Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Establishing Patient-Centered Outcomes for MCT8 Deficiency: Stakeholder Engagement and Systematic Literature Review
Authors Wilpert NM , Tonduti D, Vaia Y , Krude H , Sarret C , Schuelke M
Received 1 June 2023
Accepted for publication 7 October 2023
Published 20 October 2023 Volume 2023:19 Pages 2195—2216
DOI https://doi.org/10.2147/NDT.S379703
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Nina-Maria Wilpert,1,2 Davide Tonduti,3 Ylenia Vaia,3 Heiko Krude,4 Catherine Sarret,5 Markus Schuelke1,2,6
1Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health (BIH), Department of Pediatric Neurology, Berlin, Germany; 2Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität Zu Berlin, and Berlin Institute of Health (BIH), Center for Chronically Sick Children, Berlin, Germany; 3Unit of Pediatric Neurology, C.O.A.L.A. (Center for Diagnosis and Treatment of Leukodystrophies), V. Buzzi Children’s Hospital, Università Degli Studi Di Milano, Milan, Italy; 4Charité - Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health (BIH), Institute of Experimental Pediatric Endocrinology, Berlin, Germany; 5Centre de Compétence des Leucodystrophies et Leucoencéphalopathies de Cause Rare, Centre Hospitalier Universitaire de Clermont-Ferrand, Clermont-Ferrand, France; 6Charité - Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health (BIH), NeuroCure Clinical Research Center, Berlin, Germany
Correspondence: Markus Schuelke, Charité - Universitätsmedizin Berlin, Campus Virchow-Klinikum, Augustenburger Platz 1, Berlin, 13353, Germany, Tel +49 30 450566468, Email [email protected]
Introduction: The SCL16A2 gene encodes the thyroid hormone (TH) transporter MCT8. Pathogenic variants result in a reduced TH uptake into the CNS despite high serum T3 concentrations. Patients suffer from severe neurodevelopmental delay and require multidisciplinary care. Since a first compassionate use study in 2008, the development of therapies has recently gained momentum. Treatment strategies range from symptom-based approaches, supplementation with TH or TH-analogs, to gene therapy. All these studies have mainly used surrogate endpoints and clinical outcomes. However, the EMA and FDA strongly encourage researchers to involve patients and their advocacy groups in the design of clinical trials. This should strengthen the patients’ perspective and identify clinical endpoints that are clinically relevant to their daily life.
Methods: We involved patient families to define patient-relevant outcomes for MCT8 deficiency. In close collaboration with patient families, we designed a questionnaire asking for their five most preferred therapeutic goals, which, if achieved at least, make a difference in their lives. In addition, we performed a systematic review according to Cochrane recommendations of the published treatment trials.
Results: We obtained results from 15 families with completed questionnaires from 14 mothers and 8 fathers. Improvement in development, especially in gross motor skills, was most important to the parents. 59% wished for head control and 50% for sitting ability. Another 36% wished for weight gain, 32% for improvement of expressive language skills, and 18% for a reduction of dystonia/spasticity, less dysphagia, and reflux. Paraclinical aspects were least important (5– 9%). In a treatment trial (n=46) and compassionate use cases (n=83), the results were mainly inconclusive, partly due to a lack of predefined patient-centered clinical endpoints.
Discussion: We recommend that future trials should define a relevant improvement in “development” and/or other patient-relevant outcomes compared to natural history as treatment goals.
Keywords: MCT8 deficiency, SLC16A2, ultra-rare disease, movement disorders, Triac, stakeholder engagement
Introduction
Thyroid hormone (TH) action in the central nervous system (CNS) is essential for proper brain development and function. While TH is released from the thyroid gland into the bloodstream, its uptake by target organs must be facilitated by a complex array of different TH transporters. To reach neuronal cells, TH must be transported across multiple cell membrane barriers (endothelium, pericytes, astrocytes, neurons) of the neurovascular unit that protects the CNS cells.1–4 Once in the intracellular compartment, TH binds to its nuclear receptors THRα and THRβ to modify gene expression.5 Animal models have shown that T3 (3,3’,5-triiodothyronine)-regulated genes play a critical role in neurodevelopmental processes such as cell proliferation, cell fate decision, axonogenesis, synaptogenesis, and myelinogenesis.6 Insufficient TH supply during the first trimester of pregnancy can cause severe psychomotor retardation as seen in children born in severely iodine-deficient regions.7–9 Insufficient postnatal TH production causes intellectual and motor disability, which can be prevented in children with congenital hypothyroidism by LT4 (levothyroxine) substitution, which should be initiated immediately after birth.10 However, under conditions of impaired TH transport to the brain, TH cannot become active at its designated sites, leading to “local TH deficiency” before and after birth.
Consequences of inactivating mutations of only one TH transporter, the monocarboxylate transporter 8 (MCT8), illustrate the fragility of the spatiotemporal regulation of TH action in the brain. Although the first patients with this disease were published in 1944 by the American geneticists William Allan and Nash Herndon, and their assistant, Florence Dudley,11 the underlying molecular causes remained obscure for a long time. In 2004, two research teams finally succeeded in identifying X-chromosomal mutations in SLC16A2 (encoding MCT8) as the cause of the Allan-Herndon-Dudley syndrome (AHDS, OMIM #300523).12,13 Since then, only approximately 200 individuals with a variety of different SLC16A2 mutations have been identified worldwide (ultra-rare disease).14 Patients present with elevated peripheral T3 concentrations, resulting in a complex spectrum of hypo- and hyperthyroid symptoms depending on the cell- and organ-specific TH transporter composition. The most prominent features are severe global developmental delay, chronic and paroxysmal movement disorders (dystonia, hypo-/bradykinesia, chorea, myoclonus), spasticity, epilepsy, underweight, tachycardia and hypertension, and early death.14–18 The overall disease burden for children and families is high.
Since the first compassionate use trial with LT4 and PTU (propylthiouracil) in 2008,19 the development of therapies for MCT8 deficiency has recently gained momentum. Treatment strategies range from symptomatic interventions,15,20 replacement therapies19,21–26 and chaperone rescue27 to gene-modifying approaches.28,29 Clinical outcome measures for all these studies vary widely, often consisting of surrogate parameters. The (European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) strongly encourage researchers to involve patients and their advocacy groups in the design of clinical trials. The aim is to strengthen patients’ perspective on their disease to identify clinical endpoints that are clinically relevant to their daily lives. In this case, we involved patient families to define targets for therapies against MCT8 deficiency. In close collaboration with patient families, we designed a questionnaire asking for the five most preferred therapeutic goals that, at minimum, would bring a change in their daily lives. In addition, we performed a systematic literature review on the therapeutic options for MCT8 deficiency and evaluated treatment effects on patient-relevant outcome parameters.
Methods
Stakeholder Engagement and Patient-Oriented Outcomes
For stakeholder engagement, we involved parents (n=4) who had already shown great interest in the current therapeutic development for children with AHDS, had sufficient knowledge of English and were networking with other families with affected children (self-initiated WhatsApp group). We drafted a first proposal for a patient-oriented outcome questionnaire, which was modified in two meetings with the parents. The parents distributed and collected the questionnaires independently in their group. We interpreted the results of the survey together with the parents. Results were summarized descriptively. Ethical approval for the study was obtained from the Institutional Review Boards of Charité (EA2/026/20) and Milano Area 1 (2019/ST/221). Written informed consent was obtained from all participants. The study was conducted in accordance with the tenets of the Declaration of Helsinki.
Systematic Literature Review
Search Strategies Used to Identify Studies
We systematically searched PubMed, Google Scholar, and ClinicalTrials.gov up to October 17, 2022, using the following search terms: “[(MCT8) OR (SLC16A2) OR (Allan Herndon Dudley syndrome)] AND [(therapy) OR (treatment)]” without restrictions on publication type or language. We also searched for eligible studies by screening the reference lists of included articles and reviews. This yielded n=2,430 publications.
Criteria for Inclusion of Studies in This Review
From the above publications, only studies on the topic of treatment for AHDS patients published in peer-reviewed journals and available as full-text articles in English were included. Reviews were excluded. Only male participants with a proven pathogenic SLC16A2 mutation were included. We assessed all studies with treatment trials regardless of treatment strategy. We excluded articles reporting on studies in animal models and in vitro analyses. Intraindividual comparisons of compassionate use studies, comparisons with the natural history, and placebo controls were included. We included all studies regardless of their reported outcome parameters.
Data Collection and Analysis
A first reviewer screened the results of the above-mentioned search strategies for eligibility (see Criteria for inclusion of studies in this review) by reading titles and abstracts. In cases of uncertainty, the reviewer read the full text of the article and consulted a second reviewer. The study selection process was documented in a flow chart according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.30 First, we extracted data on the favorite twelve preferred patient-oriented outcomes from the survey. We then extracted data from full-text articles and supplementary data on patient-oriented outcome measures and outcomes. We fully considered the data provided in the supplements. For missing data, we tried to contact the corresponding author of the respective study or digitized data from published figures using ImageJ. Not all authors responded to our requests. The study criteria were described, and the risk of bias of the categories “selection”, “performance”, “detection”, “attrition”, “reporting”, and “other bias” was assessed according to the Cochrane recommendations. The arguments that led to each assessment are listed in Table 1. The overall risk of bias was classified as “low”, “moderate”, or “high”. Due to the lack of comprehensive data on age-related patient-oriented outcomes, the results could only be summarized descriptively without performing a meta-analysis.
 |
Table 1 Study Characteristics and Risk of Bias |
Results
Stakeholder Engagement and Patient-Oriented Outcomes
In close collaboration with patient families, we designed a survey that included (i) a section on the patient’s clinical phenotype to account for phenotypic heterogeneity, (ii) a list of parents’ therapy wishes, and (iii) a list of the child’s anticipated therapy wishes (Supplementary Figure 1). Free text was allowed in all sections. The survey was to be completed by each parent separately. It was distributed through a German patient advocacy group and through the Italian Leukodystrophy Center C.O.A.L.A. (Center for Diagnosis and Treatment of Leukodystrophies). We received results from 15 families completed by 14 mothers and 8 fathers.
Phenotype Spectrum of Patients
The cohort of patients (all male) ranged from infants to young adults. The median age was 10.75 years (range 2.5–19.9). All patients had a severe developmental delay (Supplementary Figure 2). At 2.5 years of age (our youngest patient), children are usually able to sit, walk, run, jump (gross motor skills), build towers with multiple cubes, scribble (fine motor skills) and combine words (verbal skills). In our cohort, however, only 4/22 (18%) were able to hold the head, 1/22 (5%) were able to sit, and no patient was able to walk. 6/22 (27%) patients could grasp objects, and 1/22 (5%) could speak words. All patients had disease-related complications: 21/22 (95%) had spasticity and/or dystonia, 21/22 (95%) had dysphagia, 19/22 (86%) were underweight, 17/22 (77%) had gastro-esophageal reflux, and 5/22 (23%) were gastric tube dependent.
The Achievement of Developmental Milestones Was Most Important
While all parents (100%) selected improvement in neurodevelopment (motor, verbal, or social skills) as their preferred patient-oriented outcome (Figure 1), the achievement of gross motor milestones was prioritized by parents: 13/22 (59%) wished for head control, 11/22 (50%) for the ability to sit and 6/22 (27%) for the ability to walk. Improving expressive and receptive language skills was also important for parents with 7/22 (32%) wishing for the ability to articulate single words, 4/22 (18%) for the ability to speak sentences, and 4/22 (18%) for the ability to understand simple questions. The patients’ choices on relevant outcomes were anticipated by their parents (due to intellectual disability) to be motor and language development (Figure 2).
Second, Parents Prioritized Dystonia, Spasticity, and Gastrointestinal Symptoms
Second, after developmental improvement, parents chose therapy goals that addressed gastrointestinal complications or the movement disorders/pyramidal signs (Figure 1). 8/22 (36%) wanted weight gain, 4/22 (18%) wanted gastric tube independence, and 4/22 (18%) wanted to reduce gastro-esophageal reflux. Reduction of dystonia/spasticity (4/22, 18%) and associated dysphagia (6/22, 27%) was also very important.
Other Outcomes
Other outcomes, such as a reduction in infectious or orthopedic complications, were considered relevant in <15% of patients. Paraclinical aspects, such as laboratory values or imaging results, were least important (5–9%).
Gender Differences of Patient-Oriented Outcomes
Interestingly, patient-oriented outcome choices differed significantly between mothers and fathers (Supplementary Figure 3). While fathers clearly prioritized improvement of gross and fine motor skills, mothers prioritized improvement of motor development as well, but the choices were more dispersed across the categories. Only mothers selected paraclinical treatment goals. Mothers were more ambitious at 5/14 (36%) versus 1/8 (13%) who wanted the child to learn walking.
Systematic Literature Review
We then performed a systematic literature review to search for available treatment trials for MCT8 deficiency. After searching the PubMed and Google Scholar databases, we identified a total 2,430 publications (Figure 3). We removed 150 duplicates and excluded an additional 2,204 studies after screening their titles and abstracts that did not address therapeutic strategies for MCT8 deficiency. We read 76 full-text articles from peer-reviewed journals and assessed their eligibility for inclusion in the systematic review. We excluded commentaries (n=3), basic research articles (n=6), natural history studies (n=15), in vitro therapeutic trials (n=8), and animal model studies (n=12). After exclusion of 23 reviews, n=9 original articles15,19–26 remained.
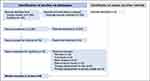 |
Figure 3 PRISMA flowchart for systematic literature review. We searched PubMed, Google Scholar and ClinicalTrials.gov for “[(MCT8) OR (SLC16A2) OR (Allan Herndon Dudley syndrome)] AND [(therapy) OR (treatment)]” until October 17, 2022. We included studies on the topic of treatment of AHDS patients from peer-reviewed journals that were published as full-text articles and in the English language. Reviews (n=23) were excluded. We considered all studies with treatment trials regardless of treatment strategy. Articles with studies in animal models (n=12) or in vitro (n=8) analyses were excluded. Studies were included irrespective of their control group or reported outcome parameters. Finally, nine studies were included in the systematic review.15,19–26 |
Study Characteristics and Risk of Bias
We summarized the study characteristics and the risk of bias of the nine included studies according to the Cochrane recommendations (Table 1).15,19–26 Overall, the studies were highly heterogenous in design, patient numbers, treatment duration and outcomes. Most studies were compassionate use or natural history studies (total of n=1-4 patients). Only the Triac treatment was tested in a total of n=113 patients. The following treatment strategies were identified:
(i) Block & Replace: The first treatment strategy for MCT8 deficiency in n=1 patient comprised PTU (“block”) plus LT4 substitution (“replace”) for a total of 9 months.19 LT4 was given to saturate the TH transport despite the MCT8 transporter defect and PTU to block the extrathyroidal conversion of T4 to T3 by deiodinases and endogenous TH synthesis with the rational of reducing peripheral elevated T3 concentrations but increasing serum T4 as the major TH supply to the brain.1 Another compassionate use study of n=1 patient was published five years later with the addition of MMI (methimazole) for approximately 2 years, which is a thyrostatic medication that blocks TH synthesis only.24
(ii) LT4 and LT3 (liothyronine) substitution: Long-term treatment with LT4 for 6.5 years and short-term treatment with LT3 for 6 months was tested to saturate TH transport in n=1 patient.21
(iii) Prenatal use of LT4: The only prenatal approach was performed in an index family, in which a mother was pregnant with a second affected child.26 The male fetus was treated with intra-amniotic LT4 from the 18th week of gestation and postnatally with LT4 and PTU as described above. Therapeutic responses were then compared with the older brother who was treated postnatally only.
(iv) Triac (3,3’,5-triiodothyroacetic acid): By far, the most extensive therapy studies have been conducted with the TH analogue Triac. Triac can cross cell membranes independently of the MCT8 transporter, bind to TH receptors and induce TH action.31 In an international open-label phase 2 trial (TRIAC I), n=46 patients with MCT8 deficiency were enrolled and treated with Triac for 12 months.22 In a subsequent “real-life” follow-up, another n=67 patients received Triac on an off-label basis for 0.2–6.2 years. Results were reported from a retrospective survey.23
(v) DIPTA (3,5-diiodothyropropionic acid): A compassionate use study with n=4 participants was conducted with DIPTA, another TH derivative.25 Patients were treated for 26–40 months.
(vi) Levodopa/Carbidopa: The use of Levodopa/Carbidopa, as standard of care to reduce dystonia and facilitate the development of voluntary movements, was only briefly mentioned in two natural history studies as an adjunctive therapy in a total of n=5 patients.15,20
(vii) Botulinum toxin A: Botulinum toxin is a potent inhibitor of the peripheral neuromuscular transmission and can reduce spasticity or dystonia, allowing for more purposeful movement. Botulinum toxin A was injected in n=3 AHDS patients, described in a natural history study.15
Treatment Effects on Development
Improvement in neuromotor development was rarely defined as a primary outcome, and investigators assessed it using different scales: GMFM88, Bayley III, VABS II, CAT/CLAMS, and clinical neurological examination (Table 2). The most promising results were achieved in the pre- and postnatally treated 31-month-old patient, who reached more advanced motor and language milestones compared to the affected 58-month-old brother: eg, 12 versus 1 month equivalent for gross motor functions and 25 versus 7 months equivalent for receptive language.26
 |
Table 2 Treatment Effects on TOP 12 Family-Oriented Outcome Measures |
The effect of Triac on development was tested as an exploratory endpoint in the Triac I study.22 The investigators found no intra-individual improvement in GMFM88 scores in patients >4 years of age (n=7). However, in patients <3.5 years-of-age (n=7), they found an increase of up to ∆15% in gross motor function scores. However, children did not achieve significantly higher scores than 20% of age-corrected values. When we compared the GMFM88 scores of Triac-treated patients with a natural history cohort,14 we found no relevant improvement with treatment (Supplementary figure 4). Age-related data for precise quantitative comparison were unfortunately not provided by the corresponding authors upon written request. In the “real-life” retrospective follow-up assessment, neurological data were not reported due to a lack of data, although n=23 patients (34%) belonged to the cohort of children who were younger than 2.5 years at treatment initiation, who may be responsive to early treatment.32
Treatment with Levodopa/Carbidopa resulted in a transient improvement in head control and spontaneous movements.20 However, there are insufficient data. No improvement in development has been reported with DIPTA,25 Block-and-Replace,19,24 or LT4, LT3 substitution.21
Treatment Effects in Dystonia and Spasticity
Hyperkinetic movement disorders (dystonia) or pyramidal signs (spasticity) have rarely been evaluated in treatment trials and potential effects have not been adequately tested (Table 2). After prenatal LT4 therapy, one patient had milder spasticity (neurological examination, no quantification) compared with the affected older brother.26 While Levodopa/Carbidopa produced a transient improvement in spontaneous movements, possibly due to reduced involuntary movements or muscle tone in the n=1 child, another n=4 patients had “poor efficacy” (not further specified) under treatment.15,20 Botulinum toxin A injections have been reported to have “some benefit on spasticity” in n=3 treated patients.15
Treatment Effects on Underweight
With respect to patient underweight, intra-individual increases in body weight (z-scores) have been reported with Triac treatment (mean ∆0.27–0.72 SD),22,23 DIPTA treatment (∆0.4–2.0 SD),25 prenatal LT4 (∆4% for age),26 and TH substitution (∆0.4 SD)21 (Table 2). In comparison, the body weight z-scores progressively decreased over time in a natural history cohort compared with normal controls.14 Other studies did not report body weight or did not relate the body weight to age (z-score).
Treatment Effects on Dysphagia, Need for Gastric Tube, and Gastro-Esophageal Reflux
Other TOP 12 patient-oriented outcomes such as dysphagia, gastro-esophageal reflux, or the need for a gastric tube were not adequately assessed in either study (Table 2).
Treatment Effects on Mortality Rate
Mortality was not part of the family survey, but can be considered an important overall and relevant outcome measure. The number of age-related deaths was reported only in one natural history study (15% deaths in patients aged 10–18 years)14 and in the “real-life” Triac compassionate use observation (20% deaths in patients aged 10–18 years).23
Discussion
Patients with MCT8 deficiency suffer from a complex spectrum of hypo- and hyperthyroid symptoms with severe neurological impairment (global developmental delay, axial hypotonia, movement disorders, spasticity, epilepsy, dysphagia), gastro-esophageal reflux, reduced body weight, infectious (recurrent infections), and orthopedic complications (scoliosis, contractures). Over the past 15 years, therapies ranging from symptomatic (Levodopa/Carbidopa, Botulinum toxin A),15,20 to TH replacement therapies (Triac, DIPTA, prenatal LT4, postnatal TH supplementation, Block & Replace)19,21–26 have been developed and tested in patients. Gene-modifying approaches have recently been tested in mouse models.28,29
To identify patient-relevant outcome measures for these multimorbid children, we conducted a stakeholder engagement and collected surveys from 15 affected families. Improvement in their children’s motor and language skills was by far the most important outcome for families (Figure 1). In addition, parents wished for weight gain and for reduced dystonia/spasticity, dysphagia, gastro-esophageal reflux and the removal of the gastric tube.
In addition, we performed a systematic literature review to collect data on therapy effects on patient-relevant outcomes of available treatment strategies and included n=9 studies (Figure 3). Most of the studies defined paraclinical endpoints as primary goals of therapy (Table 1). Although the majority of patients present with elevated serum T3 concentrations, increased heart rate, arterial hypertension, and abnormal MRI,14 these endpoints were the least important to parents. In future studies, we suggest including the TOP 12 patient-oriented treatment goals (Figure 1) as outcome measures in addition to paraclinical aspects.
To date, the most convincing evidence of language and motor improvement with therapy has been achieved by prenatal LT4 treatment of n=1 affected male fetus (Table 2).26 However, this approach is only feasible in index families and does not represent a solution for most patients. The ongoing TRIAC II trial (NCT02396459) is currently testing whether early initiation of Triac treatment before the age of 2.5 years may have a beneficial effect. In this trial, the primary outcome measure is development as assessed by GMFM88 and Bayley III.
Of note, many genetic disorders manifest in early childhood, and pediatric patients may continue to developmental milestones, albeit at a slower rate than healthy children. This apparent developmental progress may be misinterpreted as a treatment effect, if not compared to a natural history cohort or placebo group. Furthermore, outcome assessment is complicated by the heterogeneous clinical phenotype of AHDS patients, some of whom (8/24, 33%) are even able to walk as reported by Remerand and colleagues,15 whereas in our cohort no patient could walk and the majority of patients did not even reach early milestones of motor function, such as head control and anti-gravity movements. Therefore, it is necessary to establish control groups. The use of placebo controls as the gold standard in controlled trials may raise ethical concerns in severely affected children, especially when the timing of initiation of therapy needs to be as early as possible. This challenge can be addressed by using cross-over designs. As a minimum, the natural history of matched patients should be used for comparison. Describing the natural history of a disease is not trivial and needs to be done by a standardized longitudinal deep phenotyping approach. For ultra-rare diseases, international networks will be necessary to increase case numbers, facilitate data sharing and strengthen the validity of the studies. The omission of a control group may only be reasonable for compassionate off-label use or for drugs with a strong treatment effect as recently seen in gene therapy trials of spinal muscular atrophy (SMA) or aromatic L-amino acid decarboxylase (AADC) deficiency.33–35 Compassionate off-label use studies justified for individual patient benefit, but hinder knowledge gain.36
Parents prioritized weight gain as an important therapeutic goal because AHDS patients suffer from severe and progressive underweight, which is per se associated with higher mortality rates.14 All replacement therapies lead to an increase of body weight z-scores.21–23,25 Whether the reported increase is clinically relevant (eg, lower mortality rate, the gastric tube removal, fewer infections) needs to be addressed in future studies, ideally reporting the coexistence of a gastric tube, the weight, and the BMI (body mass index) z-scores compared to natural history controls. Further investigation is needed to determine whether the underweight of these patients is solely due to peripheral thyrotoxicity, as suggested by most authors, or whether the increased muscle tone due to spasticity and dystonia in association with oral dyskinesia/dysphagia may also play a role. In parallel, children with cerebral palsy, a phenotype similar to patients with MCT8 deficiency but without peripheral thyrotoxicity, suffer of comparable underweight also associated with an increased mortality risk.37
Movement disorders and spasticity as described in AHDS patients18 can cause pain, impair the development of voluntary and targeted movements, affect speech and swallowing, and significantly reduce overall quality of life. To date, movement disorders and spasticity have not been adequately assessed as outcome parameters, and we suggest that this be done in future studies.
Concluding considerations for development of therapies for MCT8 deficiency:
(i) The full downstream effects of SLC16A2 mutations are not yet fully understood. T3 regulates over 1000 genes in the mouse cortex with multiple effects on the developing brain.6 The transmembrane protein MCT8 may even have an unknown function beyond TH transport. This may hamper the therapeutic effects of downstream therapies (TH replacement therapies) and may be more broadly addressed by upstream approaches (eg gene therapies).
(ii) Species differences in TH transporter expression are known and led to the development of an MCT8-deficient mouse model that had a double knock-out of Mct8 and Oatp1c1 (organic anion transporter1 C1),38 which is another TH transporter expressed in the murine but not in the human blood-brain barrier. These Mct8/Oatp1c1 double knock-out mice show neuropathological alterations (reduced gray matter, cerebellar thickness, impaired myelination and functional connectivity, fewer interneurons) and impaired locomotion (Rotarod, hanging wire performance),38 both of which can be improved by Triac treatment.39,40 Whether the successful treatment of mice with respect to neurodevelopment would be transferable to human patients may be answered by the ongoing TRIAC II trial (NCT02396459). In general, it is a well-known phenomenon that mouse models often do not resemble pediatric neurological diseases, leading to the development of mammalian models that are evolutionarily closer to humans, as has been done for Duchenne muscular dystrophy,41 which could also be considered for MCT8 deficiency.
(iii) Immunohistological studies of human and mouse tissues indicate that MCT8 is not ubiquitously expressed, but is organ- and cell-specific. Therefore, mechanisms should be carefully selected to target specific organs and cells, and overtreatment with potentially harmful effects should be avoided.42 This is especially important for therapeutic strategies with systemic approaches (eg by TH replacement or gene replacement therapies).
(iv) Comparisons with other hypothyroid conditions (iodine deficiency, congenital hypothyroidism) highlight the fact that the timing of treatment is of paramount importance. While the timing of postnatal diagnosis has shifted to younger ages due to low-threshold whole exome sequencing, prenatal diagnosis is still a rarity and only applies to families with an index patient. Nevertheless, the postnatal time window of opportunity should be defined in future studies.
We would like to emphasize that our work was the first to involve stakeholders to identify patient-oriented outcomes for children with AHDS (strength of this study). Parents of affected children expressed that especially an improvement of the neurological phenotype, including accelerated development to promote participation/independence and reduction of muscle tone (movement disorders, spasticity) and its consequences (dysphagia, underweight), would improve the quality of life of their children. Inclusion of these patient-oriented therapeutic goals in future therapeutic trials may facilitate therapy development and reduce research costs. In addition, we recommend that patients be seen regularly in interdisciplinary centers (neuropediatrics, orthopedics, radiology, physiotherapy, speech therapy, occupational therapy) to address the above outcomes with standard of care. Another strength of the study is the systematic approach of the literature review and the targeted evaluation of previously published studies with a focus on patient-oriented outcomes. However, the analysis was limited by a lack of age-specific, comparable data on patient-oriented outcomes (limitation of this study).
In conclusion, we suggest defining improvement in neurodevelopment as the primary outcome measure and prioritizing other patient-oriented outcomes (body weight, movement disorders/spasticity, dysphagia, gastro-esophageal reflux) in future studies. Larger data with sufficient longitudinal natural history controls will be needed to finally evaluate the efficacy of treatment options for MCT8 deficiency.
Acknowledgments
We thank the families involved for sharing their patient-oriented perspective and for their involvement in the design and implementation of the survey. We also thank the QUEST Center for Responsible Research, especially Dr. Sarah Weschke, for her advice on stakeholder engagement.
Author Contributions
NMW, DT, HK, MS and CS contributed to the conception of the study. Parents of two affected patients were involved (NMW, HK, MS) in designing, sharing, collecting, and interpreting the survey. NMW, DV, YV, and MS collected the surveys and NMW performed the systematic literature review. NMW wrote the first draft of the manuscript. All authors and patient families contributed to data analysis, revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
NMW was supported by the DFG Research Unit 2841 “Beyond the Exome”. HK und MS were supported by the DFG P06 of TRR 296 “Local control of TH action” (LocoTact).
Disclosure
Professor Catherine Sarret reports personal fees from EGETIS, during the conduct of the study; personal fees from NOVARTIS, ARGENX, ORCHARD, and LUPIN, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Wirth EK, Schweizer U, Kohrle J. Transport of thyroid hormone in brain. Front Endocrinol Lausanne. 2014;5:98. doi:10.3389/fendo.2014.00098
2. Wilpert NM, Krueger M, Opitz R, et al. Spatiotemporal Changes of Cerebral Monocarboxylate Transporter 8 Expression. Thyroid. 2020;30(9):1366–1383. doi:10.1089/thy.2019.0544
3. Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi:10.1038/nrn1824
4. Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28(1):5–11. doi:10.1016/j.it.2006.11.007
5. Harvey CB, Williams GR. Mechanism of thyroid hormone action. Thyroid. 2002;12(6):441–446. doi:10.1089/105072502760143791
6. Bernal J. Thyroid hormone regulated genes in cerebral cortex development. J Endocrinol. 2017;232(2):R83–r97. doi:10.1530/joe-16-0424
7. Korevaar TI, Muetzel R, Medici M, et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol. 2016;4(1):35–43. doi:10.1016/s2213-8587(15)00327-7
8. Cao XY, Jiang XM, Dou ZH, et al. Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. N Engl J Med. 1994;331(26):1739–1744. doi:10.1056/nejm199412293312603
9. Zimmermann MB. Iodine deficiency. Endocr Rev. 2009;30(4):376–408. doi:10.1210/er.2009-0011
10. Gruters A, Krude H. Detection and treatment of congenital hypothyroidism. Nat Rev Endocrinol. 2011;8(2):104–113. doi:10.1038/nrendo.2011.160
11. Allan WH. Some examples of the inheritance of mental deficiency: apparently sex-linked idiocy and microencephaly. Am J Ment Defic. 1944;48:325–334.
12. Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem. 2003;278(41):40128–40135. doi:10.1074/jbc.M300909200
13. Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74(1):168–175. doi:10.1086/380999
14. Groeneweg S, van Geest FS, Abacı A, et al. Disease characteristics of MCT8 deficiency: an international, retrospective, multicentre cohort study. Lancet Diabetes Endocrinol. 2020;8(7):594–605. doi:10.1016/s2213-8587(20)30153-4
15. Remerand G, Boespflug-Tanguy O, Tonduti D, et al. Expanding the phenotypic spectrum of Allan-Herndon-Dudley syndrome in patients with SLC16A2 mutations. Dev Med Child Neurol. 2019;61(12):1439–1447. doi:10.1111/dmcn.14332
16. Schwartz CE, May MM, Carpenter NJ, et al. Allan-Herndon-Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am J Hum Genet. 2005;77(1):41–53. doi:10.1086/431313
17. Krude H, Biebermann H, Schuelke M, Müller TD, Tschöp M. Allan-Herndon-Dudley-Syndrome: considerations about the Brain Phenotype with Implications for Treatment Strategies. Exp Clin Endocrinol Diabetes. 2020;128(6–7):414–422. doi:10.1055/a-1108-1456
18. Masnada S, Sarret C, Antonello CE, et al. Movement disorders in MCT8 deficiency/Allan-Herndon-Dudley Syndrome. Mol Genet Metab. 2022;135(1):109–113. doi:10.1016/j.ymgme.2021.12.003
19. Wemeau JL, Pigeyre M, Proust-Lemoine E, et al. Beneficial effects of propylthiouracil plus L-thyroxine treatment in a patient with a mutation in MCT8. J Clin Endocrinol Metab. 2008;93(6):2084–2088. doi:10.1210/jc.2007-2719
20. Tonduti D, Vanderver A, Berardinelli A, et al. MCT8 deficiency: extrapyramidal symptoms and delayed myelination as prominent features. J Child Neurol. 2013;28(6):795–800. doi:10.1177/0883073812450944
21. Zung A, Visser TJ, Uitterlinden AG, Rivadeneira F, Friesema ECH. A child with a deletion in the monocarboxylate transporter 8 gene: 7-year follow-up and effects of thyroid hormone treatment. Eur J Endocrinol. 2011;165(5):823–830. doi:10.1530/EJE-11-0358
22. Groeneweg S, Peeters RP, Moran C, et al. Effectiveness and safety of the tri-iodothyronine analogue Triac in children and adults with MCT8 deficiency: an international, single-arm, open-label, phase 2 trial. Lancet Diabetes Endocrinol. 2019;7(9):695–706. doi:10.1016/s2213-8587(19)30155-x
23. van Geest FS, Groeneweg S, van den Akker ELT, et al. Long-Term Efficacy of T3 Analogue Triac in Children and Adults With MCT8 Deficiency: a Real-Life Retrospective Cohort Study. J Clin Endocrinol Metab. 2022;107(3):e1136–e1147. doi:10.1210/clinem/dgab750
24. Visser WE, Vrijmoeth P, Visser FE, Arts WFM, van Toor H, Visser TJ. Identification, functional analysis, prevalence and treatment of monocarboxylate transporter 8 (MCT8) mutations in a cohort of adult patients with mental retardation. Clin Endocrinol (Oxf). 2013;78(2):310–315. doi:10.1111/cen.12023
25. Verge CF, Konrad D, Cohen M, et al. Diiodothyropropionic acid (DITPA) in the treatment of MCT8 deficiency. J Clin Endocrinol Metab. 2012;97(12):4515–4523. doi:10.1210/jc.2012-2556
26. Refetoff S, Pappa T, Williams MK, et al. Prenatal treatment of thyroid hormone cell membrane transport defect caused by MCT8 gene mutation. Thyroid. 2020. doi:10.1089/thy.2020.0306
27. Braun D, Schweizer U. The Protein Translocation Defect of MCT8(L291R) Is Rescued by Sodium Phenylbutyrate. Eur Thyroid J. 2020;9(5):269–280. doi:10.1159/000507439
28. Iwayama H, Liao XH, Braun L, et al. Adeno Associated Virus 9-Based Gene Therapy Delivers a Functional Monocarboxylate Transporter 8, Improving Thyroid Hormone Availability to the Brain of Mct8-Deficient Mice. Thyroid. 2016;26(9):1311–1319. doi:10.1089/thy.2016.0060
29. Sundaram SM, Arrulo Pereira A, Müller-Fielitz H, et al. Gene therapy targeting the blood-brain barrier improves neurological symptoms in a model of genetic MCT8 deficiency. Brain J Neurol. 2022:awac243. doi:10.1093/brain/awac243
30. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
31. Kersseboom S, Horn S, Visser WE, et al. In vitro and mouse studies supporting therapeutic utility of triiodothyroacetic acid in MCT8 deficiency. Mol Endocrinol. 2014;28(12):1961–1970. doi:10.1210/me.2014-1135
32. van Geest FS, Groeneweg S, Visser WE. Monocarboxylate transporter 8 deficiency: update on clinical characteristics and treatment. Endocrine. 2021;71(3):689–695. doi:10.1007/s12020-020-02603-y
33. Day JW, Finkel RS, Chiriboga CA, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20(4):284–293. doi:10.1016/S1474-4422(21)00001-6
34. Hwu WL, Muramatsu S, Tseng SH, et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency. Sci Transl Med. 2012;4(134):134ra61. doi:10.1126/scitranslmed.3003640
35. Pearson TS, Gupta N, San Sebastian W, et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons. Nat Commun. 2021;12(1):4251. doi:10.1038/s41467-021-24524-8
36. Synofzik M, van Roon-Mom WMC, Marckmann G, et al. Preparing n-of-1 Antisense Oligonucleotide Treatments for Rare Neurological Diseases in Europe: genetic, Regulatory, and Ethical Perspectives. Nucleic Acid Ther. 2022;32(2):83–94. doi:10.1089/nat.2021.0039
37. Brooks J, Day S, Shavelle R, Strauss D. Low weight, morbidity, and mortality in children with cerebral palsy: new clinical growth charts. Pediatrics. 2011;128(2):e299–307. doi:10.1542/peds.2010-2801
38. Mayerl S, Muller J, Bauer R, et al. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J Clin Invest. 2014;124(5):1987–1999. doi:10.1172/jci70324
39. Chen J, Salveridou E, Liebmann L, et al. Triac Treatment Prevents Neurodevelopmental and Locomotor Impairments in Thyroid Hormone Transporter Mct8/Oatp1c1 Deficient Mice. Int J Mol Sci. 2023;24(4). doi:10.3390/ijms24043452
40. Reinwald JR, Weber-Fahr W, Cosa-Linan A, et al. TRIAC Treatment Improves Impaired Brain Network Function and White Matter Loss in Thyroid Hormone Transporter Mct8/Oatp1c1 Deficient Mice. Int J Mol Sci. 2022;23(24):15547. doi:10.3390/ijms232415547
41. Willmann R, Possekel S, Dubach-Powell J, Meier T, Ruegg MA. Mammalian animal models for Duchenne muscular dystrophy. Neuromuscul Disord NMD. 2009;19(4):241–249. doi:10.1016/j.nmd.2008.11.015
42. Bárez-López S, Obregon MJ, Martínez-de-Mena R, Bernal J, Guadaño-Ferraz A, Morte B. Effect of Triiodothyroacetic Acid Treatment in Mct8 Deficiency: a Word of Caution. Thyroid off J Am Thyroid Assoc. 2016;26(5):618–626. doi:10.1089/thy.2015.0388
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


