Back to Journals » Veterinary Medicine: Research and Reports » Volume 9
Equine degenerative myeloencephalopathy: prevalence, impact, and management
Received 15 February 2018
Accepted for publication 1 May 2018
Published 7 September 2018 Volume 2018:9 Pages 63—67
DOI https://doi.org/10.2147/VMRR.S148542
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Young Lyoo
Erin N Burns, Carrie J Finno
Department of Population Health and Reproduction, School of Veterinary Medicine, University of California-Davis, Davis, CA, USA
Abstract: Equine neuroaxonal dystrophy/equine degenerative myeloencephalopathy is an inherited neurodegenerative disorder affecting many horse breeds. Clinical signs include a symmetric ataxia and an abnormal stance at rest, similar to cervical vertebral compressive myelopathy, equine protozoal myeloencephalitis, and equine herpesvirus 1 myeloencephalopathy. This review will provide an update on the disease prevalence, management, impact, and ongoing research.
Keywords: ataxia, α-tocopherol, horse, vitamin E, gestation, foal, pasture
Introduction
Equine neuroaxonal dystrophy/equine degenerative myeloencephalopathy (eNAD/EDM) is an inherited neurodegenerative disease characterized by the development of ataxia within the first year of life.1 Histologic hallmarks of eNAD/EDM include spheroids, axonal loss, and secondary demyelination within the caudal brainstem and spinal cord.1 eNAD and EDM are clinically indistinguishable,2 with EDM being a more advanced form of the disease. The histologic lesions in eNAD are confined to the cuneate and gracile nuclei of the caudal medulla oblongata, while in EDM, the lesions are more widespread and include demyelination within the ascending tracts of the spinal cord.2 Immunohistochemical calretinin staining has defined afferent proprioceptive tracts as the primary tracts damaged in eNAD/EDM horses, and isolated soma within the dorsal root ganglia as the source of dystrophic axons.3
While a genetic susceptibility to eNAD/EDM is highly suspected,5–9 the genetic basis of eNAD/EDM currently remains unknown. Previous studies in Quarter Horses,7 Appaloosas,6 Morgans,9 and Standardbreds5 indicated an autosomal dominant and incompletely penetrant mode of inheritance.7 The disease has been reported in multiple breeds and a breed-specific susceptibility has not been identified.
The clinical signs of eNAD/EDM are an early onset of symmetric ataxia (≥ grade 2/5),10 abnormal stance at rest, prominent hypermetria when walked with the head elevated, and general proprioceptive deficits.1,5,6,11–14 Some reports also detail additional neurologic symptoms including a decreased or absent menace response,1 lack of fight or flight response,1 and increased severity of ataxia in the pelvic limbs.15 Decreased serum α-tocopherol levels within the first 4 months of life have also been reported in some eNAD/EDM cases.1,16 In rare cases, eNAD/EDM has been reported concurrently with equine motor neuron disease.17
There are other neurological diseases which are clinically indistinguishable from eNAD/EDM. For example, horses affected with cervical vertebral compressive myelopathy (CVCM), equine protozoal myeloencephalitis (EPM), or equine herpesvirus-1 myeloencephalopathy (EHM) demonstrate similar clinical signs as individuals with eNAD/EDM. EPM can be confirmed through antibody detection tests of cerebrospinal fluid and serum.18 EHM can be confirmed through virus culture of EHV-1 and molecular testing.19 A likely diagnosis of CVCM can be excluded based on standing cervical radiographs, myelography, or computed tomography. Cytological evaluation of cerebrospinal fluid does not typically reveal any abnormalities with CVCM or eNAD/EDM. Currently, the only way to definitively diagnose eNAD/EDM and CVCM is through postmortem histologic evaluation of the brainstem and spinal cord.
Vitamin E
Early studies of eNAD/EDM determined that foals raised on pasture incurred a protective effect against the development of the disease.20 It was hypothesized this protective effect was due to the increased consumption of vitamin E (vitE) as grazing pasture has a range of 45–400 IU vitE/kg of dry matter.21 This hypothesis was also supported in vitE supplementation studies, demonstrating a decrease in disease incidence within susceptible populations when supplemented.5 The average 500 kg horse living on pasture would consume between 350 and 3,000 IUs of vitE/d when consuming 1.7% of their body weight in a 90% dry matter diet. This range is variable because the vitE available on pasture is subject to change based on the forage type available and the time of year.21 Serum α-tocopherol concentrations have been shown to fluctuate seasonally, with the highest concentrations identified between June and August, and the lowest between February and May.22
There are many different products on the market to supplement vitE into a horse’s diet. D-α-Tocopherol (RRR-α-tocopherol) is the most bioactive isoform of vitE. All racemic vitE contains 2 different isoforms of vitE (D-α-tocopherol and L-α-tocopherol). It is not recommended to supplement with all racemic vitE or synthetic vitE at any dose as it is not bioavailable.33 When supplementing with D-α-tocopherol (RRR-α-tocopherol), also called natural vitE, 10 IU/kg/d should be administered for a suspect eNAD/EDM-affected horse.
Based on the authors’ clinical observations of eNAD/EDM, we recommend 10 IU/kg/d of water-soluble natural vitE (RRR-α-tocopherol), totaling 5,000 IU/d for the average adult horse. This water soluble natural vitE is absorbed rapidly and therefore increases serum concentrations most efficiently.
The mechanism by which vitE protects a foal from disease development is currently not fully understood. It is known that D-α-tocopherol (RRR-α-tocopherol) is the most potent, bioavailable isoform of vitE,23 and that oxidative stress is occurring in the brainstem and spinal cord of eNAD/EDM affected horses.4,35 Current research is ongoing to determine the exact cause of this oxidative injury and how vitE prevents such damage.
Prevalence
There are many breeds affected by eNAD/EDM including the Standardbred,5 Appaloosa,6 Morgan,9 Lusitano,24 Paint,17 Thoroughbred,17 Arabian,25 Norwegian Fjord,26 and Quarter Horse.1,7,12,27,28 As a definitive diagnosis of eNAD/EDM requires postmortem evaluation of the central nervous system, prevalence of the disease would more accurately be assessed with a genetic marker or biochemical assay. When diagnosing eNAD/EDM antemortem, it is imperative that the veterinarian rule out all other possible causes of the observed neurological deficits (CVCM, EPM, EHM, and trauma). α-Tocopherol supplementation may be indicated if the serum α-tocopherol levels are <2 µg/mL, and a history of neurological disease exists in related individuals.
A prospective study at Cornell University College of Veterinary Medicine found that EDM (n=23) was the second leading cause of equine spinal cord disease among all necropsied spinal cord disease cases between 1974 and 1976.29 In this population of horses, EPM (n=32) was the leading cause of neurologic disease, with a total of 96 horses evaluated. Eleven years later, a retrospective study of 19 ataxic horses was published by the University of Montreal College of Veterinary Medicine. In this study, CVCM was the leading cause of ataxia (n=11), and EDM was the second leading cause (n=4).30 In evaluating both these studies based on postmortem confirmed diagnoses of spinal ataxia, it can be concluded that CVCM, EPM, and eNAD/EDM account for the majority of spinal ataxia presentations in horses.
Since many neurologic horses are euthanized without postmortem evaluation, it is impossible to know the true prevalence of eNAD/EDM. The study of this disease is also complicated by the environments foals are raised in. If a susceptible foal is raised on pasture, they are less likely to demonstrate clinical signs of the disease,20 thus masking individuals who would have underlying predisposition for the disease. A 1990 study also found that application of insecticide, exposure to wood preservatives, and frequent time spent on dirt lots were risk factors for eNAD/EDM.20 Later epidemiological studies were able to confirm the risk associated with low vitE levels (ie, frequent time spent in dirt lots without access to pasture) but not the other factors.34
Case reports have demonstrated that eNAD/EDM affects horses globally. Equine NAD/EDM has been diagnosed in horses residing in New York,5,13,20 California,16 Nebraska,31 Iowa,4 the United Kingdom,26 Italy,28 and Spain.25 Researchers have also studied eNAD/EDM in Iowa,4 Minnesota,3 California,1,7,24,32 Oregon,6,11 and Florida.5 With this geographic distribution, we do not suspect there to be a specific geographic region in which eNAD/EDM cases are confined to, but for the number of cases to be increased in area where access to pasture is limited.
Impact
Any ataxic horse is not suitable for any riding purpose. Because eNAD/EDM is an inherited disorder, these individuals are also not suited for breeding. This causes an economic loss to the equine breeding and training industries.
The economic impact on the training and breeding industry can be estimated by the amount of money invested in the production of an affected horse. Although clinical cases may present between 2 and 3 years of age,1 the disease typically develops within the first year of life.16,29 The authors suspect this discrepancy to be caused by the increased workload, allowing owners and trainers to observe the lack of coordination that has existed since early in the animals’ life. As young horses enter a training regimen, they are continually asked to perform at increasingly higher levels. Affected horses would be unable to meet these expectations, and subsequently be examined by a veterinarian for possible causes.
Long-term supplementation with 10 IU/kg/d is a financial burden on the breeding and training industry that must be considered when making breeding management decisions. The cost of supplementation for 1 genetically susceptible foal over the course of 2 years is nearly $3,500 (Table 1). Both the mare and the foal must be supplemented with this high dose of D-α-tocopherol (RRR-α-tocopherol) to achieve sufficient protective effects. As the current methods to deliver water-soluble D-α-tocopherol (RRR-α-tocopherol) require daily oral administration of the most bioavailable liquid supplement, the burden of time spent dosing several horses within a breeding or training program can be substantial.
Management
Once clinical signs of ataxia are apparent, α-tocopherol supplementation will not improve the neurologic deficits. Progression of the disease can be halted through supplementation, but the general proprioceptive ataxia will remain. Clinical signs of the disease typically progress at varying rates until the horse reaches 2 years of age, at which time signs stabilize.
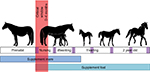
Currently the best management practice for prevention of eNAD/EDM is to maintain pregnant mares, foals, weanlings, yearlings, and 2-year-old horses on lush green pastures. This ensures adequate consumption of vitE to protect against neurodegeneration in the developing central nervous system. If adequate pasture is not available, the best practices outlined in Figure 1 should be followed.
Based on neonatal development studies at UC Davis,16 and previous research, clinical signs may be observable as early as 1–2 months of age.6,17,20,29 To provide the best treatment, foals should have a neurologic exam at 1 and 6 months of age, and every 6 months thereafter until 2 years of age for early disease detection (Figure 1). Within the first 4 months of life, the serum α-tocopherol level of the foal should be assessed.16 This critical window is the only time in which a difference exists between foals that progressed with normal neurologic function and foals that developed eNAD/EDM (Figure 1) (reference range >2 µg/mL).16 Ideally, the serum α-tocopherol level should be evaluated within the first week of life, as the difference between the 2 groups has been shown to be the largest during this time.16
Serum α-tocopherol concentrations of the dam can also be an indicator of the need for supplementation during pregnancy and lactation.16 Supplementation is indicated when serum concentrations are below 2 µg/mL in a pregnant mare.16 If eNAD/EDM is suspected in the foal, the dam and offspring should be supplemented at 10 IU/kg/d D-α-tocopherol (RRR-α-tocopherol). Supplementation in the dam should begin at the start of the third trimester and end when the foal is weaned. The foal should be supplemented from birth to 3 years of age (Figure 1).
Current work is under way to develop an antemortem genetic test for eNAD/EDM at UC Davis. A genetic test would allow breeders to make more informed decisions about their breeding and management practices, ie, not breeding-susceptible individuals in a dry climate lacking adequate access to pasture. A test would also allow veterinarians to better diagnose the cause of clinically observed ataxia.
Acknowledgment
The authors thank Anna Dahlgren and Francesca Gianino for their contributions to the manuscript.
Disclosure
Support for CJF was provided by the National Institutes of Health (NIH) (1K01OD015134 and L40 TR001136). ENB was supported by USDA NIFA National Need Fellowship Award #20143842021796. The authors report no other conflicts of interest in this work.
References
Aleman M, Finno CJ, Higgins RJ, et al. Evaluation of epidemiological, clinical, and pathological features of neuroaxonal dystrophy in Quarter Horses. J Am Vet Med Assoc. 2011;239(6):823–833. | ||
Miller MM, Collatos C. Equine degenerative myeloencephalopathy. Vet Clin North Am Equine Pract. 1997;13(1):43–52. | ||
Finno CJ, Valberg SJ, Shivers J, D’Almeida E, Armien AG. Evidence of the primary afferent tracts undergoing neurodegeneration in horses with equine degenerative myeloencephalopathy based on calretinin immunohistochemical localization. Vet Pathol. 2016;53(1):77–86. | ||
Wong DM, Ghosh A, Fales-Williams AJ, Haynes JS, Kanthasamy AG. Evidence of oxidative injury of the spinal cord in 2 horses with equine degenerative myeloencephalopathy. Vet Pathol. 2012;49(6):1049–1053. | ||
Mayhew IG, Brown CM, Stowe HD, Trapp AL, Derksen FJ, Clement SF. Equine degenerative myeloencephalopathy: a vitamin E deficiency that may be familial. J Vet Intern Med. 1987;1(1):45–50. | ||
Blythe LL, Hultgren BD, Craig AM, et al. Clinical, viral, and genetic evaluation of equine degenerative myeloencephalopathy in a family of Appaloosas. J Am Vet Med Assoc. 1991;198(6):1005–1013. | ||
Finno CJ, Famula T, Aleman M, Higgins RJ, Madigan JE, Bannasch DL. Pedigree analysis and exclusion of alpha-tocopherol transfer protein (TTPA) as a candidate gene for neuroaxonal dystrophy in the American Quarter Horse. J Vet Intern Med. 2013;27(1):177–185. | ||
Finno CJ, Kaese HJ, Miller AD, Gianino G, Divers T, Valberg SJ. Pigment retinopathy in warmblood horses with equine degenerative myeloencephalopathy and equine motor neuron disease. Vet Ophthalmol. 2017;20(4):304–309. | ||
Beech J, Haskins M. Genetic studies of neuraxonal dystrophy in the Morgan. Am J Vet Res. 1987;48(1):109–113. | ||
Lunn DP, Mayhew IG. The neurologic evaluation of horses. Equine Vet Educ. 1989;1:94–101. | ||
Blythe LL, Craig AM, Lassen ED, Rowe KE, Appell LH. Serially determined plasma alpha-tocopherol concentrations and results of the oral vitamin E absorption test in clinically normal horses and in horses with degenerative myeloencephalopathy. Am J Vet Res. 1991;52(6):908–911. | ||
Finno CJ, Aleman M, Ofri R, et al. Electrophysiological studies in American Quarter horses with neuroaxonal dystrophy. Vet Ophthalmol. 2012;15(Suppl 2):3–7. | ||
Mayhew IG, deLahunta A, Whitlock RH, Geary JC. Equine degenerative myeloencephalopathy. J Am Vet Med Assoc. 1977;170(2):195–201. | ||
Blythe LL, Craig AM. Equine degenerative myeloencephalopathy. Part 1. Clinical signs and pathogenesis. Compend Contin Educ Pract Vet. 1992;14:1215–1221. | ||
Dill SG, Hintz HF, deLahunta A, Waldron CH. Plasma and liver copper values in horses with equine degenerative myeloencephalopathy. Can J Vet Res. 1989;53(1):29–32. | ||
Finno CJ, Estell KE, Katzman S, et al. Blood and cerebrospinal fluid α-tocopherol and selenium concentrations in neonatal foals with neuroaxonal dystrophy. J Vet Intern Med. 2015;29(6):1667–1675. | ||
Finno CJ, Miller AD, Siso S, et al. Concurrent equine degenerative myeloencephalopathy and equine motor neuron disease in three young horses. J Vet Intern Med. 2016;30(4):1344–1350. | ||
Granstrom DE, Dubey JP, Davis SW, et al. Equine protozoal myeloencephalitis: antigen analysis of cultured Sarcocystis neurona merozoites. J Vet Diagn Invest. 1993;5(1):88–90. | ||
Lunn DP, Davis-Poynter N, Flaminio MJ, et al. Equine herpesvirus-1 consensus statement. J Vet Intern Med. 2009;23(3):450–461. | ||
Dill SG, Correa MT, Erb HN, deLahunta A, Kallfelz FA, Waldron C. Factors associated with the development of equine degenerative myeloencephalopathy. Am J Vet Res. 1990;51(8):1300–1305. | ||
McDowell LR. Vitamins in Animal Nutrition. Vol 1. San Diego, CA: Academic Press, Inc.; 1989. | ||
Maenpaa PH, Koskinen T, Koskinen E. Serum profiles of vitamins A, E and D in mares and foals during different seasons. J Anim Sci. 1988; 66(6):1418–1423. | ||
Mustacich DJ, Bruno RS, Traber MG. Vitamin E. Vitam Horm. 2007;76:1–21. | ||
Finno CJ, Higgins RJ, Aleman M, et al. Equine degenerative myeloencephalopathy in Lusitano horses. J Vet Intern Med. 2011;25(6):1439–1446. | ||
Siso S, Ferrer I, Pumarola M. Abnormal synaptic protein expression in two Arabian horses with equine degenerative myeloencephalopathy. Vet J. 2003;166(3):238–243. | ||
Naylor RJ, Priestnall SL, Turk AC, Summers BA, Schoniger S, Piercy RJ. Equine degenerative myeloencephalopathy in a horse in the UK. Vet Rec. 2010;167(10):380–381. | ||
Adams AP, Collatos C, Fuentealba C, Illanes O, Blanchard R. Neuroaxonal dystrophy in a two-year-old quarter horse filly. Can Vet J. 1996;37(1):43–44. | ||
Gandini G, Fatzer R, Mariscoli M, Spadari A, Cipone M, Jaggy A. Equine degenerative myeloencephalopathy in five quarter horses: clinical and neuropathological findings. Equine Vet J. 2004;36(1):83–85. | ||
Mayhew IG, deLahunta A, Whitlock RH, Krook L, Tasker JB. Spinal cord disease in the horse. Cornell Vet. 1978;68(Suppl 6):1–207. | ||
Nappert G, Vrins A, Breton L, Beauregard M. A retrospective study of nineteen ataxic horses. Can Vet J. 1989;30(10):802–806. | ||
Blatchford G, Arington H. A greater good. Equus. 2017 Dec 2:4. The Woodlands, TX: Wright’s Media. | ||
Finno CJ, Bordbari MH, Valberg SJ, et al. Transcriptome profiling of equine vitamin E deficient neuroaxonal dystrophy identifies upregulation of liver X receptor target genes. Free Radic Biol Med. 2016;101:261–271. | ||
Higgins JK, Puschner B, Kass PH, Pusteria N. Assessment of vitamin E concentrations in serum and cerebrospinal fluid of horses following oral administration of vitamin E. Am J Vet Res. 2008;69(6):785–790. | ||
Blythe LL, Craig AM, Lassen ED, Hultgren BD. Vitamin E deficiency as a causative factor in equine degenerative myeloencephalopathy. Vitamin E: biochemistry and health implications. Ann NY Acad Sci. 1989;570:415–416. | ||
Finno CJ, Estell KE, Winfield L, et al. Lipid peroxidation biomarkers for evaluating oxidative stress in equine neuroaxonal dystrophy. J Vet Intern Med. In press 2018. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.