Back to Journals » International Journal of Nanomedicine » Volume 18
Chitosan-Based Nanoformulated (–)-Epigallocatechin-3-Gallate (EGCG) Modulates Human Keratinocyte-Induced Responses and Alleviates Imiquimod-Induced Murine Psoriasiform Dermatitis [Erratum]
Authors Chamcheu JC , Siddiqui IA, Adhami VM, Esnault S, Bharali DJ, Babatunde AS , Adame S , Massey RJ, Wood GS, Longley BJ, Mousa SA , Mukhtar H
Received 6 April 2023
Accepted for publication 6 April 2023
Published 11 May 2023 Volume 2023:18 Pages 2503—2505
Chamcheu JC, Siddiqui IA, Adhami VM, et al. Int J Nanomedicine. 2018;13:4189–4206.
An error during the preparation of Figures 1 and 5 for publishing led to the inadvertent creation of duplicate regions in images from these figures on pages 4194 and 4199, respectively. The journal wishes to apologise for this error. The correct versions of Figures 1 and 5 are as follows:
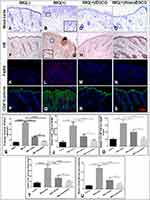 |
Figure 5 Effect of topically applied free EGCG and nanoEGCG on infiltrating immune cells and expression of differentiation markers in IMQ-treated mouse skin lesions: Mice were treated in 4 groups as described in the legends to Figure 4 and Figure S5. (A–D, F–I, K–N, P–S) Photomicrographs showing immunohistological features of: (A–D) mast cells (toluidine blue staining); (F–I) epidermis/dermis (NE, brown staining), and microabscesses (arrow); (K–N) macrophages (F4/80, red staining); and (P–S) double immunofluorescence staining for loricrin (green) and T-lymphocytes (CD4+, red staining). Nuclei were counterstained blue with DAPI. Magnification for all panels ×200. (E, J, O, T, U) Quantitative analyses of changes in immune cells: (E) mast cells; (J) NE+ cells; (O) F4/80+ cells; and (U) loricrin in the 4 treatment groups. Each data point represents the mean±SD of 4 random fields/mouse from 5 mice/group. *p, 0.05, **p, 0.01, ***p, 0.001, and ****p, 0.0001 for the indicated 2-way comparisons. Abbreviations: EGCG, (–)-epigallocatechin-3-gallate; nanoEGCG, chitosan-based polymeric nanoparticle formulation of EGCG; IMQ, imiquimod; NE, neutrophil elastase; LPV, low-power view. |
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

