Back to Journals » Biologics: Targets and Therapy » Volume 11
Epigenetic memory of oxidative stress: does nephrilin exert its protective effects via Rac1?
Authors Mascarenhas DD, Herndon DN, Arany I
Received 5 March 2017
Accepted for publication 9 May 2017
Published 17 July 2017 Volume 2017:11 Pages 97—106
DOI https://doi.org/10.2147/BTT.S136188
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Doris Benbrook
Desmond D Mascarenhas,1,2 David N Herndon,3 Istvan Arany4
1Mayflower Organization for Research & Education, Sunnyvale, CA, 2Transporin, Inc., Sunnyvale, CA, 3Department of Surgery, The University of Texas Medical Branch, and Shriners Hospitals for Children, Galveston, TX, 4Department of Pediatrics, Division of Pediatric Nephrology, University of Mississippi Medical Center, Jackson, MS, USA
Aim: Nephrilin peptide, a designed inhibitor of Rictor complex (mTORC2), exerts pleiotropic protective effects in metabolic, xenobiotic and traumatic stress models. Stress can generate enduring epigenetic changes in gene function. In this work we examine the possibility that nephrilin treatment protects against acute and enduring global changes in oxidative metabolism, with a focus on the Rictor-complex-mediated activation of Rac1, a subunit of NADPH oxidase (Nox) via PKCs, Prex1 and p66shc.
Methods: Given the wide range of animal models in which nephrilin peptide has previously demonstrated effectiveness in vivo, we chose three different experimental systems for this investigation: dermal fibroblasts, renal proximal tubule epithelial cells (PTECs), and kidney tissue and urine from an animal model of burn trauma in which nephrilin was previously shown to prevent loss of kidney function.
Results: (1) Nephrilin protects dermal fibroblasts from loss of viability and collagen synthesis after ultraviolet A (UV-A) or H2O2 insult. (2) Nephrilin reduces reactive oxygen species (ROS) formation by H2O2–treated (PTECs) with or without nicotine pretreatment. Using RNA arrays and pathway analysis we demonstrate that nicotine and H2O2-treated PTECs specifically induced Rac1 gene networks in these cells. (3) Using kidney tissue and urine from the burn trauma model we demonstrate significant elevations of [a] 8-aminoprostane in urine; [b] kidney tissue histone modification and DNA methylation; and [c] post-transcriptional phosphorylation events consistent with Rac1 activation in kidney tissue.
Conclusion: Nephrilin protects against oxidative stress, possibly by modulating the activation of Rac1.
Keywords: nephrilin, epigenetic, Rac1, burn injury, Rictor, 8-isoprostane
Introduction
Organisms respond to stress with enduring changes in metabolism, some of which can be epigenetically transmitted to offspring.1,2 It is thought that such persistent changes may involve DNA and histone modification.3,4
Nephrilin is a 40-mer peptide designed as an inhibitor of Rictor complex (also known as mammalian target of rapamycin complex 2, mTORC2). The protective effects of nephrilin have been documented in animal models of metabolic, xenobiotic and traumatic stress.5-8 In previous studies we showed that nephrilin exerts pleiotropic protective effects in a model of severe burn injury, modulating persistent changes in inflammation and sepsis, loss of lean body mass, glycemic control, kidney function and wound healing.7,8 The mechanism(s) by which nephrilin peptide exerts its protective effects have not been fully elucidated. Because of the remarkable range of efficacy of nephrilin in many disease models we decided to investigate whether oxidative stress might play a role in the action of nephrilin. We chose to investigate this question in three distinct experimental systems, two in vitro and one in vivo, as individual test systems may exhibit idiosyncrasies.
The pathways by which cells respond epigenetically to stress insult are not well understood. In this work we investigate the hypothesis that nephrilin exerts its pleiotropic protective effects by modulating epigenetic and post-transcriptional changes that conspire to stimulate oxidative stress.
Rho-family GTPases are activated by a variety of extracellular signals. Some well described targets include RhoA (Ras homolog gene family, member A), Cdc42 (cell division control protein 42), and Rac1 (Ras-related C3 botulinum toxin substrate 1). Rac1, an obligate subunit of activated NADPH oxidase, plays a central role in oxidative metabolism in skin and other tissues. Some substrates activated by Rictor complex, such as Prex1/2 and PKC-alpha, may play a role in Rac1 and ERK activation.
Reactive oxygen species (ROS) play an important role in skin cells. UV-A radiation, in particular, is thought to stimulate oxidative damage.9,10 Nicotine pre-exposure, especially in combination with hydrogen peroxide, enhances oxidative stress in renal proximal tubule cells, a finding that may be relevant to the clinical observation that smokers experience poorer outcomes in surgery and transplant settings. Activation of the key adaptor protein p66shc has been shown to play a role in this process.16 p66shc, which is activated by PKC-beta-2 (a substrate of Rictor complex) is known to activate Rac1.12,13
In this work we show the protective effects of nephrilin on UV-A stress in dermal fibroblasts, and on H2O2–triggered oxidative stress in both dermal fibroblasts and nicotine-pre-exposed renal proximal tubule cells. We further investigate epigenetic and phosphorylation events in kidney tissues derived from a well-established rat scald model in which we previously demonstrated the protective effect of nephrilin on the pleotropic effects of burn injury, including loss of kidney function.8,14 In this work we analyze frozen kidney tissue from these earlier studies to show that the action of nephrilin in vivo is consistent with its protective effects in vitro.
Materials and Methods
Reagents: Nephrilin peptide, a 40-mer peptide carrying a sequence derived from PRR5/Protor (the sequence is conserved in human, rat and mouse species) was synthesized by LifeTein Inc (Hillsborough, NJ, USA) and purified to >80% purity by HPLC. The design and characterization of nephrilin have been previously described.5 The sequence of nephrilin peptide and the scrambled control sequence peptide are shown below:
Nephrilin:Ac-RGVTEDYLRLETLVQKVVSKGFYKKKQCRPSKGRKRGFCW-amide
Scrambled:Ac-RDLEGYRVLTETLVQKVVSKGFYKKKQSRPSKGRKRGFSW-amide
BCA Protein Kit was from Pierce (Rockford, IL, USA). Antibodies for ELISAs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) except for anti-phospho-p66shc-S36 and anti-collagen-I antibodies (Abcam, Cambridge, MA, USA). CelLytic M cell lysis reagent was obtained from Sigma (St. Louis, MO, USA). Urinary 8-isoprostane ELISA assay kit was from Cayman Chemical (Ann Arbor, MI, USA).
Institutional Review Board Approval: All experiments reported in this study received formal approval from the Mayflower Organization for Research & Education Ethics/Institutional Review Board.
Cell Lines and Treatment: All assays were done in triplicate. Human HS27 skin fibroblasts were purchased from ATCC (Manassas, VA, USA) and grown at 37°C in DMEM+10% FBS and penicillin/streptomycin to log phase, then seeded in 96-well flat-bottom plates at 8,000 cells/well. After 24 hr incubation cells were treated with 10 uM H2O2 in PBS for 2 hrs, or UV irradiation as described.15 After insult, the cells were washed and fresh cell culture medium was added along with 20 ug/ml peptide, or buffer control. Cells were incubated for 5 days in a 37°C humidified 5% CO2 incubator, supernatants were harvested for ELISA and cells were tested for viability (sulforhodamine B, SRB assay). The protocol from the supplier (GeneCopoeia, Inc., Rockville, MD, USA) was followed. 50 uL/well of cold fixation solution was added and the cells were incubated at 4 °C for 1 hr. Cells were washed 5 times with distilled water and stained with 50 uL/well SRB stain at RT for 30 min, then washed four times to remove unbound dye. 50 uL solubilization buffer was added per well (5 min, agitation) and the plate was read at 560 nm using a Tecan M200 Plate Reader (Tecan Trading AG, Switzerland). PTECs (NRK52E) were purchased from ATCC and maintained in DMEM supplemented with 10% fetal bovine serum (Life Technologies, Grand Island, NY, USA). Some cells were pre-treated with 200 uM nicotine overnight prior to treatment with either 10 mg/ml nephrilin or scrambled peptide followed by H2O2.
Determination of intracellular ROS production. For determination of ROS production cells were grown in T25 flasks and pre-treated with 200 uM nicotine as needed. After trypsinization, cells were counted and loaded with the oxidant-sensitive 2’,7’-dichlorofluorescein-diacetate (100 mM, DCFDA; Life Technologies) as described elsewhere.11 After 30 mins of incubation at 37oC the dye was washed away with fresh HBSS and cells were placed in wells of a 96-well-plate (0.2x106 cell/well) and treated with 400 uM H2O2. ROS production was determined by recording increase in fluorescence at 485nmexc/530nmem in 30-minute-intervals for up to 120 minutes in a plate reader (Fluorocount, Packard). ROS production was calculated as changes in fluorescence/30 minutes/0.2x106 cells and expressed as percentage of the corresponding untreated values.
Kidney tissue: Frozen rat left kidney tissue and rat urine were gifts from Dr. Celeste Finnerty at UTMB. The experiments from which these tissues were collected have been described in published reports.7,8 The rat scald burn model is a modified Walker-Mason model that induces inflammation and hypermetabolism in line with what severely burned patients experience.14 In the previously reported experiments, scalded animals showed significant reductions in eGFR that were reversed by nephrilin treatment. Group size for animal treatment groups was n=8 for saline-treated burn control (B) group and n=9 for nephrilin-treated burn group (N1, animals treated daily for 1 week post-burn). In addition, a sham control group (S) had four animals.
Cell extracts for ELISAs: Preparation of kidney tissue cell extracts for ELISAs was performed as previously described.6 Specific immunoreactivity was measured by ELISA according to the kit manufacturer’s recommendations.
RNA extraction: 30-50 mg of tissue was homogenized in TRIzol® (Invitrogen, Carlsbad CA, USA) and RNA extracted according to the manufacturer’s protocol. Linear Acrylamide (AMRESCO, Solon OH, USA) was added as a co-precipitant at a final concentration of 25μg/mL. Concentration and purity of the RNA was determined using the NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
RNA microarray analysis. Total RNA was extracted from approximate 10e7 PTECs cultured either in the presence or absence of H2O2, or with both Nic+ H2O2. RNA samples were prepared for microarray analysis following Agilent’s one-color microarray-based gene expression analysis Low Input Quick Amp Labeling v6.0. Briefly, an input of 100ng of high quality total RNA (RIN values from 9.8 to 10) was amplified to generate Cyanine-3 labeled cRNA. An amount of 1.65μg of labeled cRNA was hybridized on Agilent Rat Gene Expression Microarrays 4x44K (AMDID 014879). Microarrays were scanned with the Agilent DNA Microarray Scanner at a 3um scan resolution. Data was processed with Agilent Feature Extraction 11.0.1.1 and quantile normalized with Agilent GeneSpring 12.0.
Quantitative PCR: Quantitative polymerase chain reaction (rt-qPCR) for gene transcripts were performed using RNA extracted from kidney tissue and expressed relative to transcripts of GAPDH, a housekeeping gene. The cDNA synthesis reaction was carried out using 1,000 nanograms of RNA in a final volume of 20 uL following manufacturer’s instructions (High –Capacity cDNA Reverse Transcription kit, Applied Biosystems, Foster City, CA, USA). qPCR was carried out with the Fast Real Time 7500 (Applied Biosystems). The final reaction volume was 20 ul and contained: each primer at a final concentration of 200 nM, Power Sybr Green (Applied Biosystems) 1X and 2 ul of template (dilution 1:4 of cDNA synthesis reaction). Samples and standards were run in triplicate. Primer sequences for detecting gene transcripts and control primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are proprietary. The thermo cycle conditions were as follows: one cycle at 50°C for 20 sec, one activation cycle at 95°C for 10 minutes, followed by 40 cycles of 15 seconds at 95°C, 45 seconds at 60°C. Melting curve analysis was carried out using the continuous method from the 7500 Software (Applied Biosystems) conducted at 60°C, with increments of 1°C for 15 seconds. Data analysis was carried out with 7500 Software (Applied Biosystems). The auto threshold and baseline options were used for the calculations of Ct values per well. The linear equation for the standard curve (i.e., for preparations containing known quantities of DNA) was then used to interpolate the numbers of copies present in unknown samples.
Statistical analysis: Probability values (p values) were computed using Student’s t-test and expressed relative to sham group or saline-treated group.
Results
Effect of nephrilin on UV-treated skin fibroblasts
UV treatment of skin fibroblasts is known to generate enhanced oxidative stress.9,10 Dermal fibroblasts were irradiated with UV-A or treated with H2O2 and cultured as described in Methods. Cell viability (SRB assay) and collagen-I immunoreactivity in supernatants (ELISA) were measured. As shown in Figure 1, both UV-A and H2O2 treatments resulted in significantly reduced cell viability and collagen synthesis in fibroblasts. Co-treatment with 20 ug/ml nephrilin reversed these deficits in all cases. In the non-irradiated control condition too, nephrilin treatment improved cell viability and collagen synthesis in fibroblasts.
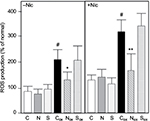
Effect of nephrilin on renal PTECs treated with hydrogen peroxide
Treatment of cultured rat PTECs with H2O2 has been shown to generate p66shc-mediated ROS and oxidative stress. Pre-treatment with nicotine enhances these effects.16 We treated PTECs with H2O2 with or without nicotine pretreatment (Nic) as described in Methods. The results shown in Figure 2 show that H2O2 treatment significantly enhances cellular ROS in both cases. Co-treatment with 10 ug/ml nephrilin (but not a scrambled peptide control) at the time of H2O2 addition significantly reverses ROS generation.
RNA microarray analysis of genes induced by oxidative stress in PTECs.
Total RNA was extracted from PTECs cultured either in the presence or absence of H2O2, or with both Nic+H2O2. These RNAs were analyzed on Agilent Rat Gene Expression Microarrays 4x44K (AMDID 014879) as described in Methods. Of the 41,105 genes interrogated we identified 49 upregulated (>3-fold threshhold) genes of known function, for which treatment with H2O2 alone was >30% over control, and treatment with the Nic+ H2O2 combination elevated expression an additional >30%. These genes are listed in Table 1. Of these, 29% are associated with Wnt and ERK signaling and 21% are associated with GTP-mediated signaling. Ingenuity Pathway software analysis (Palo Alto, CA, USA) using a 3057 gene set in which changes of expression of any magnitude were reported, identified a Rac1/PKC/Prex1 cluster as the network generating a high score (=32) using their proprietary algorithms (Figure 3). These results, taken together, point to the possible involvement of Prex1, PKCs (both are known to be activated by Rictor complex) in Rac1-mediated effects on oxidative metabolism. Prex1 and (indirectly through p66shc) PKC-beta-2 are both known to activate Rac1.
  | Figure 3. Pathway analysis using Ingenuity Systems software. Major network (score=32) is shown. Dataset was 3057 genes exhibiting differential expression in the Agilent RNA arrays (see text). |
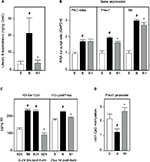
Analysis of phosphorylation events in kidney tissue from animals treated with nephrilin in vivo
Frozen left kidney tissue slices from burned animals treated with saline or 4mg/kg/day nephrilin were analyzed by ELISA. These animals showed significantly reduced kidney function (eGFR) as a result of injury.8 The results of the ELISAs are shown in Figure 4. As suggested by the RNA array analysis, several key signaling molecules involved in the oxidative stress response were dramatically up-regulated by burn injury. These included the key molecules Rac1 and p66shc activated by phosphorylation at Ser71 and Ser36 respectively. In addition, PKC-beta (Ser660) and PKC-alpha (Ser657) were phosphorylated at cognate positions. Total PKC was also elevated. PKC-beta is responsible for activation of p66shc and PKC alpha is a key participant in ERK/Wnt pathway signaling. Nephrilin treatment significantly reversed all of these elevations in phosphorylation but not in total PKC-beta.
Impact of nephrilin treatment on whole body oxidative stress
Urine collected from animal donors of kidney tissue was analyzed for 8-isoprostane, a measure of lipid peroxidation. The result is shown in Figure 5a. A dramatic elevation in 8-isoprostane in burned animals is significantly reversed by treatment with nephrilin.
Expression of PKC-beta, Prex1 and Bik genes in kidney tissue
Two of the top five up-regulated genes in the PTEC RNA microarray experiment (Table 1) are known participants in the oxidative stress response: Prex1 is a key GEF for Rac 1/2 — a subunit of NADPH oxidase — and Bik is a BH3-only pro-apoptotic molecule induced in oxidative (but not ER-) stress.18-21 As noted above, PKC-beta is a key molecule in the activation of p66shc. We examined the expression of these three genes in whole kidney tissue slices by qPCR (Figure 5b). The expression of all three genes is dramatically elevated in burn injury. Nephrilin treatment causes minor though significant reductions in Prex1 and Bik expression, but no impact on the level of PKC-beta transcripts. The latter result is consistent with the observed immunoreactivity of total PKC-beta in kidney tissue (Figure 4).
Global changes in histone 3 phosphorylation and acetylation
In human populations severe burn stress produces a range of enduring dysfunctions that outlive the healing of the external burn wound by months and even years. Phosphorylation of Ser10 in the tail of Histone 3 is a well-documented early response to a variety of stress stimuli.22 It is believed to be a gating event for more lasting changes in acetylation of this histone, notably at residues Lys9/Lys14.23 We examined these histone 3 modifications by ELISA of protein extracts from kidney slices (Figure 5c). In the 24 hours immediately following burn insult, Ser10 phosphorylation is elevated (this elevation having occurred by 6 hours post-burn). Treatment of animals with nephrilin reverses this elevation in H3-Ser10 phosphorylation. At Day 14 post-burn, enduring elevations in acetylation of histone 3 at residues Ly9/Lys14 are observed in saline-treated burned animals but not in the animals treated with nephrilin. This results suggests that enduring changes post-burn could be mediated, at least in part, by global changes in histone acetylation.
Methylation changes in Prex1 promoter region
We examined CpG islands in the region of DNA immediately upstream of the Prex1 gene. We focused on cytosine methylation in a CpG dinucleotide located at -601 (antisense strand) relative to the initiation codon. Figure 5d shows that methylation at this residue is dramatically reduced in burned animals, consistent with derepression of Prex1 gene expression. This reduction in methylation does not occur in animals treated with nephrilin.
Discussion
In this study we showed that nephrilin peptide specifically reverses the detrimental effects of oxidative stress in (a) cultured fibroblasts (b) cultured PTECs and (c) tissues of rats that had experienced burn injury.
Nephrilin reverses the pronounced elevation of urinary 8-isoprostane in burned animals. Elevation of this marker in human subjects has been linked to increased NADPH oxidase activity in tissues.24,25 This result supports our hypothesis that nephrilin works by reversing the effects of oxidative stress.
Deficits in cell viability and collagen I synthesis in fibroblasts caused by UV-A or H2O2 insult are reversed by 20 ug/ml nephrilin. These insults are believed to increase oxidative stress in these cells and the reversal of their effects by nephrilin supports our hypothesis that nephrilin works by reversing the effects of oxidative stress.9,10 Protective effects are even seen in ‘unstressed’ controls, perhaps indicating the fact that all cultured cells are ‘stressed’ to some degree.
These results obtained with fibroblast cultures are also intriguing in the context of skin aging, which is believed to be at least partly driven by oxidative stress triggered by sun exposure.10 The addition of nephrilin peptide to skin via cosmetic formulations is a possible area of future inquiry, though unaided penetration of the skin barrier by peptides is generally poor.
It was previously shown that long-term treatment with NIC exacerbates renal ischemia/reperfusion-dependent oxidative stress in vivo and oxidant (H2O2)-dependent ROS production in vitro.16,17 Here, we show that 10 µg/ml nephrilin peptide but not a control (scrambled sequence) peptide protects PTECs by reducing nicotine-dependent ROS generation. Nephrilin peptide reverses the ROS-generating effects of H2O2 on renal PTECs with or without pre-exposure to nicotine. This result also supports our hypothesis that nephrilin works by reversing the effects of oxidative stress. Differential analysis of RNA array data from treated PTECs (± H2O2; ± Nic;) further provided a gene list that supported the possible role of Rac1 and ROS generation.
Using rat kidney tissues previously harvested in a well-characterized burn model we were able to show burn stress-related (a) global elevations in histone-3 phosphorylation (Ser10) and acetylation (Ser9/14); (b) elevation of PKC-beta, Prex1 and Bik gene transcripts; (c) specific methylation decreases in the promoter region of Prex1; and (d) elevated phosphorylation of Rac1-Ser71, PKC-beta-Ser660, total PKC-beta, PKC-alpha-Ser657 and p66shc-Ser36. It is notable that the elevation of Bik was previously shown to correlate with oxidative stress.20,21 These results are consistent with and supportive of our main hypothesis.
In all cases except total PKC-beta and PKC-alpha protein and transcripts, treatment of burned animals with nephrilin reversed the effects of burn stress. Taken together with the array data, these results seem to implicate Rac1 activation in nephrilin’s mechanism of action via Rictor complex. Additional studies will be needed to prove this point conclusively.
Rictor complex is implicated in the activation of Prex1, PKCs alpha and beta-2 and — indirectly through PKC-beta-2 — p66shc.12,18,26,27 Our results, taken together, suggest at least two pathways (PKC-beta and Prex1) through which Rictor complex might control the activation of Rac1/2, and possibly a third via activated PKC-alpha and ERK.26 One may further speculate that Rictor (mTORC2) complex acts as a central regulator of oxidative metabolism in mammalian cells in response to stress and may even influence apoptosis through effectors like Bik. The documented pleiotropic protective effects of nephrilin peptide in numerous and diverse rodent models of metabolic, xenobiotic and traumatic stress.5-8 could thus be explained if the Rictor-controlled generation of oxidative stress via Rac1 and NADPH oxidase in these disease models amplifies the disease state (for example, by inducing elevated levels of ROS-triggered inflammation stress or Bik-triggered apoptosis).
Additionally, the effect of nephrilin treatment on the enduring effects of burn stress could be related to stress-triggered changes in epigenetic programs of histone modification and DNA methylation. As we show in this study, nephrilin treatment of animals reverses significant elevations in histone phosphorylation and acetylation as well as CpG methylation changes in the Prex1 promoter region. This observation, combined with our data showing substantial elevations of transcription for PKC-beta and Prex1 as well as total immunoreactive PKCs, and bearing in mind the role of these proteins in Rac1 activation leads us to speculate that perhaps the initial traumatic stress event generates persistent changes via epigenetic programs that affect gene expression levels and protein pools. Interestingly, methylation of Prex1 and PKC genes have previously been shown to affect gene transcription by others.28,29 Such transcriptionally-derived changes in ‘set point’ by increasing the steady state pool of protein could produce exaggerated responses to subsequent stressful stimuli when that pool of protein is post-translationally activated by phosphorylation, thereby setting the stage for a chronic feed-forward state. As Rictor complex regulates the activation of PKC-alpha, PKC-beta, p66shc and Prex1, inactivation of Rictor complex with its designed inhibitor nephrilin would (in theory) allow cells to exit this chronic feed-forward state, despite the presence of elevated PKC and Prex1 substrate pools. This, in turn, might allow a gradual return to the epigenetic baseline initially disrupted by the traumatic stress.
Further studies will be required to show the generality of nephrilin’s protective effects on tissues other than kidney. Of particular interest to us is the possible effect of this agent on protecting the CNS, a known target of stress-related damage that can persist for a lifetime e.g. in PTSD and even, possibly, several lifetimes.1-3
Acknowledgements
We thank Dr. Celeste Finnerty and Dr. Amina ElAyadi for their generous gift of frozen rat tissues used in this study. This work was supported in part by the Department of Pediatrics at the University of Mississippi Medical Center and the Bower Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
Exposome review: Bale TL. Lifetime stress experience: transgenerational epigenetics and germ cell programming. Dialogues Clin Neurosci. 2014 Sep;16(3):297–305. | ||
Zucchi FC, Yao Y, Metz GA. The secret language of destiny: stress imprinting and transgenerational origins of disease. Front Genet. 2012 Jun 4;3:96. | ||
Bowers ME, Yehuda R. Intergenerational Transmission of Stress in Humans. Neuropsychopharmacology. 2016 Jan;41(1):232–244. | ||
Yehuda R, Daskalakis NP, Bierer LM, Bader HN, Klengel T, Holsboer F, Binder EB. Holocaust Exposure Induced Intergenerational Effects on FKBP5 Methylation. Biol Psychiatry. 2016 Sep 1;80(5):372–380. | ||
Singh BK, Singh A, Mascarenhas DD. A nuclear complex of rictor and insulin receptor substrate-2 is associated with albuminuria in diabetic mice. Metab Syndr Relat Disord. 2010 Aug; 8(4): 355–363. | ||
Mascarenhas D, Routt S, Singh BK. Mammalian target of rapamycin complex 2 regulates inflammatory response to stress. Inflamm Res. 2012 Dec; 61(12): 1395–1404. | ||
Mascarenhas DD, El Ayadi A, Singh BK, Prasai A, Hegde SD, Herndon DN, Finnerty CC. Nephrilin peptide modulates a neuroimmune stress response in rodent models of burn trauma and sepsis. Int J Burns Trauma. 2013 Nov 1; 3(4): 190–200. | ||
Mascarenhas DD, Ayadi AE, Wetzel M, Prasai A, Mifflin R, Jay J, Herndon DN, Finnerty CC. Effects of the nephrilin peptide on post-burn glycemic control, renal function, fat and lean body mass, and wound healing. Int J Burns Trauma. 2016 Nov 30;6(3):44–50. | ||
Parat MO, Richard MJ, Leccia MT, Amblard P, Favier A, Beani JC. Does manganese protect cultured human skin fibroblasts against oxidative injury by UVA, dithranol and hydrogen peroxide? Free Radic Res. 1995 Oct;23(4):339–351. | ||
Seité S, Colige A, Piquemal-Vivenot P, Montastier C, Fourtanier A, Lapière C, Nusgens B. A full-UV spectrum absorbing daily use cream protects human skin against biological changes occurring in photoaging. Photodermatol Photoimmunol Photomed. 2000 Aug;16(4):147–155. | ||
Arany I, Faisal A, Clark JS, Vera T, Baliga R, Nagamine Y. p66SHC-mediated mitochondrial dysfunction in renal proximal tubule cells during oxidative injury. Am J Physiol Renal Physiol. 2010 May;298(5):F1214–21. | ||
Pinton P, Rizzuto R. p66Shc, oxidative stress and aging: importing a lifespan determinant into mitochondria. Cell Cycle. 2008 Feb 1;7(3):304–308. | ||
De Marchi E, Baldassari F, Bononi A, Wieckowski MR, Pinton P. Oxidative stress in cardiovascular diseases and obesity: role of p66Shc and protein kinase C. Oxid Med Cell Longev. 2013;2013:564961. | ||
Herndon DN, Wilmore DW, Mason AD Jr. Development and analysis of a small animal model simulating the human postburn hypermetabolic response. J Surg Res. 1978 Nov;25(5):394–403. | ||
Song J, Liu P, Yang Z, Li L, Su H, Lu N, Peng Z. MiR-155 Negatively Regulates c-Jun Expression at the Post-transcriptional Level in Human Dermal Fibroblasts in vitro: Implications in UVA Irradiation-induced Photoaging. Cell Physiol Biochem. 2012;29(3-4):331–340. | ||
Arany I, Clark J, Reed DK, Juncos LA. Chronic nicotine exposure augments renal oxidative stress and injury through transcriptional activation of p66shc. Nephrol Dial Transplant. 2013 Jun;28(6):1417–1425. | ||
Arany I, Hall S, Reed DK, Dixit M. The pro-oxidant gene p66shc increases nicotine exposure-induced lipotoxic oxidative stress in renal proximal tubule cells. Mol Med Rep. 2016 Sep;14(3):2771–2777. | ||
Hernández-Negrete I, Carretero-Ortega J, Rosenfeldt H, Hernández-García R, Calderón-Salinas JV, Reyes-Cruz G, Gutkind JS, Vázquez-Prado J. P-Rex1 links mammalian target of rapamycin signaling to Rac activation and cell migration. J Biol Chem. 2007 Aug 10;282(32):23708–23715. | ||
Welch HC. Regulation and function of P-Rex family Rac-GEFs. Small GTPases. 2015;6(2):49–70. | ||
Trejo-Vargas A, Hernández-Mercado E, Ordóñez-Razo RM, Lazzarini R, Arenas-Aranda DJ, Gutiérrez-Ruiz MC, Königsberg M, Luna-López A. Bik subcellular localization in response to oxidative stress induced by chemotherapy, in two different breast cancer cell lines and a non-tumorigenic epithelial cell line. J Appl Toxicol. 2015 Nov;35(11):1262–1270. | ||
Bodet L, Ménoret E, Descamps G, Pellat-Deceunynck C, Bataille R, Le Gouill S, Moreau P, Amiot M, Gomez-Bougie P. BH3-only protein Bik is involved in both apoptosis induction and sensitivity to oxidative stress in multiple myeloma. Br J Cancer. 2010 Dec 7;103(12):1808–1814. | ||
Huang W, Mishra V, Batra S, Dillon I, Mehta KD. Phorbol ester promotes histone H3-Ser10 phosphorylation at the LDL receptor promoter in a protein kinase C-dependent manner. J Lipid Res. 2004 Aug; 45(8):1519–1527. | ||
Sharma AK, Mansukh A, Varma A, Gadewal N, Gupta S. Molecular Modeling of Differentially Phosphorylated Serine 10 and Acetylated lysine 9/14 of Histone H3 Regulates their Interactions with 14-3-3ζ, MSK1, and MKP1. Bioinform Biol Insights. 2013 Aug 26;7:271–288. | ||
Ohashi N, Urushihara M, Kobori H. Activated intrarenal reactive oxygen species and renin angiotensin system in IgA nephropathy. Minerva Urol Nefrol. 2009 Mar;61(1):55–66. | ||
Del Ben M, Fabiani M, Loffredo L, Polimeni L, Carnevale R, Baratta F, Brunori M, Albanese F, Augelletti T, Violi F, Angelico F. Oxidative stress mediated arterial dysfunction in patients with obstructive sleep apnoea and the effect of continuous positive airway pressure treatment. BMC Pulm Med. 2012 Jul 23;12:36. | ||
Kumar A, Chambers TC, Cloud-Heflin BA, Mehta KD. Phorbol ester-induced low density lipoprotein receptor gene expression in HepG2 cells involves protein kinase C-mediated p42/44 MAP kinase activation. J Lipid Res. 1997 Nov;38(11):2240–2248. | ||
Kirmani D, Bhat HF, Bashir M, Zargar MA, Khanday FA. P66Shc-rac1 pathway-mediated ROS production and cell migration is downregulated by ascorbic acid. J Recept Signal Transduct Res. 2013 Apr; 33(2):107–113. | ||
Barrio-Real L, Benedetti LG, Engel N, Tu Y, Cho S, Sukumar S, Kazanietz MG. Subtype-specific overexpression of the Rac-GEF P-REX1 in breast cancer is associated with promoter hypomethylation. Breast Cancer Res. 2014 Sep 24;16(5):441. | ||
Hagiwara K, Ito H, Murate T, Miyata Y, Ohashi H, Nagai H. PROX1 overexpression inhibits protein kinase C beta II transcription through promoter DNA methylation. Genes Chromosomes Cancer. 2012 Nov;51(11):1024–1036. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.