Back to Journals » Biologics: Targets and Therapy » Volume 8
Epoetin zeta in the management of anemia associated with chronic kidney disease, differential pharmacology and clinical utility
Authors Davis-Ajami ML, Wu J, Downton K, Ludeman E, Noxon V
Received 12 October 2013
Accepted for publication 13 December 2013
Published 16 April 2014 Volume 2014:8 Pages 155—167
DOI https://doi.org/10.2147/BTT.S27578
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Mary Lynn Davis-Ajami,1 Jun Wu,2 Katherine Downton,3 Emilie Ludeman,3 Virginia Noxon4
1Organizational Systems and Adult Health, University of Maryland School of Nursing, Baltimore, MD, USA; 2South Carolina College of Pharmacy, University of South Carolina, Greenville, SC, USA; 3Health Sciences and Human Services Library, University of Maryland, Baltimore, MD, USA; 4Department of Clinical Pharmacy and Outcomes Science, South Carolina College of Pharmacy, University of South Carolina, Columbia, SC, USA
Abstract: Epoetin zeta was granted marketing authorization in October 2007 by the European Medicines Agency as a recombinant human erythropoietin erythropoiesis-stimulating agent to treat symptomatic anemia of renal origin in adult and pediatric patients on hemodialysis and adults on peritoneal dialysis, as well as for symptomatic renal anemia in adult patients with renal insufficiency not yet on dialysis. Currently, epoetin zeta can be administered either subcutaneously or intravenously to correct for hemoglobin concentrations ≤10 g/dL (6.2 mmol/L) or with dose adjustment to maintain hemoglobin levels at desired levels not in excess of 12 g/dL (7.5 mmol/L). This review article focuses on epoetin zeta indications in chronic kidney disease, its use in managing anemia of renal origin, and discusses its pharmacology and clinical utility.
Keywords: biosimilar, chronic kidney disease, epoetin alfa, erythropoiesis, renal anemia, Retacrit®
Introduction
Renal anemia occurs as a common complication of chronic kidney disease (CKD). CKD is a complex disease characterized by impaired renal function. The Kidney Disease Improving Global Outcomes (KDIGO) initiative defines CKD as the presence of structural or functional abnormalities of the kidneys resulting in kidney damage, for instance, pathologic abnormalities or markers of kidney damage to include proteinuria, renal tubular syndromes or imaging abnormalities, or level of kidney function measured by glomerular filtration rate (GFR) <60 mL/minute/1.73 m2 lasting ≥3 months.1 There are five stages to disease progression based on estimated GFR levels calculated from serum creatinine levels and levels of proteinuria. These stages range from kidney damage with normal or increased GFR (stage I) to kidney failure (stage 5).2 Table 1 shows the KDIGO classifications for the five stages of CKD. The term CKD refers to the presence of any stage of CKD (stage 1 through 5) with or without kidney transplant, and includes both nondialysis and dialysis dependent disease.3
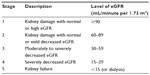  | Table 1 Stages of CKD, from the Kidney Disease Improving Global Outcomes (KDIGO) initiative |
Prevalence, disease burden, and treatment costs for CKD are increasing in the US and globally. Overall, in the US, an estimated one in ten adults, or about 20 million individuals, have CKD, and, during the years 1999–2004, data show a higher prevalence among females than males (females 15.0% versus males 11.1%).4–6 Although, in 2011, new cases of end stage renal disease (ESRD) declined for the first time in 30 years in the US, there was an overall increase in patients receiving treatment for ESRD.6 In the UK, CKD affects an estimated 6% of the population.7 Little data exist for European prevalence.8 The Global Burden of Disease project’s ranking of leading causes of disability-adjusted life years (DALYs) for 291 specific diseases ranks CKD 29th overall globally with regional geographical rankings ranging from 8–44 (see Figure 1).9 Between 1990 and 2010, DALYs (per 100,000) for CKD show an overall increase of 16.7%, with an even greater percent increase for CKD due to diabetes mellitus (36.1%) or hypertension (42.2%).9
  | Figure 1 2010 ranking of CKD by regional rank order of leading cause of DALY(s). |
Risk factors for developing CKD include age, gender, race, diabetes, and genetic makeup, as well as modifiable factors such as hypertension, proteinuria, anemia, metabolic disturbances, and dyslipidemia.10,11 Disease progression to CKD increases the risk for cardiovascular disease, hospitalization, and death,12,13 with some suggesting an increased risk for a cardiorenal syndrome.14
Hemoglobin (Hb) levels often gradually decline with the decline in renal function.15 The prevalence of anemia increases as kidney function declines.16 Renal anemia is associated with adverse patient outcomes, including decreased exercise capacity17 and quality of life,18 and increased hospitalization, cardiovascular events, and chance of death.19–21 Renal anemia arises from CKD-induced oxidative stress, inflammation, and a relative deficiency in the renal production of erythropoietin (EPO)22–25 due to loss of EPO synthesis or inhibitors of EPO.2,26 EPO is an endogenous protein produced in the kidneys to stimulate red blood cell production under hypoxic conditions.24,27–29 Similar to the anemia of chronic disease, renal anemia is normochromic, normocytic, and characteristically hypoproliferative, however different from anemia of chronic disease, renal anemia also shows low EPO and some iron deficiency.1,15
The definition of renal anemia has evolved over the years. Currently, the KDIGO, the European Renal Best Practice group (ERBP), and the National Kidney Foundation (NKF) Kidney Disease Outcomes Quality Initiative (KDOQI) give Hb levels to serve as a guide when diagnosing anemia. The current KDIGO guidelines use the World Health Organization definition of anemia while the ERBP position statement takes into consideration Hb set point differences for European populations.30 In the US, the NKF KDOQI continues to use the 2006 KDOQI definition.31 Table 2 shows the differences between guidelines for diagnosing renal anemia among NKF KDOQI 2006 US guidelines, KDIGO updated 2012 guidelines, and the ERBP 2013 position statement.30,31
After ruling out other causes of symptomatic anemia in the presence of CKD, and once renal anemia has been diagnosed, renal anemia treatment guidelines suggest a trial of iron therapy before beginning erythropoiesis-stimulating agent (ESA) therapy and based on transferrin saturation (TSAT) and ferritin levels. Iron supplementation improves Hb levels and cellular response to ESA stimulation, which may in turn help reduce the ESA dosage. This ESA response to iron supplementation may arise from how the hepcidin-ferroportin axis helps regulate iron homeostasis by controlling the entry of iron from dietary sources into the bloodstream. The iron mediated hepcidin pathway is suppressed in the presence of hypoxia and anemia, while ESA administration causes hepcidin excess. Hepcidin excess impedes the absorption of dietary iron and the release of iron stores. Therefore, administering iron supplementation, either intravenously for hemodialysis patients, or through an oral agent for nondialyzed CKD patients, for TSAT <30% and ferritin level <300 ng/mL is recommended. Iron supplementation should be used with caution for TSAT >30% and ferritin levels exceeding 500 ng/mL.30,32
In 1988 in Europe, and subsequently for the year 1989 in the US, recombinant human erythropoietin (rHuEPO) was introduced to treat renal anemia. Its ability to normalize Hb led to rHuEPO eventually replacing blood transfusions and the use of androgenic steroids as the mainstay treatment for renal anemia. Moreover, renal anemia was independently associated with the development of left ventricular hypertrophy (LVH) and heart failure. Normalizing Hb with epoetin alfa arrested the progression of LVH in CKD with associated improvements in cardiovascular-related morbidity and mortality.33 Additionally, early rHuEPO research reported improved quality of life scores in CKD contributing to the eventual widespread use of rHuEPO in treating renal anemia.34–37 Over time, various epoetin analogues (epoetin alfa, epoetin beta, darbepoetin alfa) were developed that effectively increased Hb levels. Patent expirations for rHuEPO biopharmaceuticals led to the development of biosimilars, including epoetin zeta.38
Currently, epoetin zeta carries indications for treating symptomatic renal anemia, for intravenous (IV) and subcutaneous administration in adults and pediatric patients undergoing hemodialysis and in adult patients on peritoneal dialysis, or for those not yet undergoing dialysis.39,40 Contraindications include hypersensitivity, patients who develop epoetin-induced antibody-mediated pure red cell aplasia (PRCA) following treatment, uncontrolled hypertension, and patients who for any reason cannot receive adequate antithrombotic prophylaxis.39 The purpose of this article is to review the differential pharmacology and clinical utility of epoetin zeta.
Methods
We began by identifying general information about epoetin zeta; that this biosimilar was a rHuEPO preparation of epoetin alfa with a Chemical Abstracts Service registry number 604802-70-2 and a chemical name 1-165-Erythropoetin (human clone B03XA01) marketed under various brand names in different regions of the world (Retacrit® [HOSPIRA Inc, Lake Forest Illinois, USA, for distribution in Europe], Silapro® [STADA Arzneimittel AG, Bad Vilbel, Germany], Epobel® [STADA Arzneimittel AG, Bad Vilbel, Germany, licensed to NOBEL, IIac Pazarlama ve Sanayii Lt STI, for distribution inTurkey]), see Table 3. Based on this information, we developed a search incorporating concepts related to “epoetin zeta” and “chronic renal failure.” Our final PubMed search strategy used the following terms: (epoetin zeta OR EPO zeta OR erythropoietin zeta OR Retacrit OR Silapro) AND (kidney* OR renal OR uremia OR uremic OR ESRD OR ESRF OR CRD OR CRF OR CKD) AND (chronic OR insufficien* OR sufficien* OR disease OR fail* OR damage* OR irreversible OR end stage* OR anemia OR anemic). Searches were conducted in PubMed (publication n=10), Embase (publication n=61), Cochrane CENTRAL (publication n=4), Web of Science (publication n=24), ClinicalTrials.gov (publication n=3), and Google Scholar (publication n=278). Additionally, we gathered information from regulatory authorities including the European Medicines Agency (EMA), US Food and Drug Administration (FDA), and US Centers for Medicare and Medicaid Services (CMS), as well as from the professional association The National Kidney Foundation, Inc. Additional pertinent references were identified from the bibliographies of retrieved publications. Finally, we excluded non-English language publications and duplicates.
  | Table 3 Epoetin zeta, substance, cell line, brand name and manufacturer |
Background
Patent expirations in 2004 for epoetin alfa and 2005 for epoetin beta in the European Union led to the development of biosimilar epoetin. The term “biosimilar”, as used in Europe (termed “follow-on-biologics” in the US and Japan, and called “subsequent-entry biologic” in Canada),40 refers to officially-approved subsequent versions of innovator biotechnological products with the same primary amino acid sequence as the referent product used at the same dose to treat the same disease.24,28,41,42 Biosimilars differ from low molecular weight pharmaceutical generics in respect to the size and complexity of the active substance, the heterogeneity of the originator materials, and variability in manufacturing processes.38 Pharmaceutical generics are low molecular weight formulations with similar pharmacokinetic and pharmacodynamic profiles as the original that are synthesized from commercially available reagents using standardized procedures.43
Biotechnologically derived proteins have highly complex large molecular structures and a high degree of heterogeneity. This creates challenging formulation and manufacturing production processes that prevent the formulation and manufacture of exact copies of the original.44,45 Unlike generics with commercially available reagents, the biotechnologically derived biosimilars engineer their cellular clones in-house using living organisms or extracted tissues.44–47 Further, both originator biotechnologicals and biosimilars can undergo changes such as glycosylation or amino acid sequence variation (contamination), among others, that can induce immune effects.43,48–50 PRCA, an anti-EPO antibody-associated severe anemia that results in transfusion dependence, is one such immune effect.50,51 Complex manufacturing processes can influence the chemical and clinical characteristics of a biosimilar or affect biosimilar quality. The complex molecular structure, as well as other issues including formulation and manufacture, results in biosimilars being similar to the referent product but not identical. However, biosimilars demonstrate comparable pharmacokinetics and therapeutic equivalence to the reference product.33,45,52–54 Minor differences in the microheterogeneity pattern of the molecule between the reference product and biosimilar are acceptable only when the difference does not impact safety and efficacy.55
Regulatory approval designed to ensure therapeutic equivalence for biosimilars differs from that for generic drug formulations and addresses the complex formulation and manufacture processes. Since the formulation and manufacturing process can affect molecular similarity, regulators face challenges in establishing efficient and appropriate regulatory review and approval pathways to ensure equivalent therapeutic efficacy and safety.47 The EMA provides regulatory oversight for biosimilars for the EU, with Australia also adopting the EMA comparability and quality guidelines.56 The EMA guidelines require comparability studies between an authorized originator (termed reference product) biotechnologically derived product and the biosimilar in regards to clinical and nonclinical quality, safety, efficacy,44,45,53,57–60 and delineate standards for immunogenicity assessment.58,61,62 Further, the EMA provides specific epoetin guidelines for recombinant EPO biosimilars.62 In the US, the Biosimilar Price Competition and Innovation Act, passed as part of the 2010 Patient Protection and Affordable Care Act, establishes the framework for the FDA regulatory pathway for biosimilars. To date, the FDA guidance is formative, with the fourth guidance draft released in the spring of 2013.63 The EMA and FDA have a biosimilar cluster to enhance global development.63 Also in the US, the CMS issues rules and regulations for physician fee schedule and other Medicare Part B payment systems for biosimilar biological products,64 as well as billing procedures and other policies affecting the use of EPO stimulating agents in the US Medicare and Medicaid population.65,66
Pharmacology
Epoetin zeta is a rHuEPO biosimilar preparation to epoetin alfa. In adults, endogenous EPO production occurs primarily in interstitial kidney cells, as well as in smaller amounts in the liver and central nervous system.67 Although anemia associated with renal impairment is multifactorial, in the presence of hypoxia, endogenous EPO production declines. Several theories give rationale explaining why hypoxic conditions in renal disease result in reduced EPO production instead of the normal increase in EPO production.24,30,68,69 Some theories suggest dysfunctional hypoxic cell signaling,30,69 whereas others suggest shortened life span of circulating red blood cells, nutritional deficiencies, or inflammation.24 Dysfunctional hypoxic cell signaling in the kidneys appears to play an important role in the lack of upregulation of EPO in CKD patients. Normally, tissue hypoxia increases the expression of hypoxia inducible transcription factors that bind to a hypoxia-responsive portion of the EPO gene that, in turn, results in increased EPO production.67,69 In CKD, the normal oxygen dependent hydroxylation of hypoxia-inducible transcription factors are impaired.70 Instead of increasing EPO expression, there is an inhibition of EPO production or loss of synthesis of EPO67,70 with no corresponding increase in red blood cell production.70
Iron deficiency also contributes to renal anemia due to insufficient iron and chronic inflammation.71,72 Iron facilitates new red blood cell production whereas iron insufficiency limits the quantity of new red blood cells.71,72 Chronic inflammation reduces the amount of iron released from storage when it is needed.72 Supplementing EPO treatments with iron reduces the risk for iron deficiency. Epoetin zeta is a rHuEPO therapy that can be supplemented with iron.68
Epoetin zeta is a derivative of the endogenous protein erythropoietin68 and is a biosimilar of the rHuEPO epoetin alfa, the first commercialized rHuEPO (Epogen®, AMGEN Inc., Thousand Oaks, CA, USA; Procrit®, JANSSEN Biotech, Inc., Horsham, PA, USA; Eprex®, JANSEEN-CILAG Ltd., Buckinghamshire, UK; Epoetin®, AMGEN Inc., Thousand Oaks, CA, USA). As a biosimilar to epoetin alfa, epoetin zeta has the same 165-amino-acid structure and comparable carbohydrate composition with only minor differences in the glycosylation pattern.24,41,48,74 Glycosylation patterns largely influence immunogenicity and half-life.48 More complex glycosylation patterns have a higher number of sialic acid residues with longer half-lives.68,74 Sialic acid carbohydrate content affects serum half-life, with increased sialic acid content resulting in longer half-life. Epoetin alfa and zeta both have 14 sialic acid residues,68 and are short acting drugs with a half-life of 6–8 hours when administered intravenously or 19–24 hours if administered subcutaneously.75 The short half-life allows epoetin zeta to be administered up to three times per week to treat anemia due to CKD.24,76
Epoetin alfa and epoetin zeta are pharmacodynamically comparable both in vitro and in in vivo tests. Pharmacokinetic studies also report bioequivalence. Krivoshiev et al28,41 report the pharmacokinetics of epoetin zeta and epoetin alfa (the reference product) in healthy volunteers after intravenous and subcutaneous administration, respectively. The pharmacokinetic study assessing intravenous administration was a monocentric, open, randomized, single dose, and two-period crossover trial in 24 volunteers28 with 21 volunteers included for statistical analysis. Compared to epoetin alfa, the biosimilar epoetin zeta showed nearly identical pharmacokinetic parameters including area under the concentration/time curve, maximal concentration after administration, volume of distribution, total plasma clearance of drug after administration, mean residence time, and terminal half-life.28 A second study assessing subcutaneous administration of epoetin zeta reported results for a monocentric, double-blind, randomized, single dose, three-period crossover trial in 48 volunteers. This study evaluated the subcutaneous bioavailability and pharmacokinetic properties of epoetin zeta compared with epoetin alfa (reference product).41 The subcutaneous bioavailability of epoetin zeta is 24%, which is equivalent to epoetin alpha 20%.41 Additionally, no significant differences in the aforementioned pharmacokinetic parameters were observed between subcutaneous epoetin zeta and epoetin alfa.41 The nearly identical pharmacokinetic characteristics suggest bioequivalence of epoetin zeta to epoetin alfa.
The dosage for epoetin zeta is 93–97 IU/kg/week to treat anemia due to loss of endogenous production of EPO in patients with chronic renal failure.41 Despite its differences in the glycosylation pattern from epoetin alfa, epoetin zeta stimulates erythropoiesis by binding to the EPO receptor on cells.68 Once epoetin zeta is bound to the receptor, the receptor dimerizes and activates the Janus kinase 2 signal pathway, which goes on to activate the transcription 5 pathway.68 This pathway continues to activate the EPO gene needed for proliferation and maturation of red blood cells and to treat anemia.77 As a biosimilar using the endogenous pathway, there is concern that epoetin zeta could lead to neutralizing antibodies.48,57 Neutralizing antibodies react against endogenous EPO and result in PRCA. PRCA occurs when there is no endogenous production of EPO due to antibodies clearing endogenous EPO and rHuEPO. The clearing of all EPO results in severe anemia requiring blood transfusion intervention.77 However, epoetin zeta shows a different immunogenicity profile,48 mitigating the likelihood of PRCA.41,52
Efficacy
Efficacy studies test the comparability of biosimilars to the referent product for specific endpoints as well as product interchangeability. Epoetin zeta demonstrates comparable therapeutic efficacy to the reference product (epoetin alfa) for IV and subcutaneous routes of administration in the correction and maintenance phases of treating renal anemia.24,27,28,78
Hemoglobin endpoints were measured in two premarket authorization studies for the correction and maintenance phases of treating renal anemia in patients receiving hemodialysis. These premarket studies were designed to assess the clinical efficacy of SB309 (epoetin zeta) to the referent product, epoetin alfa (Erypo® formulation, Johnson & Johnson, New Brunswick, NJ, USA). One randomized, double-blind, verum controlled, multiple dose, parallel group, multicenter study (study 411-54-04-05-0000) assessed the therapeutic equivalence of SB309 to the referent product in the correction phase of renal anemia when administered IV. Another randomized, double-blind, crossover, verum controlled, multiple dose, multination Phase III trial (study 411-54-04-04-0000) tested the therapeutic equivalence of SB309 to the referent product in maintaining Hb levels, also when administered IV. Both studies met designated therapeutic Hb endpoints in correcting and maintaining target Hb levels when administered IV compared to the referent product.61 Additional supportive open-label uncontrolled safety trials were conducted, reporting similar safety profiles between epoetin zeta and the referent product.61 These premarket studies, demonstrating a high degree of similarity between epoetin zeta and the referent product, led to the approval of epoetin zeta as a biosimilar to epoetin alfa. After receiving authorization for IV administration, a later premarket authorization randomized trial assessed the therapeutic equivalence of subcutaneously administered epoetin zeta versus epoetin alfa. This study showed therapeutic equivalence in maintaining target Hb levels (epoetin zeta mean Hb 10.94 ± 0.84 g/dL, epoetin alfa mean Hb 11.02±0.94 g/dL [95% CI –0.28 g/dL to 0.12 g/dL]) and safety profiles.41
Postmarket authorization research reports epoetin zeta as meeting or maintaining target Hb levels when administered IV, over the long term, or when patients switch from other ESAs to epoetin zeta. A study assessing the long-term safety and tolerability of IV epoetin zeta administration reports maintaining target Hb levels of 10.5–12.5 g/dL (after 56 weeks in n=745 patients, mean Hb=11.3–11.5 g/dL; after 108 weeks in n=164 patients, mean Hb=11.1–11.6 g/dL) with stable dosing and no neutralizing antibodies against erythropoietin.79 One German postmarketing surveillance study of IV administered epoetin zeta in 322 eligible elderly (age ≥75 years) patients from 40 centers showed epoetin zeta as effective at maintaining Hb levels with no significant difference in mean dose requirements or safety concerns.80 A study by Bajraktar et al81 assessing interchangeability switched 33 patients in two dialysis centers from various ESAs to epoetin zeta. The Bajraktar et al study reported that epoetin zeta maintained target Hb with equivalent mean dose, no PRCA, and a safety profile in line for the study population.
Safety
Several studies report adverse events associated with using erythropoiesis stimulating agents (ESAs), including epoetin alfa, when trying to achieve supraphysiologic target Hb levels or when using high doses to achieve normalization of Hb levels in poorly responsive renal anemia.30,60,62,82 When treating anemia in CKD with ESAs, using high or supraphysiologic Hb thresholds, and/or high target Hb levels, and/or high ESA dosing levels to guide therapy a significant association is shown, with an increased risk for mortality, morbidity, cardiovascular events and stoke, and tumor progression. Also, supraphysiologic Hb thresholds significantly increase the risk for an immunogenic response termed ESA-induced PRACA.30,60,62,82
Three important studies – the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR),82 Cardiovascular Reduction Early Anemia Treatment Epoetin beta (CREATE),83 and Trial to Reduce cardiovascular Events with Aranesp Therapy (TREAT)84 – reported pivotal findings influencing current thinking about safety and practice guidelines related to target Hb ranges and dosing for all ESAs, including biosimilars such as epoetin zeta when treating renal anemia. CHOIR and CREATE reported findings from an open-label research design, while the TREAT study reported results from a placebo controlled randomized double-blind controlled trial. The CHOIR study (EPO alfa) included 1,432 CKD patients, approximately 50% of whom had coexistent diabetes, and had an intention-to-treat Hb range of 13.5 g/dL versus 11.3 g/dL. The study was stopped due to the number and severity of adverse safety events in the high Hb group.82 Another study, CREATE, assessed EPO beta with Hb intention-to-treat (13–15 g/dL versus 10.5–11.5 g/dL) in 603 patients with CKD and anemia (n=301 and n=302, respectively) with results showing significant progression to ESRD in the higher target Hb group.83 The study also showed no significant difference between groups for cardiovascular outcomes and LVH.83 A third study, TREAT, included patients with CKD, type 2 diabetes, and anemia (n=2,012 treated with darbepoetin alfa targeting Hb 13 g/dL versus the placebo control group n=2,026 treated with rescue doses of darbepoetin alfa for Hb <9 g/dL) and reported no significant difference in hazard rates for cardiovascular events, death, myocardial ischemia, or ESRD, and a significant nearly two-fold increased risk of stroke.84
Current clinical practice recommendations suggest lower than previously accepted parameters for target Hb ranges in CKD for those not yet on dialysis and for patients on dialysis, as well as for low risk and high risk patients with other comorbid conditions. Guidelines continue to evolve in light of the CHOIR, CREATE, and TREAT findings and subsequent secondary analysis of their data, among other research using secondary analysis of the data, with revisions being made to previous standards for appropriate Hb levels when initiating treatment as well as target Hb ranges for maintenance therapy. To give context, the 2008 ERBP guidelines for the management of anemia in CKD revised earlier guidelines and emphasize safety concerns when using ESAs.85 More recently, in April 2013, the KDIGO position paper on anemia management guidelines in CKD summarizes, in chapter 3, the recent evidence about earlier EPO safety concerns and recommends adapting the guidelines for the European population.29 When considering the risks and benefits of initiating ESA therapy, the KDIGO recommends caution in high risk groups (stroke, vascular access loss, or hypertension) and great caution for those with CKD and active malignancy, and suggests Hb levels for low-risk patients (no higher than 12 g/DL) and high risk patients (Hb between 9 and 10 g/dL) when initiating therapy.29 The report discusses Hb normalization and suggests individualized therapy, but cautions against using ESAs to maintain Hb levels above 11.5 g/dL.30 The KDIGO group goes on to say ESAs should not be used to intentionally increase Hb concentrations above 13 g/dL (130 g/L).30 In the US, in March 2007, the FDA issued a public health advisory with an updated black box warning for approved ESAs in the US (epoetin alfa and darbepoetin alfa) with the recommendation to monitor Hb levels and adjust ESA dose to the lowest possible to avoid blood transfusion.62 Collaterally, the CMS put forth a position statement for ESA use in the treatment of anemia in adults with CKD, including patients on dialysis and patients not on dialysis, after reviewing the evidence related to safety.65,66
The safety profile for epoetin zeta is similar to epoetin alfa, the reference product. Reports of ESA-induced PRACA in all commercially available ESAs are a key safety concern. Although the mechanism behind ESA-induced PRACA remains unknown, the highest incidence arose from epoetin alfa manufactured outside the US (Eprex®/Erypro®; Ortho-biologics, LLC, Manati, Puerto Rico).86 Our review found no reports for ESA-induced PRACA related to manufacture site. The Baldamus et al study assessing the long-term safety of IV administered epoetin zeta showed no incidence of PRACA.79 Three other postmarket clinical trials reported no cases of anti-epoetin antibodies or PRCA.24,28,41
A few studies have compared epoetin alfa’s safety profile to that of epoetin zeta. Del Vecchio and Locatelli’s review article examined cardiovascular safety (in particular stroke and hypertension), cancer progression, and immunogenicity associated with using ESA to treat anemia in patients with chronic disease.57 To avoid high ESA dosing, the authors suggest using a conservative and individualized approach based on a risk/benefit evaluation, and targeting intermediate Hb levels.75,86
Eight studies reported the safety of epoetin zeta in patients with renal anemia. Of those, there are four clinical trials,24,28,41,79 three observational studies,76,81,87 and one post hoc analysis based on two clinical trials.88 Table 4 summarizes the common adverse events observed in more than 5% of the patients in the four clinical trials. Infections and infestations were the most common adverse events reported in the clinical trials (12.5%–34.1%) followed by gastrointestinal disorders (5.2%–21.9%) and injury, poisoning, and procedural complications (7.2%–25.8%). Baldamus et al79 reported 715 serious adverse events in 278 patients with cardiac disorders (8.6% of patients), vascular disorders (6.6% of patients), and injury, poisoning, and procedural complications (7.0% of patients) as the most common groups of serious adverse events occurring in more than 5% of patients. Krivoshiev et al41 assessed epoetin zeta administered subcutaneously for maintenance treatment of renal anemia. The study reported 38 patients as having 91 serious adverse events, with the three most common serious adverse events being medical surgical and medical procedures (4.7% of patients), cardiac disorders (3.4% of patients), and nervous system disorders (3.4%). Krivoshiev et al28 also compared therapeutic effects of epoetin zeta and epoetin alfa in the correction of renal anemia, where 54 out of 305 patients treated with epoetin zeta reported serious events. Wizemann et al’s24 crossover design clinical trial showed 43 patients experiencing 71 serious adverse events, with injuries, poisoning, and procedural complications as the most reported serious adverse events (23.3% of patients).
  | Table 4 Adverse events reported in epoetin zeta clinical trials |
Lonnemann and Wrenger’s76 small clinical observational study assessing the use of epoetin zeta in ESRD reported 17 out of 18 patients as switching from various ESAs to epoetin zeta with no side effects after 6 months of epoetin zeta treatment. Bajraktar et al81 evaluated the safety of epoetin zeta in 33 dialysis patients and reported that 3% of the patients develop hypotension, in-dialyzers clotting, or thrombosis of the arteriovenous fistula. Perani et al87 examined the immunological reaction of epoetin zeta in 12 patients with CKD. This study showed no adverse events after switching from methoxy polyethylene glycol epoetin beta to epoetin zeta.
In a post hoc analysis of three large Phase III clinical trials, Wiecek et al88 reported the safety of switching epoetin alfa and epoetin zeta. The post hoc analysis includes 481 patients from the maintenance trial (n=239) as well as from the induction trial (n=242). The results show no difference in the treatment-emergent adverse event profiles when patients switch from epoetin alfa to epoetin zeta. The same type of treatment-emergent events occurred as would be seen in ≥10% of the patient population.
Overall, the biosimilar epoetin zeta shows tolerable adverse effects in patients with renal anemia compared to the reference product epoetin alfa.52,89 However, regulations require a detailed risk management plan to monitor serious and severe safety events and unknown potential safety issues for pharmacovigilance.77 For instance, assessing safety issues in the populations underrepresented in clinical trials, such as elderly patients or patients with various comorbidities, would add to risk management plan requirements. Additional postmarketing pharmacovigilant activities to evaluate unanticipated issues that may happen when patients switch between a biosimilar and their referent product could include the close monitoring of PRCA and thromboembolic events, as well as drug utilization and tracking of rare but serious adverse events.57,89 Table 4 illustrates the adverse events reported in epoetin zeta clinical trials.
Current treatment in CKD
Patients with symptomatic renal anemia subsequent to ESRD or CKD of at least stage 3 (estimated GFR <60) whose Hb levels are less than or equal to 10 g/dL are eligible for treatment.
For all CKD indications, current EMA guidelines recommend specific target Hb ranges and dosing regimens for initiating treatment (termed the correction phase) as well as for maintenance therapy. Target Hb levels are particularly important due to the safety concerns surrounding the increased risk for mortality and morbidity associated with epoetin use to achieve relatively high target Hb levels.90 The target Hb ranges are 10–12 g/dL (6.2–7.5 mmol/L) for adults and 9.5–11 g/dL (5.9–6.8 mmol/L) in pediatric patients. Clinicians should avoid maintaining Hb levels >12 g/dL (7.5 mmol/L) and should be vigilant to avoid a rise in Hb levels >2g/dL (1.25 mmol/l) over a 4 week period.90
Licensed health care providers face patient care challenges related to the interchangeability of biotherapeutics and ESA drug safety concerns including those for epoetin zeta.91,92 Interchangeability can occur between biosimilar products and the reference product, as well as among a biopharmaceutical product class (eg, any ESAs). Salem and Harvie discuss the nurse’s role and responsibilities regarding biosimilar medicines and their clinical use.93 Inadequate knowledge of biosimilar products increases the risks for medication errors and adverse events, and/or may affect desired treatment outcomes. Due to the complex nature of biopharmaceutical products, the study authors suggest providing additional professional training and educational opportunities for healthcare providers who prescribe, dispense, or administer biopharmaceutical products or biosimilars.93 Health care providers should be well informed about the differences between biosimilars and their reference products, gain evidence-based knowledge about biosimilar use in clinical practice, and track adverse events when patients switch between biosimilar and reference products.78,92,93
Pharmacoeconomic considerations
ESAs bring significant clinical benefits to patients with renal anemia. However, ESAs are very expensive and access to them is likely to be restricted. With the advent of biosimilars, drug access and treatment options improved adding potential health economic benefits over the reference biopharmaceutical ESA therapy.87,88,93,94,96 In one survey in Spain, the epoetin zeta cost analysis reported a total cost saving of nearly 45% with respect to epoetin alfa.97 Costs per patient per month declined from €298 for epoetin alfa therapy to €177 per patient per month for epoetin zeta.96 Additionally, a UK cost minimization study showed that epoetin zeta minimizes the costs for treating renal anemia compared to epoetin alfa.98 Epoetin zeta decreased the cost by £7.9 per week for a hemodialysis patient in the Hb correction phase and by £3.9–£15.6 per week in a hemodialysis patient at Hb maintenance phase, based on a 70 kg patient.98 Low acquisition costs for epoetin zeta contribute to the potential cost savings. However, the risk of immunogenicity and the safety profile for serious adverse events raises uncertainties for the long-term safety and effectiveness of biosimilars. Future epoetin zeta studies could assess patient outcomes and costs for target Hb levels and further compare results between epoetin zeta and epoetin alfa. Additionally, Simoens suggests conducting further pharmacoeconomic analyses at multiple time points throughout the life cycle of biological pharmaceutical products.99
Utility
Utility measures the value of health states from a specified perspective (eg, society, payer, or patient perspective) using a standard measure such as a quality adjusted life year (QALY). Our search did not find any formal cost utility analysis or direct elicitation measures, such as standard gamble or time trade-off, for epoetin zeta in CKD. We found only one study reporting quality of life (QOL) for epoetin zeta in chemotherapy-induced anemia.100
Early research suggests improved health outcomes and symptom management for rHuEPO (EPO alfa), such as exercise capacity,36 and health related QOL in patients with renal anemia.37,101,102 A recent post hoc analysis of the Canadian Erythropoietin Study Group trial ([EPO alfa] included patients on dialysis >3 months with anemia (Hb <9 g/dL), randomized to (n=38, epoetin zeta intravenous treatment group versus 40, placebo), intention-to-treat Hb range 9.5–11 g/dL EPO alfa treatment group versus Hb 11.5–13 g/dL placebo control) showed significantly that fatigue, shortness of breath, and weakness were lessened, and energy was improved in the epoetin alfa treatment group compared to controls.103 Another post hoc, multicenter, open-label prospective study using epoetin alfa to treat anemia in patients with nondialysis CKD showed that, across every 2 g/dL change in Hb, the greatest incremental improvement in QOL occurred when the Hb level reached 11–12 g/dL.102 A meta-analysis of epoetin alfa clinical trials by Jones et al reported improvements in Hb levels and QOL, and reductions in hospitalizations, overall transfusion rate, and number of units of blood transfused.104 Another study showed improvements in QOL when treating anemia with epoetin alfa in patients with human immunodeficiency virus infection/acquired immunodeficiency syndrome and coexistent CKD.105
However, the CHOIR trial reported serious increased risk of death, myocardial infarction, congestive heart failure, and stroke with no incremental improvement in QOL when correcting renal anemia with epoetin alfa with target Hb levels of 13.5 g/dL.83 Target Hb levels remain an important consideration in the risk benefit profile for epoetin alfa and in the relationship to utility measures and health outcomes. A review by Leaf and Goldfarb to assess the magnitude and nature of ESA-associated improvements in health related QOL found the maximal increase in health related QOL occurs for Hb ranging between 10 and 12 g/dL, with health-related quality of life (HRQL). improvements blunted beyond that range.106 Additional utility research is needed to assess QALYs, symptom management using the current standard of care for appropriate Hb levels when initiating therapy, and Hb target ranges to correct renal anemia with epoetin zeta compared to other EPAs or biosimilars.
Although we did not find any studies reporting QOL outcomes generally or in terms of QALY, or by symptom management for epoetin zeta in managing anemia in CKD, one study reported improvements in QOL when treating patients with chemotherapy-induced anemia with epoetin zeta.100 Future research could further our knowledge about utility outcomes for epoetin zeta in CKD, by the different stages of CKD, or among patients with different risk profiles as compared to the reference product (EPO alfa), or as compared to other ESAs or other biosimilars.
Conclusion
Our review of the biosimilar epoetin zeta found that epoetin zeta has therapeutic equivalence and a comparable safety profile to the reference product (EPO alfa). Epoetin zeta shows comparable efficacy to epoetin alfa in the correction and maintenance phases of CKD anemia treatment for both IV and subcutaneous administration, as well as when interchanged with other ESAs. Although two studies report cost savings, the small number of rigorous studies devoted to cost effectiveness limits the generalizability of suggesting epoetin zeta as a cost effective alternative to current standard treatment or other ESAs.107 Although one study assessed health related QOL in cancer, to date, we found no utility studies assessing measures of health related QOL, when treating renal anemia with epoetin zeta. Evidence based practice guidelines for ESAs for both originator products and biosimilars, including epoetin zeta, continue to evolve in light of safety concerns, and include recent recommendations about contraindications, when to initiate therapy, dosing, and parameters for Hb levels and target maintenance Hb ranges. Further research is needed to expand our knowledge of the cost effectiveness and health related QOL outcomes for epoetin zeta.
Disclosure
The authors report no conflicts of interest in this work.
References
KDOQI Clinical Practice Guidelines: KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease [webpage on the Internet]. New York: National Kidney Foundation; 2006. Available from: http://www.kidney.org/professionals/kdoqi/guidelines_anemia/cpr12.htm. Accessed September 09, 2013. | |
KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. New York: National Kidney Foundation; 2002. Available from: http://www.kidney.org/professionals/kdoqi/guidelines_ckd/bibliography.htm#267. Accessed September 09, 2013. | |
Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int. 2012;2:279–335. | |
Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. | |
El Nahas AM, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005;365(9456):331–340. | |
US Government. Renal Data System, USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013. | |
Roderick P, Roth M, Mindell J. Prevalence of chronic kidney disease in England: findings from the 2009 health survey for England. J Epidemiol Community Health. 2011;65(Suppl I):A12. | |
Zoccali C, Kramer A, Jager K. Chronic kidney disease and end-stage renal disease a review produced to contribute to the report ‘status of health in the European union: towards a healthier Europe’. NDT Plus. 2010;3(3):213–224. | |
Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010; a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. | |
Levin A. Identification of patients and risk factors in chronic kidney disease: evaluating risk factors and therapeutic strategies. Nephrol Dial Transplant. 2001;16 Suppl 7:57–60. | |
Chadban S, Howell M, Twigg S, et al; CARI. The CARI guidelines. Cost-effectiveness and socioeconomic implications of prevention and management of chronic kidney disease in type 2 diabetes. Nephrology (Carlton). 2010;15 Suppl 1:S195–203. | |
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalizations. N Engl J Med. 2004;351(13):1296–1305. | |
Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15(5):1307–1315. | |
Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardol. 2008;52(19):1527–1539. | |
Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with aneamia; The Third national Health and Nutrition Examination Survey (1988–1994). Arch Intern Med. 2002;162(12):1401–1408. | |
McClellan W, Aronoff SL, Bolton WK, et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin. 2004;20(9):1501–1510. | |
Painter P, Moore G, Carlson L, et al. Effects of exercise training plus normalization of hematocrit on exercise capacity and health-related quality of life. Am J Kidney Dis. 2002;39(2):257–265. | |
Perlman RL, Finkelstein FO, Liu L, et al. Quality of life in chronic kidney disease (CKD); a cross-sectional analysis in the Renal Research Institute-CKD study. Am J Kidney Dis. 2005;45(4):658–666. | |
Baigent C, Burbury K, Wheeler D. Premature cardiovascular disease in chronic renal failure. Lancet. 2000;356(9224):147–152. | |
Portolés J, Gorriz JL, Rubio E, et al; for NADIR-3 Study Group. The development of anemia is associated to poor prognosis in NKF/KDOQI stage 3 chronic kidney disease. BMC Nephrol. 2013;14:2. | |
Thorp ML, Johnson ES, Yang X, Petrik AF, Platt R, Smith DH. Effect of anaemia on mortality, cardiovascular hospitalization and end-stage renal disease among patients with chronic kidney disease. Nephrology (Carlton). 2009;14(2):240–246. | |
Locatelli F, Andrulli S, Memoli B, et al. Nutritional-inflammation status and resistance to erythropoietin therapy in haemodialysis patients. Nephrol Dial Transplant. 2006;21(4):991–998. | |
Vaziri ND. Anemia and anemia correction: surrogate markers or causes of morbidity in chronic kidney disease? Nat Clin Pract Nephrol. 2008;4(8):436–445. | |
Wizemann V, Rutkowski B, Baldamus C, Scigalla P, Koytchev R; Epoetin Zeta Study Group. Comparison of the therapeutic effects of epoetin zeta to epoetin alfa in the maintenance phase of renal anaemia treatment. Curr Med Res Opin. 2008;24(3):625–637. | |
Eandi M. Erythropoiesis-stimulating agents in patients with chronic kidney disease. Rev Health Care. 2012;3(2):113–125. | |
McGonigle RJ, Wallin JD, Shadduck RK, Fisher JW. Erythropoietin deficiency and inhibition of erythropoiesis in renal insufficiency. Kidney Int. 1984;25(2):437–444. | |
Lonnemann G, Macdougall IC. Biosimilar epoetins in renal anaemia â current status and insights from European practice. Eur Nephrol. 2011;5(2):101–107. | |
Krivoshiev S, Todorov VV, Manitius J, et al. Comparison of the therapeutic effects of epoetin zeta and epoetin alfa in the correction of renal anaemia. Curr Med Res Opin. 2008;24(5):1407–1415. | |
Macdougall IC. Recent advances in erythropoietic agents in renal aneamia. Semin Nephrol. 2006;26(4):313–318. | |
Locatelli F, Báránay P, Covic A, et al. Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statement. Nephrol Dial Transplant. 2013;28(6):1346–1359. | |
KDOQI. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–S154. | |
Zumbreanen-Bullogh K, Babitt JL. The iron cycle in CKD: from genetics and experimental models to CKD patients. Nephrol Dial Transplant. Epub November 13, 2013. | |
Jones M, Schenkel B, Just J. Epoetin alfa’s effect on left ventricular hypertrophy and subsequent mortality. Int J Cardiol. 2005;100(2):253–265. | |
Benz RL, Pressman MR, Hovick ET, Peterson DD. A preliminary study of the effects of correction of anemia with recombinant human erythropoietin therapy on sleep, sleep disorders, and daytime sleepiness in hemodialysis patients (the SLEEPO study). Am J Kidney Dis. 1999;34(6):1089–1095. | |
Evans RW. Recombinant human erythropoietin and the quality of life of end-stage renal disease patients: a comparative analysis. Am J Kidney Dis. 1991;18(4 Suppl 1):62–70. | |
Painter P. The importance of exercise training in rehabilitation of patients with end-stage renal disease. Am J Kidney Dis. 1994;24(1 Suppl 1):S2–S9; discussion S31–S32. | |
Revicki DA, Brown RE, Feeny DH, et al. Health-related quality of life associated with recombinant human erythropoietin therapy for predialysis chronic renal disease patients. Am J Kidney Dis. 1995;25(4):548–554. | |
Roger SD. Biosimilars: How similar or dissimilar are they? Nephrology (Carlton). 2006;11(4):341–346. | |
Guideline on non-clinical and clinical development of similar biological medicinal products containing recombinant erythropoietins (Revision). London: European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP); 2010 Oct[about 8 p.] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/04/WC500089474.pdf. Accessed September 12, 2013. | |
Rossert J. EMEA guidelines on biosimilars and their clinical implications. Kidney Blood Press Res. 2007;30 Suppl 1:13–17. | |
Krivoshiev S, Wizemann V, Czekalsk S, et al. Therapeutic equivalence of epoetin zeta and alfa, administered subcutaneously, for maintenance treatment of renal anemia. Adv Ther. 2010;27(2):105–117. | |
Triolo G; Italian Society of Nephrology. Guidelines for the treatment of aneamia in chronic renal failure. G Ital Nefrol. 2003;20 Suppl 24: S61–S82. Italian. | |
Barosi G, Bosi A, Abbroacchio MP, et al. Key concepts and crucial issues on epoetin and filgrastim biosimilars. A position paper from the Italian Society of Hematology, Italian Society of Experimental Hematology, and Italian Group for Bone Marrow Transplantation. Haematologica. 2011;96(7):937–942. | |
Waller CF. Biosimilars and their use in hematology and oncology. Commun Oncol. 2012;9(6):198–205. | |
Tsiftsoglou AS, Ruiz S, Schneider CK. Development and regulation of biosimilars: Current status and future challenges. Bio Drugs. 2013; 27(3):203–211. | |
Borges L. Different modalities of erythropoiesis stimulating agents. Port J Nephrol Hypert. 2010;24(2):137–145. | |
Sitte HH. Biologicals and biosimilars. Port J Nephrol Hypert. 2009;23(2):135–139. | |
Tamilvanan S, Raja NL, Sa B, Basu SK. Clinical concerns of immunogenicity produced at cellular levels by biopharmaceuticals following heir parenteral administration into human body. J Drug Target. 2010;18(17):489–498. | |
Schellekens H. Biosimilar therapeutics – what do we need to consider? NDT Plus. 2009;2(Suppl 1):i27–i36. | |
Casadevall N, Echardt KU, Rossert J. Epoetin-induced autoimmune pure red cell aplasia. J Am Soc Nephrol. 2005;16 Suppl 1:S67–S69. | |
Casadevall N. What is antibody-mediated pure red cell aplasia (PRCA)? Nephrol Dial Transplant. 2005;20 Suppl 4:iv3–iv8. | |
Abraham I, Sun D, Bagalagel A, et al. Biosimilars in 3D: Definition, development and differentiation. Bioengineered. 2013;4(4):203–206. | |
Ahmed I, Kaspar B, Sharma U. Biosimilars: Impact of biologic product life cycle and European experience on the regulatory trajectory in the United States. Clin Ther. 2012;34(2):400–419. | |
Combe C, Tredree RL, Schellekens H. Biosimilar epoetins: An analysis based on recently implemented European medicines evaluation agency guidelines on comparability of biopharmaceutical proteins. Pharmacother. 2005;25(7):954–962. | |
Weise M, Bielsky MC, De Smet K, et al. Biosimilars: what clinicians should know. Blood. 2012;120(26):5111–5117. | |
Non-clinical guidelines: European Union guidelines adopted in Australia [webpage on the Internet]. Symonston: Department of Health: Therapeutic Goods Administration; 2012. Available from: http://www.tga.gov.au/industry/pm-euguidelines-adopted-nonclinical.htm#nonclinicalsimilar. Accessed Sept 10, 2013. | |
Del Vecchio L, Locatelli F. Safety issues related to erythropoiesis-stimulating agents used to treat anemia in patients with chronic kidney disease. Expert Opin Drug Saf. 2012;11(6):923–931. | |
European Medicines Agency: Committee for Medicinal Products for Human Use (CHMP). Guidelines on Immunogenicity Assessment of Biotechnology-Derived Therapeutic Proteins. London: European Medicines Agency; 2007. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003947.pdf. Accessed September 09, 2013. | |
European Medicines Agency: Committee for Medicinal Products for Human Use (CHMP). Similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. London: European Medicines Agency; 2006. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003920.pdf. Accessed September 09, 2013. | |
Jelkmann W. Biosimilar epoetins and other “follow-on” biologics: update on the European experiences. Am J Hematol. 2010;85(10):771–780. | |
European medicines Agency. Scientific discussion. London: European medicines Agency; 2007. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000872/WC500054374.pdf. Accessed September 10, 2013. | |
MP389 Immunogenicity of epoetin zeta in the treatment of renal anaemia [webpage on the internet]. Casadevall N, Kromminga A, Scigalla P. Available from: http://www.abstracts2view.com/era_archive/view.php?nu=ERA08L_1714. Accessed September 14, 2013. | |
Guidance for industry formal meetings between the FDA and biosimilar biological product sponsors or applicants [webpage on the Internet]. Silver Spring: U.S. Food and Drug Administration. Available from: http://www.fda.gov/Drugs/GuidancecomplianceRegulatory Information/Guidances/default.htm. Accessed March 2013. | |
U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER). Draft guidance for industry formal meetings between FDA and biosimilars biological product sponsors or applicants. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM345649.pdf. Accessed January 8, 2014. | |
Decision memo for erythropoiesis stimulating agents (ESAs) for treatment of anemia in adults with CKD including patients on dialysis and patients not on dialysis (CAG-00413N) [webpage on the Internet]. Baltimore: Centers for Medicare and Medicaid Services (CMS); 2011. Available from: http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=245&bc=AAAAAAAACAAAAA%3d%3d&. Accessed September 20, 2013. | |
Erythropoietin stimulating agents policies [webpage on the Internet]. Baltimore: Centers for Medicare and Medicaid Services (CMS); 2013. Available from: http://www.cms.gov/Medicare/Coverage/CoverageGenInfo/esapolicies.html. Accessed September 20, 2013. | |
Kapitsinou PP, Liu Q, Unger TL, et al. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood. 2010;116(16):3039–3048. | |
Macdougall IC. Novel agents for anaemia management in chronic kidney disease. Europiean Nephrol. 2010;4(1):37–41. | |
Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013;27(1):41–53. | |
Bernhardt WM, Wiesener MS, Scigalla P, et al. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol. 2010;21(12):2151–2156. | |
Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. N Engl J Med. 1987;316(2):73–78. | |
Adamson JW, Eschbach JW. Management of the anaemia of chronic renal failure with recombinant erythropoietin. Q J Med. 1989;73(272):1093–1101. | |
Hörl WH. Differentiating factors between erythropoiesis-stimulating agents: An update to selection for anaemia of Chronic Kidney Disease. Drugs. 2013;73(2):117–130. | |
Walsh G. Post-translational modifications in the context of therapeutic proteins: An introductory overview. Post-translational Modification of Protein Biopharmaceuticals. West Sussex, UK: Wiley-Blackwell. 2009:1–14. | |
Locatelli F, Del Vecchio L. Erythropoiesis-stimulating agents in renal medicine. Oncologist. 2011;16 Suppl 3:19–24. | |
Lonnemann G, Wrenger E. Biosimilar epoetin zeta in nephrology: Effect of injection frequency on weekly dose. Int J Clin Med. 2012;3: 598–602. | |
Ebbers HC, Muenzberg M, Schellekens H. The safety of switching between therapeutic proteins. Expert Opin Biol Ther. 2012;12(11):1473–1485. | |
Casadevall N, Edwards IR, Felix T, et al. Pharmacovigilance and biosimilars: Considerations, needs and challenges. Expert Opinion Biol Ther. 2013;13(7):1039–1047. | |
Baldamus C, Krivoshiev S, Wolf-Pflugmann M, Siebert-Weigel M, Koytchev R, Bronn A. Long-term safety and tolerability of epoetin zeta, administered intravenously, for maintenance treatment of renal anemia. Adv Ther. 2008;25(11):1215–1228. | |
Bachmakov I, MeiBner R, Benkenstein C. Sa423 Comparison of long-term efficacy and safety of epoetin zeta in elderly ‘real-word’ patients with chronic kidney disease (CKD) and renal anaemia on dialysis aged ≥ and <75 years. Poster Session: Anaemia in CKD 5. | |
Bajraktar J, Lazarova B, Najdovska E, Mihajlova L. DSL-011 efficacy and safety of epoetin zeta in dialysis patients. Eur J Hosp Pharm Sci Pract. 2013;20:A91. | |
Singh A, Saczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. New Engl J Med. 2006;355(20):2085–2098. | |
Drüeke TB, Locatelli F, Clyne N, et al; for the CREATE investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355(20):2071–2084. | |
Pfeffer MA, Burdmann EA, Chen C, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361(21):2019–2032. | |
Zockali C, Abramowicz D, Cannata-Andia JB, et al. European best practice quo vadis? From European best practice guidelines (EBPG) to European renal best practice (ERBP). Nephrol Dial Transplant. 2008;23:2162–2166. | |
Pollock C, Johnson DW, Hörl WH, et al. Pure red cell aplasia induced by erythropoiesis-stimulating agents. Clin J Am Soc Nephrol. 2008;3(1):193–199. | |
Perani L, Scolari C, Braus A, Galli E. GRP-179 switch from CERA to EPO zeta in patients with anaemia and chronic kidney disease. Eur J Hos Pharm Sci Pract. 2013;20:A64. | |
Wiecek A, Ahmed I, Scigalla P, Koytchev R. Switching epoetin alfa and epoetin zeta in patients with renal anemia on dialysis: Posthoc analysis. Adv Ther. 2010;27(12):941–952. | |
Abraham I, MacDonald K. Clinical safety of biosimilar recombinant human erythropoietins. Expert Opin Drug Saf. 2012;11(5):819–840. | |
Retacrit: epetin zeta. Product information: Annex I. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000872/human_med_001031.jsp&mid=WC0b01ac058001d124. Accessed March 4, 2014. | |
Lee JS, Ha TK, Lee SJ, Lee GM. Current state and perspectives on erythropoietin production. Appl Microbiol Biotechnol. 2012;95(6):1405–1416. | |
Lee JF, Litten JB, Grampp G. Comparability and biosimilarity: Considerations for the healthcare provider. Curr Med Res Opin. 2012;28(6):1053–1058. | |
Salem L, Harvie B. Biosimilar medicines and their use: The nurse’s role and responsibility. Ren Soc Aust J. 2010;6(2):76–80. | |
Goldsmith D, Gesualdo L. Biosimilar epoetins in nephrology – where are we now. Eur Nephrol. 2012;6:21–24. | |
Simoens S, Verbeken G, Huys I. Biosimilars and market access: A question of comparability and costs? Targeted Oncol. 2012;7(4):227–231. | |
Goldsmith D. 2009: A requiem for rHuEPOs – but should we nail down the coffin in 2010? Clin J Am Soc Nephrol. 2010;5(5):929–935. | |
Lopez MJ, Antonino G, Vicente I, Mejia L, Garcia P, Sanchez A. Erythropoietic factors in renal chronic disease: Economic management. Int J Clin Pharm. 2012;34(1):221–222. | |
Pearson IV, Johnson KI. A cost minimization analysis of epoetin zeta for the treatment of anemia associated with chronic kidney disease. Value Health. 2008;11(3):A304. | |
Simoens S. Biosimilar medicines and cost-effectiveness. Clinicoecon Outcomes Res. 2011;3:29–36. | |
Tzekova V, Mihaylov G, Elezovic I, Koytchev R; Epoetin Zeta Oncology Study Group. Therapeutic effects of epoetin zeta in the treatment of chemotherapy-induced anaemia. Curr Med Res Opin. Jul 2009;25(7):1689–1697. | |
Soni RK, Weisbord SD, Unruh ML. Health-related quality of life outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19(2):153–159. | |
Lefebvre P, Vekeman F, Sarokhan B, Enny C, Provenzano R, Cremieux PY. Relationship between hemoglobin level and quality of life in anemic patients with chronic kidney disease receiving epoetin alfa. Curr Med Res Opin. 2006;22(10):1929–1937. | |
Keown PA, Churchill DN, Poulin-Costello M, et al. Dialysis patients treated with epoetin alfa show improved anemia symptoms; A new analysis of the Canadian Erythropoietin Study Group trial. Hemodial Int. 2010;14(2):168–173. | |
Jones M, Ibels L, Schenkel B, Zagari M. Impact of epoetin alfa on clinical end points in patients with chronic renal failure: a meta-analysis. Kidney Int. 2004;65(3):757–767. | |
Kimel M, Leidy NK, Mannix S, Dixon J. Does epoetin alfa improve health-related quality of life in chronically ill patients with anemia? Summary of trials of cancer, HIV/AIDS, and chronic kidney disease. Value Health. 2008;11(1):57–75. | |
Leaf DE, Goldfarb DS. Interpretation and review of health-related quality of life data in CKD patients receiving treatment for anemia. Kidney Int. 20089;75(1):15–24. | |
All Wales Medicines Strategy Group [homepage on the Internet]. Epoetin zeta (Retacrit®):Reference No. 130, final appraisal recommendation. Available from: http://www.awmsg.org/awmsgonline/app/appraisalinfo/130. Accessed March 6, 2014. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

