Back to Journals » Nature and Science of Sleep » Volume 15
Establishment and Application Evaluation of an Improved Obstructive Sleep Apnea Screening Questionnaire for Chinese Community: The CNCQ-OSA
Authors Wang D , Ren Y, Chen R, Zeng X, Gan Q , Zhuang Z, Su X , Wu K, Zhang S, Tang Y, Li S, Zhang H, Zhou Y , Zhang N , Zhao D
Received 8 November 2022
Accepted for publication 28 February 2023
Published 13 March 2023 Volume 2023:15 Pages 103—114
DOI https://doi.org/10.2147/NSS.S396695
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Donghao Wang,1,* Yingying Ren,2,* Riken Chen,1,* Xiangxia Zeng,1,* Qiming Gan,1,* Zhiyang Zhuang,1 Xiaofen Su,1 Kang Wu,1 Sun Zhang,1 Yongkang Tang,1 Shiwei Li,1 Haojie Zhang,1,3 Yanyan Zhou,1 Nuofu Zhang,1 Dongxing Zhao1
1State Key Laboratory of Respiratory Disease, Sleep Medicine Center, Guangzhou Institute of Respiratory Health, National Center for Respiratory Disease, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, People’s Republic of China; 2Medical Records and Statistics Room, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, People’s Republic of China; 3The Clinical Medicine Department, Henan University, Zhengzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dongxing Zhao; Nuofu Zhang, State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Sleep Medicine Center, Guangzhou Institute of Respiratory Health, National Center for Respiratory Disease, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, People’s Republic of China, Tel +86-13650901411 ; +86-13600460056, Email [email protected]; [email protected]
Objective: Obstructive sleep apnea (OSA) is a common sleep-disordered breathing disease. We aimed to establish an improved screening questionnaire without physical examinations for OSA named the CNCQ-OSA (Chinese community questionnaire for OSA).
Methods: A total of 2585 participants who visited sleep medicine center and underwent overnight polysomnography were grouped into two independent cohorts: derivation (n = 2180) and validation (n = 405). The CNCQ-OSA was designed according to the baseline of patients in derivation cohort. We comprehensively analyzed the data to evaluate the predictive value of the CNCQ-OSA, compared to the GOAL questionnaire, STOP-Bang questionnaire (SBQ) and NoSAS questionnaire.
Results: The CNCQ-OSA included seven variables: loud snoring, BMI ≥ 25 kg/m2, male gender, apnea, sleepiness, hypertension and age ≥ 30, with a total score ranging from 7 to 16.7 points (≥ 13.5 points indicating high risk of OSA, ≥ 14.5 points indicating extremely high risk). In the derivation and validation cohorts, the areas under the curve of the CNCQ-OSA were 0.761 and 0.767, respectively. In the validation cohort, the sensitivity and specificity of a CNCQ-OSA score ≥ 13.5 points for the apnea–hypopnea index (AHI) ≥ 5/h were 0.821 and 0.559, respectively (Youden index, 0.380), and the score ≥ 14.5 points were 0.494 and 0.887, respectively (Youden index, 0.375). The CNCQ-OSA had a better predictive value for AHI ≥ 5/h, AHI > 15/h and AHI > 30/h, with the highest Youden index, compared to the other questionnaires.
Conclusion: The CNCQ-OSA can effectively identify the risk of OSA, which is appropriate for self-screening at home without physical examinations.
Keywords: obstructive sleep apnea, screening, GOAL, STOP-Bang, NoSAS
Introduction
Obstructive sleep apnea (OSA) is a common sleep-disordered breathing with condition characterized by complete or partial airway collapse during sleep, as well as decreased oxygen saturation and fragmented sleep.1 OSA is known to generate adverse changes such as nocturnal intermittent hypoxemia, thoracic negative pressure fluctuation, and metabolic problems, which can lead to multisystem chronic damage.2,3 Although the prevalence of OSA ranges from 9% to 38% and increases with age, the low adherence to treatment along with subsequent complications are a constant concern for physicians.4,5 As the proportion of elderly individuals is increasing gradually, early screening, diagnosis, and therapy are critical for improving the prognosis of OSA.
Polysomnography (PSG) is the gold standard for diagnosing OSA. Nevertheless, the popularity and use of PSG are unsatisfactory because it is an expensive and time-consuming process. As an alternative, OSA screening questionnaires, including the STOP-Bang questionnaire (SBQ), NoSAS score and the recently developed GOAL questionnaire, are most frequently utilized.6–8 Clinicians can rapidly identify individuals at risk for OSA by using multivariate screening questionnaires in sleep clinics.
To quickly evaluate the risk of OSA in patients before surgery, Chung et al developed and validated the SBQ.6 According to a meta-analysis, the overall sensitivity and specificity of the SBQ for detecting AHI ≥ 5/h were 0.88 and 0.44, respectively.9 The predictive efficiency of the SBQ has been demonstrated in patients from sleep clinics, surgical patients, and the general population, and the SBQ was later translated into multiple languages for wider usage.10–12 Another study found that the AUC of the SBQ for the global population and the European population were 0.76 and 0.78, respectively, while it was only 0.56 for the East Asian population.13 Several studies have indicated that the type of obesity and the location of fat accumulation are closely related to OSA.14 Central obesity is more common in Chinese individuals, and the waist–hip ratio and waist–height ratio are better screening indicators for OSA than neck circumference.15–17 It implied that the parameter of neck circumference and the screening cut-off points of age, BMI, and total score on the SBQ may not be entirely applicable to the Chinese population. Patients with OSA from Asian are younger, predominantly male, with lower obesity measures, compared to people from other continents.18
As previous study suggested that nearly 176 million adults aged 30–69 years in China have OSA,19 which might underestimates the prevalence of OSA because it does not cover all age groups. The community population in China has insufficient awareness of OSA and related screening instruments, so that a large number of potential OSA patients do not receive timely diagnosis and treatment.20 At the meanwhile, the majority of the questionnaires require clinicians to perform physical examination on the patients, such as measurement of the neck circumference (NC) for the SBQ and NoSAS. These may not be amenable to primary self-screening, particularly among elderly individuals and those living alone. Taken together, a simple and effective OSA screening questionnaire without a physical examination requirement that is applicable to Chinese patients is warranted. Based on such concerns, we aimed to developed an improved screening questionnaire for OSA, named the CNCQ-OSA (Chinese community questionnaire for OSA).
Methods
Study Participants
All participants were consecutively recruited from the Sleep Medical Center of the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China, from May 2018 to March 2022, including adults referred for PSG evaluation due to suspected sleep disordered breathing by their attending physicians or self-requested check-up. Patients were retrospectively recruited in the derivation cohort (from May 2018 to March 2021) and prospectively recruited in the validation cohort (from April 2021 to March 2022). This study complies with the Declaration of Helsinki, and was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University with Ethical Approval No. 05, 2017, and all patients gave and signed their informed consent. The inclusion criteria were (1) age >18 years; (2) complete independent behaviour and cognitive abilities; and (3) ability to answer the questionnaire completely and accurately. The exclusion criteria were (1) previous diagnosis of or treatment for OSA; (2) respiratory events dominated by central or mixed sleep apnea; (3) patients with a total sleep time <240 min by PSG; (4) patients with other chronic respiratory diseases (such as chronic obstructive pulmonary disease, bronchiectasis, interstitial lung disease, pulmonary fibrosis, lung tumours, etc.); (5) patients with acute and chronic diseases of the heart, liver, kidney and digestive system; and (6) patients with chronic insomnia, restless leg syndrome, narcolepsy and other sleep disorders. All participants from derivation cohort and validation cohort both met inclusion and exclusion criterion. The flow chart of this study is shown in Figure 1.
 |
Figure 1 Flow chart. Abbreviations: PSG, polysomnography; OSA, obstructive sleep apnea. |
Data Extraction
We retrospectively (derivation cohort) and prospectively (validation cohort) collected the self-reported variables, which are often associated with OSA from patient:21,22 (1) demographic characteristics, such as age, gender, height, weight; (2) previously diagnosed comorbidities, such as hypertension, diabetes, cardiovascular and cerebrovascular diseases, and nasopharyngeal diseases; and (3) OSA-related symptoms, such as loud snoring (louder than speech or heard through the door), apnea (conscious or observed by others), and sleepiness (daytime tiredness and dozing). Questions about comorbidities and OSA-related symptoms were answered with “yes” or “no”. BMI was calculated by participants or sleep physicians according to the formula (BMI = 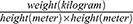 ). In derivation cohort and validation cohort, above information was collected 1 hour before PSG monitoring from patients who met the inclusion criteria but not the exclusion criteria.
). In derivation cohort and validation cohort, above information was collected 1 hour before PSG monitoring from patients who met the inclusion criteria but not the exclusion criteria.
Polysomnography
All participants underwent overnight polysomnography monitoring with an Alice 5 PSG (Philips Wellcome, USA) for at least 7 hours, and the use of alcohol, coffee, sedatives and hypnotics was prohibited on the same day. The monitoring indicators included electroencephalogram, electromyography, blood oxygen saturation, electrooculogram, electrocardiogram, snoring, mouth airflow, nasal airflow, chest breathing and body position.23 The raw data were automatically read by the instrument. Two trained sleep professionals separately manually analysed the parameters, such as sleep duration and sleep breathing events, based on the Manual for the Scoring of Sleep and Associated Events published by the American Academy of Sleep Medicine in 2012. Apneas were classified from a drop ≥90% of baseline airflow lasting at least 10s, while hypopnea were classified from a ≥30% pre-event drop over ≥10s associated with desaturation of oxygen ≥3% or an arousal.21 Patients with an apnea–hypopnea index (AHI) score of ≥5 events/h were defined as having OSA.21
Questionnaire
We aimed to develop an OSA screening questionnaire without physical examination focused on the Chinese community population (the CNCQ-OSA). The STOP-Bang questionnaire allocates 1 point for 8 items with positive results (loud snoring, tiredness, observed apnea, hypertension, body mass index (BMI) >35 kg/m2, age >50 years, NC > 40 cm, and male gender), 3 or more points indicates a high risk of OSA.6 NoSAS score is consisted of 5 questions with a final score of 0–17 points, with a threshold for high risk of OSA of 8 points or more. Four points for having NC > 40 cm, 3 points for having a BMI of 25 kg/m2 to less than 30 kg/m2 or 5 points for having a BMI ≥ 30 kg/m2, 2 points for habitual snoring, 4 points for age >55, and 2 points for the male gender.7 The GOAL questionnaire is a newly developed screening instrument that does not require physical examinations and was used to compare the predictive value of the CNCQ. The GOAL questionnaire includes 4 items (male, age ≥50, BMI ≥ 30 kg/m2, loud snoring), and each item needs to be answered as yes or no (scored 1 or 0 points, respectively, total points range from 0 to 4). If the assessment of 2 or more topics was positive, then the patient was considered at high risk for OSA.8
Statistical Analysis
All analyses were conducted using SPSS version 26.0. The results are expressed as the mean ± standard deviation for continuous variables and as number (n) and percentage (%) for categorical variables. The chi-square test was used for categorical variables in the comparison group, while Student’s t-test and one-way ANOVA were used for continuous variables. The original screening model was based on self-reported risk factors for OSA, including 11 factors: male gender, age, BMI, loud snoring, apnea, sleepiness, hypertension, diabetes, coronary heart disease, cerebrovascular disease, and nasopharyngeal disease. BMI was calculated based on reported height and weight from patients. The odds ratio (OR) of the above 11 variables was obtained by logistic regression analysis, and the variables showing statistical significance (P < 0.05) were selected as the next screening indicators.
Furthermore, the optimal cut-off points of age and BMI were determined according to the receiver operating characteristic (ROC) curve and Youden index; thus, age and BMI were converted into categorical variables. Multivariate logistic regression analysis was used to calculate the adjusted OR value of the next included indicators, which subsequently served as the corresponding score for the questionnaire. If the OR of the indicator for OSA was 1.5, then this item would receive 1.5 points. Otherwise, all negative answers received 1 point. The area under the ROC curve (AUC) was used to evaluate the final screening model, and the cut-off point for OSA screening was defined according to the sensitivity, specificity and the Youden index. Finally, 2×2 contingency tables were used to evaluate the sensitivity, specificity, Youden index, positive predictive value (PPV) and negative predictive value (NPV) of the CNCQ-OSA, GOAL, SBQ and NoSAS, comparing their predictive value for AHI ≥ 5/h, AHI > 15/h and AHI > 30/h. P values < 0.05 were considered indicative of statistical significance.
Results
Characteristics
A total of 2585 participants were ultimately included in the study and consecutively allocated into a derivation cohort (n = 2180) and validation cohort (n = 405). The mean age of all subjects was 46.9±14.2 years old, the mean BMI was 26.3±4.2 kg/m2, the mean NC was 38.4 ± 4.0 cm, and there were 2020 (78.1%) males. A total of 2388 (92.4%) participants had loud snoring, 1074 (41.5%) felt daytime sleepiness, and 992 (38.4%) had sleep apnea. The numbers of participants with hypertension, diabetes, cerebrovascular disease, coronary heart disease, and nasopharyngeal disease were 713 (27.6%), 203 (7.9%), 82 (3.2%), 130 (5.0%) and 822 (33.0%), respectively. Overall, 1859 (71.9%) patients were diagnosed with OSA, and the mean AHI value was 25.3 ± 25.6 events/h. There were statistically significant differences in age, BMI, snoring, sleepiness, cerebrovascular disease, nasopharyngeal disease, OSA diagnosis and AHI between the derivation and validation cohorts. The GOAL scores of the derivation and validation cohorts were 2.3 ± 0.8 and 2.3 ± 0.7, respectively, showing no significant difference. While there are statistical significance in SBQ and NoSAS between derivation and validation cohorts (Table 1).
 |
Table 1 The Characteristics of Including Participants with OSA |
Derivation Cohort
Establishment of the CNCQ-OSA
Initially, we conducted a statistical analysis of the data from the derivation cohort. In the multivariate logistic regression analysis with 11 variables included, there was no significant difference between diabetes mellitus, coronary heart disease, nasopharyngeal disease and cerebrovascular disease and the diagnosis of OSA (P>0.05, Figure 2A); thus, these four variables were excluded from the subsequent analysis. In the ROC curve of age and BMI for OSA diagnosis, “age ≥ 30 years old” (Youden index: 0.044; sensitivity: 0.901; specificity: 0.143) and “BMI ≥ 25 kg/m2” (Youden index: 0.262; sensitivity: 0.716; specificity: 0.546) were the best cut-off points for screening. The cut-off points used in the GOAL questionnaire were “age ≥ 50 years old” (Youden index: 0.008; sensitivity: 0.417; specificity: 0.591) and “BMI ≥ 30 kg/m2” (Youden index: 0.112; sensitivity: 0.208; specificity: 0.904), which had low sensitivity and Youden index values.
 |
Figure 2 Odds ratio for variables associated with OSA diagnosis. (A) Univariate logistic regression models; (B) Multivariate logistic regression. Abbreviation: BMI, body mass index. |
Based on the results, a multivariate logistic regression analysis was performed on seven variables: age ≥ 30 years, BMI ≥ 25 kg/m2, male gender, loud snoring, apnea, sleepiness, and hypertension. In descending order, the adjusted OR values are loud snoring (OR: 5.03; 95% CI: 3.45–7.33), BMI 25 ≥ kg/m2 (OR: 2.30; 95% CI: 1.87–2.83), male gender (OR: 2.21; 95% CI: 1.75–2.79), apnea (OR: 2.08; 95% CI: 1.65–2.62), sleepiness (OR: 1.91; 95% CI: 1.52–2.39), hypertension (OR: 1.64; 95% CI: 1.52–2.39), and age ≥ 30 years, which were all statistically significant (P < 0.001, Figure 2B). Finally, the CNCQ-OSA contains seven dichotomous elements, and the adjusted OR value was the score for each item (one decimal place reserved). As indicated in Table 2, a “yes” answer in “male” earns 2.2 points, while a “no” answer earns 1 point. The total CNCQ-OSA score ranges from 7 to 16.7. The final version of CNCQ-OSA is shown in Table 3.
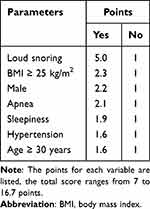 |
Table 2 The Scores of CNCQ-OSA |
 |
Table 3 The Final Version of Chinese Community Questionnaire for OSA, CNCQ-OSA |
Comparison of the AUCs and Other Predictive Value
The AUC of the CNCQ-OSA in the derivation cohort was 0.761 (95% CI: 0.739–0.783). The Youden index was calculated according to the sensitivity and specificity of each cut-off point, and the maximum score was 14.1 (Youden index: 0.388; sensitivity: 0.688; specificity: 0.700). A practical screening questionnaire should be considered to have a higher sensitivity and lower risk of missed diagnosis among OSA patients. Therefore, 13.5 points were selected as the screening cut-off point (Youden index: 0.382; sensitivity: 0.811; specificity: 0.571), and the Youden index did not considerably decrease, but the sensitivity was dramatically improved. In addition, a cut-off point of 14.5 with a specificity > 0.8 and the highest Youden index was selected for further screening for OSA (Youden index: 0.346; sensitivity: 0.540; specificity: 0.806).
In the comparison of CNCQ-OSA and the other screening tools, the AUC of the CNCQ-OSA is similar to the SBQ for AHI ≥ 5/h, AHI > 15/h and AHI > 30/h (0.761 vs 0.735, 0.745 vs 0.721, 0.746 vs 0.717, respectively), while the GOAL and NoSAS presented lower AUCs. As the OSA severity increased, the sensitivity of CNCQ-OSA gradually improved, ranging from 0.813 to 0.915, and the specificity dropped from 0.568 to 0.407. The results of AUCs and predictive value of various questionnaires are shown in Figure 3, Table 4 and Table 5.
 |
Table 4 The AUCs of Various Questionnaires in Different OSA Severity |
 |
Table 5 The Comparison of Predictive Value Between CNCQ-OSA and the Other Questionnaires for AHI ≥ 5/h, AHI > 15/h and AHI > 30/h (Derivation Cohort) |
Validation Cohort
The ROC curve was used to analyse the CNCQ-OSA, GOAL, SBQ and NoSAS (validation cohort, Figure 3) for AHI ≥ 5/h, and the AUCs were 0.767 (95% CI: 0.701–0.832), 0.727 (95% CI: 0.659–0.795), 0.637 (95% CI: 0.562–0.711) and 0.687 (95% CI: 0.615–0.760), respectively. Table 4 compares the AUCs for AHI > 15/h and AHI > 30/h in all questionnaires. As shown in Table 6, compared with a GOAL score of 2 for AHI ≥ 5/h, the sensitivity of a CNCQ-OSA score of 13.5 was slightly lower (0.821 vs 0.926), but the specificity and Youden index value were significantly higher than those of the GOAL questionnaire (0.559 vs 0.339, 0.380 vs 0.267). When a CNCQ-OSA score of 14.5 was used as the second screening cut-off point, the specificity of the CNCQ-OSA for AHI ≥ 5/h was slightly lower than that of a GOAL score of 3 (0.881 vs 0.911), but the sensitivity and Youden index were higher than those of GOAL questionnaire (0.494 vs 0.393, 0.375 vs 0.304). The AUCs of the CNCQ-OSA, GOAL and NoSAS presented stable results in derivation and validation cohort for different OSA severity, while the AUC of the SBQ dramatically declined with decreasing specificity in validation cohort (Table 4). In both cohorts, the CNCQ-OSA had a better predictive value for AHI ≥ 5/h, AHI > 15/h and AHI > 30/h, with the highest Youden index. The GOAL questionnaire always showed a highest sensitivity, and the NoSAS score showed a highest specificity.
 |
Table 6 The Comparison of Predictive Value Between CNCQ-OSA and the Other Questionnaires for AHI ≥ 5/h, AHI > 15/h and AHI > 30/h (Validation Cohort) |
Discussion
In this study, the screening model (CNCQ-OSA) was established using self-reported OSA-related parameters from patients, and scores were assigned based on the weight of each indicator (including age ≥30 years, BMI ≥ 25 kg/m², male gender, loud snoring, sleepiness, apnea and hypertension) by rigorous scientific and statistical methods. Our study revealed that the predictive value of the CNCQ-OSA was superior to that of the previous validated questionnaires: GOAL, STOP-Bang, and NoSAS, effectively identifying the high risk of OSA.
BMI is closely related to height and weight. People in China frequently visit primary hospitals for check-ups and follow-ups every two weeks or once a month. The majority of drugstores offer free devices to measure height and weight. Therefore, members in Chinese community can easily acquire their standard height and weight to evaluate the BMI as a self-reported demographic characteristic. According to the reports, the mean BMI of Chinese population and patients with OSA was 24.4 kg/m2 and 25.4–28.4 kg/m2, respectively, which is considerably lower than that of Caucasians.16,18,24 Obesity is a main cause of OSA, with Chinese being particularly sensitive to increases in weight.25 In OSA screening questionnaires, it has been shown that the cutoff of BMI, age and the total scores do not entirely apply to Chinese population.26–28 Compared to Caucasians, Chinese patients with OSA appear to be younger, predominant in male and have a lower BMI. It is recommended that the threshold of BMI in SBQ should be lowered to 25–30 kg/m2 for an improving predictive accuracy.26,29 At the primary cutoff in NoSAS score, consistent with our research, the predictive value showed a low sensitivity and high specificity, which is not applicable for screening a large amount of population.27,28
The GOAL questionnaire, which was recently developed by Duarte et al, is a simple OSA screening tool ranging from 0 to 4 points.8 Since the GOAL includes only self-reported characteristics, it is more comparable to CNCQ-OSA. In their study, the sensitivity, specificity and AUC of the GOAL for screening AHI ≥ 5/h were 0.837, 0.634 and 0.794, respectively. The predictive efficiency of the GOAL was similar to that of the SBQ, NoSAS, and other questionnaires. Nevertheless, the sensitivity, specificity, and Youden index of a GOAL score ≥2 in our study (derivation cohort and validation cohort) were 0.953, 0.265, and 0.218, respectively. Despite the high sensitivity, the low specificity is likely to result in misdiagnoses and waste of resources. One of the main reasons might be that middle-aged and older males were almost directly identified as being at a high risk for OSA in this questionnaire. Additionally, the GOAL questionnaire simply converted the four indicators into dichotomous variables without taking into account the weight of different variables and common comorbidities, such as hypertension, which eventually led to limited specificity.
Many questionnaires require physical examinations, such as measurements of neck circumference and waist circumference, which make it challenging for people to complete the questionnaires at home. To increase the follow-up rate and questionnaire response rate, a simple and effective screening tool suitable for primary care must be created. The community population was the target for CNCQ-OSA, and the corresponding score for each parameter was objectively assigned based on their correlation with OSA. After examining the sensitivity and specificity, the best screening cut-off point was determined. The seven indicators ultimately included in the questionnaire were comparable to those of the SBQ, but we employed age ≥30 years and BMI ≥25 kg/m² for the cut-off point after analysing the data given the characteristics of the Chinese population. BMI and neck circumference are measures of obesity and share a similar association with the high risk of OSA.30 In the validation cohort, the cut-off point of a CNCQ-OSA score of 13.5 had a higher specificity than the SBQ and GOAL while maintaining a high sensitivity. With a CNCQ-OSA score of 14.5 points as the cut-off, the sensitivity was 0.494 (0.442–0.547), and its specificity increased to 0.881 (0.799–0.964). Although the score increased by only 1 point, the predictive ability dramatically improved. The Youden index derived at the two cut-off points exhibited superior predictive performance to that of the GOAL questionnaire (score of 2 or 3 points). Compared to the GOAL, which ranges from 0 to 4 points, the CNCQ-OSA is more accurate in score distribution. In the future, the cut-off point can be dynamically changed in response to validation research in a community population, potentially improving the screening accuracy. Since our study have showed that the CNCQ-OSA had a better predictive value compared to the SBQ and NoSAS, we believe that the predictive value for OSA would be significantly promoted when adding physical examination to our screening model.
The research has some limitations that should be emphasized. (1) Due to the prevalence of COVID-19, the number of people coming to the sleep medicine center for check-up was significantly lower than before. Thus, the sample size of the validation cohort was insufficient, accounting for some significant differences at baseline between the derivation cohort and validation cohort. Nevertheless, the AUC did not significantly change between the two cohorts (0.761 vs 0.767, P<0.05), displaying reasonable stability. (2) The individuals included in this study were inpatients or outpatients come from sleep medicine center for variable reasons, such as suspected sleep disordered breathing by clinicians and check-up, which may lead to selection bias. The parameter of age ≥30 years in questionnaire might have limited value in practice since the mean age of all subjects was 46.9 years. The predictive value in the community population will be evaluated and verified in the next phase of research. (3) The CNCQ-OSA consists of 7 variables with different scores, resulting in probable trouble in self-evaluation and a loss of practicality. According to the CNCQ-OSA, we are designing a electronic screening system established on the platform of WeChat Mini Program to promote the practical performance and utility.
In conclusion, the screening questionnaire called CNCQ-OSA, which consists of seven self-report indicators, has considerable predictive value for OSA. Future work will focus on improving the questionnaire mainly for physicians by attempting to expand the screening system with physical measurements, such as the waist–hip ratio, body fat rate, and pharyngeal cavity evaluation.31,32 Considering the characteristics of a large population and high prevalence of OSA in China, a simple, effective and accurate screening procedure and the corresponding electronic screening instruments should be further developed.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
We would also like to thank Professor Nanshan Zhong from State Key Laboratory of Respiratory Disease for the constructive advice he gave.
Funding
This research project was supported by Basic Research Project (Dengfeng hospital) jointly Funded by Guangzhou City and the School (No.202201020586), Independent Project of State Key Laboratory of Respiratory Diseases (SKLRD-Z-202005) and the Natural Science Foundation of Guangdong Province China (No. 2019A1515010981).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zinchuk A, Yaggi HK. Phenotypic subtypes of OSA: a challenge and opportunity for precision medicine. Chest. 2020;157(2):403–420. doi:10.1016/j.chest.2019.09.002
2. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383(9918):736–747. doi:10.1016/S0140-6736(13)60734-5
3. Kim DH, Kim B, Han K, et al. The relationship between metabolic syndrome and obstructive sleep apnea syndrome: a nationwide population-based study. Sci Rep. 2021;11(1):8751. doi:10.1038/s41598-021-88233-4
4. Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi:10.1016/j.smrv.2016.07.002
5. Testelmans D, Spruit MA, Vrijsen B, et al. Comorbidity clusters in patients with moderate-to-severe OSA. Sleep Breath. 2022;26(1):195–204. doi:10.1007/s11325-021-02390-4
6. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. doi:10.1097/ALN.0b013e31816d83e4
7. Marti-Soler H, Hirotsu C, Marques-Vidal P, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med. 2016;4(9):742–748. doi:10.1016/S2213-2600(16)30075-3
8. Duarte RL, Magalhães-da-Silveira FJ, Oliveira-E-Sá TS, et al. Obstructive sleep apnea screening with a 4-item instrument, named GOAL questionnaire: development, validation and comparative study with No-Apnea, STOP-Bang, and NoSAS. Nat Sci Sleep. 2020;12:57–67. doi:10.2147/NSS.S238255
9. Chiu HY, Chen PY, Chuang LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70. doi:10.1016/j.smrv.2016.10.004
10. Miller JN, Kupzyk KA, Zimmerman L, et al. Comparisons of measures used to screen for obstructive sleep apnea in patients referred to a sleep clinic. Sleep Med. 2018;51:15–21. doi:10.1016/j.sleep.2018.06.007
11. Duarte RLM, Fonseca LBM, Magalhães-da-Silveira FJ, et al. Validation of the STOP-Bang questionnaire as a means of screening for obstructive sleep apnea in adults in Brazil. J Bras Pneumol. 2017;43(6):456–463. doi:10.1590/s1806-37562017000000139
12. Tan A, Yin JD, Tan LW, et al. Predicting obstructive sleep apnea using the STOP-Bang questionnaire in the general population. Sleep Med. 2016;27:66–71. doi:10.1016/j.sleep.2016.06.034
13. Pivetta B, Chen L, Nagappa M, et al. Use and performance of the STOP-Bang questionnaire for obstructive sleep apnea screening across geographic regions: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(3):e211009. doi:10.1001/jamanetworkopen.2021.1009
14. Zhao X, Xu H, Qian Y, et al. Abdominal obesity is more strongly correlated with obstructive sleep apnea than general obesity in china: results from two separated observational and longitudinal studies. Obes Surg. 2019;29(8):2535–2547. doi:10.1007/s11695-019-03870-z
15. Lim YH, Choi J, Kim KR, et al. Sex-specific characteristics of anthropometry in patients with obstructive sleep apnea: neck circumference and waist-Hip ratio. Ann Otol Rhinol Laryngol. 2014;123(7):517–523.
16. Lee RW, Vasudavan S, Hui DS, et al. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep. 2010;33(8):1075–1080. doi:10.1093/sleep/33.8.1075
17. Zou H, Jia Q, Yang W, et al. Clinical and polysomnographic characteristics of nonobese and obese Chinese patients with obstructive sleep apnea. J Clin Neurophysiol. 2021;2021. doi:10.1097/WNP.0000000000000831
18. Sutherland K, Keenan BT, Bittencourt L, et al. A global comparison of anatomic risk factors and their relationship to obstructive sleep apnea severity in clinical samples. J Clin Sleep Med. 2019;15(4):629–639. doi:10.5664/jcsm.7730
19. Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi:10.1016/S2213-2600(19)30198-5
20. Su L, Xiao Y. Application of personalized medicine to obstructive sleep apnea in China. Sleep Med. 2021;87:22–29. doi:10.1016/j.sleep.2021.08.014
21. Sunderram J, Weintraub M, Black K, et al. Chronic rhinosinusitis is an independent risk factor for OSA in world trade center responders. Chest. 2019;155(2):375–383. doi:10.1016/j.chest.2018.10.015
22. Lyons MM, Bhatt NY, Pack AI, et al. Global burden of sleep-disordered breathing and its implications. Respirology. 2020;25(7):690–702. doi:10.1111/resp.13838
23. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2012;8(5):597–619. doi:10.5664/jcsm.2172
24. Zhang Z, Cheng J, Yang W, et al. Gender differences in clinical manifestations and polysomnographic findings in Chinese patients with obstructive sleep apnea. Sleep Breath. 2020;24(3):1019–1026. doi:10.1007/s11325-019-01943-y
25. Lim DC, Pack AI. Obstructive sleep apnea: update and future. Annu Rev Med. 2017;68:99–112. doi:10.1146/annurev-med-042915-102623
26. Wang L, Zhao W, Liang C, et al. Accuracy and modification of the STOP-bang questionnaire for screening patients with obstructive sleep apnea in China. J Sleep Res. 2022;13:e13781.
27. Zhang Z, Yang D, Wang H, et al. Effects of age and sex on the performance of the NoSAS score as a screening tool for obstructive sleep apnea: a hospital-based retrospective study in China. Sleep Breath. 2021;25(3):1407–1417. doi:10.1007/s11325-020-02254-3
28. Chen R, Zhang Y, Luo Y, et al. Application value of joint NoSAS score and Epworth Sleepiness Scale for assessment of obstructive sleep apnea hypopnea syndrome. Sleep Med. 2022;97:36–42. doi:10.1016/j.sleep.2022.05.845
29. Waseem R, Chan MTV, Wang CY, et al. Diagnostic performance of the STOP-Bang questionnaire as a screening tool for obstructive sleep apnea in different ethnic groups. J Clin Sleep Med. 2021;17(3):521–532. doi:10.5664/jcsm.8940
30. Kawaguchi Y, Fukumoto S, Inaba M, et al. Different impacts of neck circumference and visceral obesity on the severity of obstructive sleep apnea syndrome. Obesity. 2011;19(2):276–282. doi:10.1038/oby.2010.170
31. Li T, Yao ZM, Wang L, et al. The role of body fat rate in the evaluation of obstructive sleep apnea. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019;54(6):427–431. doi:10.3760/cma.j.issn.1673-0860.2019.06.006
32. Laharnar N, Herberger S, Prochnow LK, et al. Simple and unbiased OSA prescreening: introduction of a new morphologic OSA prediction score. Nat Sci Sleep. 2021;13:2039–2049. doi:10.2147/NSS.S333471
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

