and one on your right foot, so that you can compare the effect between right and left.
We also point out that if later during the treatment phase there is any doubt or adverse event, the patient can stop the treatment directly and we will explore alternative options. And we can offer such options as we have a number of compounded creams available for prescription.
Lichtenberg et al also pointed out that in their opinion the administration of a placebo should be considered if patients are refractory to treatment, suffer from adverse events, or are in a situation where specific standard treatments do not exist.14 It is exactly this situation, we frequently see in our clinic, and this led us to develop the placebo-controlled response tests as described above.
Institutional review board
To perform an N-of-1 treatment paradigm, the critical point of discussion is whether the trial has to be reviewed by the IRB. Clearly the McMasters group pointed out that an N-of-1 study can also be a part of routine clinical practice, it thus would not be a scientific study, and a IRB review would not be required (vide infra).16
The process of involving an IRB also has some drawbacks: 1) creating a great time loss for starting the treatment, which in itself could be considered as unethical; and 2) increasing the costs considerably (eg, costs of the IRB review [€1,500], costs of good manufacturing practice (GMP) produced topical analgesics [€10,000], making the approach unfeasible, also in itself unethical).
There are several key arguments not to include IRB review in short response tests or longer N-of-1 treatment paradigms.
1.In the publication, Design and Implementation of N-of-1 Trials: A User’s Guide25 one of the key statements is that “the introduction of an N-of-1 trial service into care the sole purpose of giving individual clinicians better tools to care for individual patients, and no larger research agenda is addressed, it may be reasonable to assume that no external IRB review is needed”.
2.In the Netherlands, according to the Dutch legislation and regulations for medical-scientific research with people. (https://wetten.overheid.nl/BWBR0009408/2018-08-01) IRB approval is obligatory when two criteria are fulfilled: 1) is the intervention medical scientific research; and 2) will the participants (read patients) be submitted to certain (extra) actions (eg, blood testing) or have to obey certain behavioral rules (eg, strict diet). Whether a certain approach is defined as “medical scientific research” or not is related to the following context: “medical-scientific research is research that aims to answer a question in the area of disease and health (etiology, pathogenesis, symptoms/symptoms, diagnosis, prevention, outcome or treatment of disease), by systematically collecting and studying data. The research aims to contribute to medical knowledge that also applies to populations outside the direct research population”. Clearly, our response tests have different aims: to identify the most optimal treatment for a given, individual patient. In this respect it is comparable to a diagnostic pain block. The response tests are not aimed to answer a question in the area of disease and health by systematically collecting and studying data but must also contribute to medical knowledge that also applies to populations outside the direct research population. The same holds true for N-of-1 treatment paradigms, from which results cannot be translated to the general population. Furthermore, according to the Dutch legislation and regulations for medical-scientific research with people, interventions could be defined as research, if the intervention would infringe upon the physical or psychological integrity of the test subject. This is also not the case for the response tests. On the contrary, both the response tests and the N-of-1 treatment paradigm provide the treating physician and patient with the insight of how much the pain reducing effect can be attributed to the active compound, and helps in identifying a more certain personalized analgesic treatment fast.
Conclusion
The use in the everyday practice of single-blind and double-blind test paradigms in the treatment of peripheral neuropathic pain seems feasible and is ethically justified. The use of a double-blind test in particular can subsequently be followed by a more extended double-blind placebo-controlled N-of-1 treatment paradigm of 2 to several weeks. A fast response test (of 30 minutes), possibly followed by an extended double-blind placebo-controlled N-of-1 cross over treatment paradigm, will lead to quicker identification of the optimal therapy for our patients. Both initial fast response tests as well as the extended response test qualify for use in the clinical practice. The use of such paradigms is not only in line with the McMasters criteria, but is also supported by secondary medical ethical literature on the use of placebos in the clinical practice, and by the formal criteria of the 2008 American Medical Association Council on Ethical and Judicial Affairs. For such practical test paradigms, there would not be the requirement of an IRB approval, on the contrary, as we argued, this would induce new ethical problems, related to unnecessary delays in therapy and feasibility problems.
Double-blind placebo-controlled test paradigms therefore can be seen as an intrinsic part of our clinical practice, outside of the context of a formal clinical trial. Both our single-blind and double-bind test paradigms illustrate the value of such approach in the clinic.
Disclosure
JMKH and DJK are holders of two patents: 1) topical phenytoin for use in the treatment of peripheral neuropathic pain and 2) topical pharmaceutical composition containing phenytoin and a (co-)analgesic for the treatment of chronic pain. The authors report no other conflicts of interest in this work.
References
van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654–662. | ||
Pérez C, Ribera MV, Gálvez R, et al. High prevalence of confirmed, but also of potential and believed, neuropathic pain in pain clinics. Eur J Pain. 2013;17(3):347–356. | ||
Jacovides A, Bogoshi M, Distiller LA, et al. An epidemiological study to assess the prevalence of diabetic peripheral neuropathic pain among adults with diabetes attending private and institutional outpatient clinics in South Africa. J Int Med Res. 2014;42(4):1018–1028. | ||
Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the UK. Diabetes Care. 2011;34(10):2220–2224. | ||
Ruble J. Off-label prescribing of medications for pain: maintaining optimal care at an intersection of law, public policy, and ethics J Pain Palliat Care Pharmacother. 2012;26(2):146–152. | ||
Langedijk J. Continuous Innovation in the Drug Life Cycle. Patient Needs and the Marketing Authorization for Medicinal Products: An Analysis of EU Law. Chapter 4.1. Utrecht: Utrecht University; 2016. | ||
Kopsky D, Keppel Hesselink J. Phenytoin cream for the treatment for neuropathic pain: case series. Pharmaceuticals. 2018;11(2):53. | ||
Vavra MW, Rubin DI. The peripheral neuropathy evaluation in an office-based neurology setting. Semin Neurol. 2011;31(1):102–114. | ||
EMA. Guideline on clinical medical products intended for the treatment of neuropathic pain. London: EMA; 2007. Available from: https://www.ema.europa.eu/documents/scientific-guideline/guideline-clinical-medicinal-products-intended-treatment-neuropathic-pain_en.pdf. Accessed January 7, 2019. | ||
Farrar JT, Young JP, Lamoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. | ||
Touwen DP, Engberts DP. Those famous red pills – deliberations and hesitations. Ethics of placebo use in therapeutic and research settings. Eur Neuropsychopharmacol. 2012;22(11):775–781. | ||
Fässler M, Meissner K, Schneider A, Linde K. Frequency and circumstances of placebo use in clinical practice – a systematic review of empirical studies. BMC Med. 2010;8(1):1741–7015. | ||
Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375(9715):686–695. | ||
Lichtenberg P, Heresco-Levy U, Nitzan U. The ethics of the placebo in clinical practice. J Med Ethics. 2004;30(6):551–554. | ||
Arnold MH, Finniss DG, Kerridge I. Medicine’s inconvenient truth: the placebo and nocebo effect. Intern Med J. 2014;44(4):398–405. | ||
Guyatt G, Sackett D, Adachi J, et al. A clinician’s guide for conducting randomized trials in individual patients. CMAJ. 1988;139(6):497–503. | ||
Mirza RD, Punja S, Vohra S, Guyatt G. The history and development of N-of-1 trials. J R Soc Med. 2017;110(8):330–340. | ||
Lillie EO, Patay B, Diamant J, Issell B, Topol EJ, Schork NJ. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Per Med. 2011;8(2):161–173. | ||
Guyatt GH, Heyting A, Jaeschke R, Keller J, Adachi JD, Roberts RS. N of 1 randomized trials for investigating new drugs. Control Clin Trials. 1990;11(2):88–100. | ||
Punja S, Schmid CH, Hartling L, Urichuk L, Nikles CJ, Vohra S. To meta-analyze or not to meta-analyze? A combined meta-analysis of N-of-1 trial data with RCT data on amphetamines and methylphenidate for pediatric ADHD. J Clin Epidemiol. 2016;76:76–81. | ||
Barsky AJ, Saintfort R, Rogers MP, Borus JF. Nonspecific medication side effects and the nocebo phenomenon. JAMA. 2002;287(5):622–627. | ||
Dworkin RH, Turk DC, Peirce-Sandner S, et al. Considerations for improving assay sensitivity in chronic pain clinical trials: IMMPACT recommendations. Pain. 2012;153(6):1148–1158. | ||
Keppel Hesselink JM, Kopsky DJ. The individualized N-of-1 trial: dose-response in a single-blind cross-over response test of phenytoin 10% and 30% cream in neuropathic pain. EC Anaesthesia. 2018;4(6):1–6. | ||
Bostick NA, Sade R, Levine MA, Stewart DM; American Medical Association Council on Ethical and Judicial Affairs. Placebo use in clinical practice: report of the American Medical Association Council on Ethical and Judicial Affairs. J Clin Ethics. 2008;19(1):58–61. | ||
Panel tDMCN-o-G. Design and Implementation of N-of-1 Trials: A User’s Guide. In: Kravitz RL, Duan N, editors. Rockville, MD: Agency for Healthcare Research and Quality: AHRQ Publication No. 13(14)-EHC122-EF; 2014. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
