Back to Journals » Drug Design, Development and Therapy » Volume 15
Epigallocatechin-3-Gallate Provides Protection Against Alzheimer’s Disease-Induced Learning and Memory Impairments in Rats
Authors Nan S, Wang P, Zhang Y, Fan J
Received 15 November 2020
Accepted for publication 26 February 2021
Published 13 May 2021 Volume 2021:15 Pages 2013—2024
DOI https://doi.org/10.2147/DDDT.S289473
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Shanji Nan, Peng Wang, Yizhi Zhang, Jia Fan
Department of Neurology, The Second Hospital of Jilin University, Changchun, 130041, People’s Republic of China
Correspondence: Jia Fan
Department of Neurology, The Second Hospital of Jilin University, No. 218, Ziqiang Street, Changchun, 130041, Jilin Province, People’s Republic of China
Tel +86-13843028656
Email [email protected]
Purpose: Recent evidence has highlighted the anti-inflammatory properties of the constituent of Green Tea Polyphenols (GTP), epigallocatechin-3-gallate (EGCG) which has been suggested to exert a neuroprotective effect on Alzheimer’s disease (AD). The current study aimed to elucidate the effect of EGCG on memory function in rats with AD.
Methods: AD rat models were initially established through an injection with Aβ 25– 35 solution, followed by gavage with EGCG at varying doses to determine the effect of EGCG on learning and cognitive deficits in AD. Morris water maze test was conducted to evaluate the spatial memory function of the rats. Immunohistochemistry and Western blot analysis were performed to identify Tau phosphorylation. The expression of β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) mRNA and protein in rat hippocampus was measured by reverse transcription quantitative polymerase chain reaction (RT-qPCR) and Western blot analysis. Acetylcholinesterase (AchE) activity, Aβ 1-42 expression and Ach content were all detected using enzyme-linked immunosorbent assay (ELISA).
Results: EGCG intervention brought about a decrease in the escape latency period while increasing the time at the target quadrant among the AD rats. EGCG decreased the hyperphosphorylation of Tau in hippocampus. BACE1 expression and activity as well as the expression of Aβ 1-42 were suppressed by EGCG. Moreover, EGCG promoted Ach content by diminishing the activity of AchE.
Conclusion: The current study demonstrates that EGCG may diminish the hyperphosphorylation of the Tau protein, downregulate BACE1 and Aβ 1-42 expression to improve the antioxidant system and learning and memory function of rats with AD.
Keywords: Aβ 1-42, ACh, AChE, BACE1, epigallocatechin-3-gallate, learning and memory function, tau hyperphosphorylation
Introduction
As the most common cause of dementia in the elderly, Alzheimer’ s disease (AD) is a devastating neurodegenerative disorder characterized by cognitive deficits, memory impairment, disorientation, and behavioral abnormalities.1 Age represents a chief risk factor for AD, with individuals aged 65 years or greater reported to be more susceptible to AD.2 At present, no existing therapy has displayed an ability to either cure or slow the progression of AD.3 Polyphenols phytochemicals have been reported to play a positive role in various pathologies.4 Polyphenolic compounds found in wine have been reported to influence the gut microbiota of AD patients.5 Multiple polyphenols, such as quercetin and gallocatechin could inhibit cholinesterase, serving as a potential treatment for AD.6 Meanwhile, notable components found in coffee have been shown to exert anti-diabetic and hepatoprotective functions.7 Green tea polyphenols (GTPs), the natural flavonoids in green tea, are composed of epigallocatechin-3-gallate (EGCG), epigallocatechin, epicatechin, epicatechin gallate, and catechin.8 Existing literature has provided evidence demonstrating that GTPs can improve okadaic acid-induced spatial learning and memory impairment in rats by down-regulating Tau phosphorylation.9 In vitro studies have also suggested that GTPs may serve as effective free-radical scavengers and antioxidants.10 Furthermore, previous reports have documented that EGCG can selectively enhance the degradation of phosphorylated Tau species in primary neurons, potentially by means of increasing the adaptor protein expression.11
It has been well established that tau and extracellular deposition of β-amyloid peptide (Aβ) represent crucial components central to the pathological features associated with AD.12,13 Proteolytic cleavage of amyloid precursor protein (APP) produces Aβ, which aggregates into amyloid plaques, one of the main hallmarks of AD.14 EGCG attenuates Aβ-triggered cognitive impairment in transgenic mice in AD.15 Additionally, EGCG has also been demonstrated to alleviate Aβ-induced cytotoxicity.16 As the main feature of AD, amyloid plaque is an extracellular aggregate, the formation of which has been strongly attributed to abnormally folded Aβ 40 and Aβ 42, and neurofibrillary tangles, which is another important feature of AD mainly composed of pairs of hyperphosphorylated Tau filaments.17 Oxidative stress has been strongly implicated in the etiology of AD through Aβ peptide and redox properties resulting in the production of reactive oxygen species (ROS).18 β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) represents a key enzyme associated with the formation of Aβ in the hippocampus of rats.19 A previous study provided evidence demonstrating exposure to Aβ induces tau phosphorylation in hippocampal neurons.20 Existing literature has revealed that acetylcholinesterase (AchE) is co-localized with hyperphosphorylated tau (P-tau) within neurofibrillary tangles in AD, and that high tau phosphorylation could result in increased AchE content during the initial phases of AD.21 AChE influences the function of acetylcholine-interceded neurotransmission during AD progression.22 Inhibition of electrophorus electricus AChE by phenserine has been speculated to attenuate AD in a dose-dependent fashion.23
Based on the aforementioned literature, we subsequently asserted the hypothesis that EGCG intervention might improve the learning and memory function of AD rats by reducing Tau hyperphosphorylation, attenuating the activity and expression of BACE1 and Aβ1-42, improving the antioxidant system and reducing AchE activity in the hippocampus. Thus, the current study was performed to elucidate the effect of EGCG on learning and memory function in AD using an AD rat model and treating them with different doses of EGCG.
Materials and Methods
Ethics Statement
The animal experimental protocol was conducted with the approval of the ethics committee of the Second Hospital of Jilin University (approval number: 201908012). All procedures in the animal experiments strictly followed the guidelines for the care and use of laboratory animals issued by the National Institutes of Health. Extensive efforts were made to the suffering and number of animals used in the current study.
Bioinformatics Analysis
The secondary structure of EGCG was predicted through the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) while its targets were identified using the SwissTarget Prediction database (http://www.swisstargetprediction.ch/). Meanwhile, AD-related genes were obtained via CTD database (http://ctdbase.org/detail.go?acc=C452899&type=chem). The intersection of the common targets shared between EGCG and AD was identified using the jvenn (http://jvenn.toulouse.inra.fr/app/example.html). The drug-target network was finally visualized by Cytoscape 3.5.1 software.
AD Model Establishment
A total of 75 healthy and clean adult male Sprague-Dawley (SD) rats (weighing 190–220 g, with an average weight of 204.29 ± 8.06 g) were purchased from Shanghai Sippe-Bk Lab Animal Co., Ltd. (Shanghai, China). The rats were housed at the animal experimental center under controlled conditions at 23.5 ± 0.5°C with a relative humidity 47%–48%, a 12-h light/dark cycle, and provided free access to food and water. After one week period of adaptive feeding, the rats were randomly arranged into five groups (n = 15 rats/group): sham, AD, and AD + EGCG in different doses of 100 mg/(kg•d) (low dose), 250 mg/(kg•d) (moderate dose) and 625 mg/(kg•d) (high dose). The 1 nmol/µL Aβ 25–35 solution (Sigma-Aldrich Chemical Company, St Louis, MO, USA) was prepared with sterilized normal saline, stored at 37°C for 7 days, and placed in a refrigerator at 4°C following incubation into an aggregated state. The AD model was established after adaptive feeding for one week (the rats fasted 12 hours before operation). The rats were anesthetized with 3.0% pentobarbital sodium (40 mg/kg) and subsequently fixed in a brain stereotaxic device (TSE Systems Company, Thuringia, Germany, 540060). As per the brain stereotaxic map, the position of CA1 area of bilateral hippocampus was accurately marked and drilled, after which 4 μL Aβ 25–35 solution was injected at a speed of 2 μL/min in a consecutive manner using a micro-injector into the AD modeled rats (the sham-operated rats were injected with the same amount of sterilized normal saline). After 3–5 minutes had elapsed, the needle was slowly removed, and the rats were sterilized and sutured.
Three days after successful AD model establishment, the rats underwent gavage with EGCG (named as Teavigo, EGCG ≥ 90%, DSM company, Geleen, Netherlands) in a continuous fashion based on their respective body weight.24 At the 8th week, the rats were subjected to Morris water maze testing. The rats were then euthanized with brain tissues separated in a swift manner and cleaned by normal saline. The hippocampus and cortex tissues were subsequently collected into a 1.5 mL centrifuge tube at −80°C for 30 minutes. The water maze test was conducted between 8:00 and 10:00 a.m.
Morris Water Maze Test
Morris water maze test was conducted in order to evaluate learning, memory processes as well as the spatial positioning ability of the rats. The Morris water maze DMS-2 system was developed and produced by the Institute of Materia Medica, Chinese Academy of Medical Sciences, comprised of a stainless steel circular pool with a diameter of 120 cm and a height of 50 cm. The inner pool wall was marked with red, indicative of four water entry points in all directions, while the circular pool was divided into four separate quadrants by two imaginary vertical lines passing through the center of the circle. A movable cylindrical hidden platform (diameter of 9 cm, height of 27 cm) was placed in the middle water area of the target quadrant, and its position remained unchanged during the whole experiment. In the event that the platform was lower than the water surface (more than 1 cm), 1 kg of skimmed milk powder was added into the water pool to eliminate other factors except the space positioning. The water temperature was maintained at a controlled temperature of 20 ± 1°C. The head of SD rats was dyed black using a hair dye in a bid to facilitate camera shooting. During the experiment, the reference around the water maze remained unchanged. A camera connected to the display system was arranged above the water maze to record the motion track in time. Morris water maze data acquisition and analysis software was used to record the relevant data and image results. 1. Positioning navigation experiment. On the 22nd day post gavage, the navigation experiment was performed, namely the hidden-platform acquisition training. Each rat was trained 4 times each day (each for 60 seconds) for 5 days. Initially, the rats were placed on the platform for 20 seconds, and then randomly placed into the water from the east, west, south, and north, respectively, facing the central point of the pool wall. The escape latency which was the time that required for the rats to climb the platform was recorded. In the event that rats were unable to locate the platform within the allotted 60 seconds, they would be placed on the platform for an additional 20 seconds and the escape latency was recorded as 60 seconds. 2. Probe trial testing: after 5 days of training, the platform was removed on the 6th day, and rats were placed in the water at any of the entry points, with the retention time of rats in the target quadrant recorded.
Immunohistochemistry
The brain tissues of rats were fixed, embedded in paraffin and sliced (4 μm). The slices were dewaxed routinely, hydrated, incubated with 3% methanol hydroperoxides at room temperature for 15 minutes followed by antigen retrieval with 0.01 mol/L citric acid for 15 minutes. The slices were subsequently incubated with the primary antibody Tau-pSer396 (phosphorylated Tau-pSer396 mouse monoclonal antibody, 1:100, Cell Signaling Technologies, Beverly, MA, USA) at 4°C overnight and with horseradish peroxidase (HRP)-labeled goat anti-rabbit immunoglobulin G (IgG) (1:500, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) at room temperature for 1 hour. Finally, freshly prepared 0.05% diaminobenzidine (DAB) solution (containing 0.01% H2O2, 3,3ʹ-diaminobenzidine, Sigma-Aldrich Chemical Company, St Louis MO, USA) was added to the slices for color development, followed by gradient ethanol dehydration, xylene clearing, neutral gum sealing, and observation and photographing under an optical microscope. Phosphate buffer saline (PBS) was employed as the negative control (NC) instead of the primary antibody. Tau staining was considered positive for protuberance or cytoplasm brownish yellow. Five slices were selected from each rat (n = 15/group) and 5 randomly selected fields were observed under high power microscope with 100 cells counted in each field. Positive cells were reflected by a degree of staining greater than 25%, accompanied by the presence of evident brown or brown particles in the nucleus or cytoplasm. Positive expression rate = (number of positive cells/total number of cells) × 100%.
Western Blot Analysis
The hippocampal tissues of the rats were extracted, followed by protein collection and quantification using bicinchoninic acid kit (Beyotime Biotechnology Co., Ltd., Shanghai, China). The samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane at a constant current of 0.8 mA/cm2 for 50 minutes. The membrane was then probed with primary goat anti-rat antibodies (Abcam Inc., Cambridge, UK) to Tau-Pser396 (1:1000), BACE1 (1:1000), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:1000) at 4°C for 24 hours. The membrane was subsequently re-probed with HRP-labeled goat anti-rabbit IgG secondary antibody (1:1000, Abcam Inc.) for 2 hours at room temperature. The membrane was added with enhanced chemiluminescence fluorescence test kit (Beyotime Biotechnology Co., Shanghai, China) for color development with the ratio of each sample to the internal reference calculated accordingly.
RNA Isolation and Quantitation
The total RNA of the rat hippocampus tissues was extracted by Trizol (Invitrogen, Car, Cal, USA). The RNA was reversely transcribed into complementary DNA (cDNA) as per the instructions provided by the PrimeScript RT reagent Kit (RR047A, Takara, Tokyo, Japan). Fast SYBR Green PCR kit (Applied biosystems) and ABI PRISM 7300 RT-PCR system (Applied biosystems) were adopted for reverse transcription quantitative polymerase chain reaction (RT-qPCR), with 3 replicates in each well. The relative expression of BACE1 gene was calculated using the 2−ΔΔCt method, which was standardized by GAPDH. The primer sequences that were synthesized by Shanghai Sangon Biotechnology Co. Ltd. (Shanghai, China) are depicted in Table S1.
Determination of Biochemical Indexes
Next, 10% brain homogenate of 1 g rat brain tissues was prepared by adding iced normal saline at a ratio of 1:10, followed by centrifugation at 4°C for 10 minutes at 3000 r/min. The activity of AchE and BACE1 in the supernatant was subsequently measured using basic hydroxylamine colorimetry, with the experiment performed in strict accordance with the instructions of the respective kits (AchE kit, A024, Nanjing Jiancheng Bioengineering Institute, Nanjing, China; BACE1 kit, ab267637, Abcam, Cambridge, UK). The Aβ1-42 content was determined using enzyme-linked immunosorbent assay (ELISA) in strict accordance with the instructions of the kit (rat β amyloid protein 1–42 ELISA kit, CSB-E10786r, CUSABIO, Wuhan, China).
After acetylcholine (Ach) had been measured, 10% brain homogenate of 1 g rat brain tissues was prepared with the addition of iced normal saline at a ratio of 1:10, and subsequently centrifuged at 4°C for 10 minutes at 3000 r/min. The supernatant was then removed, followed by the prompt addition of 0.25 mL of 8.1% sodium dodecyl sulfonate, 0.10 mL of 30% trichloroacetic acid and 1.00 mL of basic hydroxylamine. The solution was placed at room temperature for 15 minutes and added with 0.5 mL of 4N hydrochloric acid and 0.5 mL of 10% ferric chloride. The absorbance was detected by a spectrophotometer at a wavelength of 530 nm.
Total superoxide dismutase (T-SOD), glutathione peroxidase (GPx), methane dicarboxylic aldehyde (MDA) were then determined. More specifically, 1 g of the rat brain tissues was used to prepare 10% brain homogenate by adding ice normal saline at a ratio of 1:10, and centrifuged at 3000 r/min for 10 minutes. The supernatant was then removed and the content of each index was determined based on the provided kit instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Statistical Analysis
All statistical analyses were conducted using SPSS 22.0 statistical software (IBM SPSS Statistics, Chicago, IL, USA). Measurement data were expressed as the mean ± standard deviation. Data obeying normal distribution and homogeneity of variance among multiple groups were checked by one-way analysis of variance (ANOVA) with Tukey’s post hoc test. Data comparison among multiple groups at different time points was conducted using repeated measurement with Bonferroni’s post hoc test. p < 0.05 was considered to be indicative of statistically significant difference.
Results
EGCG Shortened the Escape Latency and Prolonged the Stay Time on Target Quadrant
A flowchart illustrating the experimental findings is depicted in Figure 1. In order to investigate the effect of EGCG in AD, we established an AD rat model via an injection of Aβ 25–35 solution, followed by gavage with EGCG in different doses of 100 mg/(kg•d), 250 mg/(kg•d) and 625 mg/(kg•d) after 3 days of successful AD modeling, while the sham-operated rats were regarded as the control group. The escape latency of rats was gradually shortened with an increase in training times (Figure 2A). The escape latency of the AD rats was elevated when compared to the sham-operated rats. Compared with the AD rats, the escape latency of the AD rats administered with EGCG was shorter. At the same total time, the retention time of sham-operated rats, AD rats, and AD rats treated with 100 mg/kg EGCG, 250 mg/kg EGCG, and 625 mg/kg EGCG in the target quadrant was 19.05 ± 2.28 s, 12.34 ± 2.17 s, 21.34 ± 1.52 s, 23.26 ± 3.16 s and 27.48 ± 2.15 s, respectively.
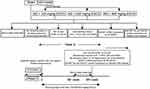 |
Figure 1 A schematic representation of the experiments. |
The one-way ANOVA results indicated that the retention time of AD rats notably decreased relative to the sham-operated rats. However, the retention time of AD rats administered with EGCG was notably higher than that of the AD rats in a dose-dependent manner (P < 0.01, Figure 2B). The percentages of swimming distance at the target quadrant of sham-operated rats, AD rats, and AD rats treated with 100 mg/kg EGCG, 250 mg/kg EGCG, and 625 mg/kg EGCG were 33.38%, 19.45%, 30.17%, 28.41% and 25.32%, respectively. Relative to the AD rats, the percentage of swimming distance at the target quadrant of AD rats with EGCG treatment increased in a dose-dependent manner (P < 0.01, Figure 2C). The aforementioned findings suggest that EGCG shortened the escape latency of the AD rats in addition to extending the retention time at the target quadrant, suggesting an improvement in the memory function of the AD rats.
EGCG Reduced Hyperphosphorylation of Tau and BACE1 Expression in Hippocampus of AD Rats
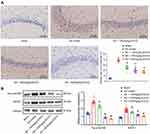
Tau-pSer396 is a Tau phosphorylation dependent antibody.25 The expression of phosphorylated Tau in hippocampal neuronal somata and processes was detected by immunohistochemistry. When compared to the sham-operated rats, the AD rats presented with an increase area of brownish signal. The brownish signal was diminished in the AD rats with EGCG intervention when compared to the AD rats, especially in the AD rats administered with 250 mg/kg and 625 mg/kg EGCG (Figure 3A), suggesting that EGCG reduced the Tau-pSer396 in AD rats in a dose-dependent manner.
As detected by Western blot analysis (Figure 3B), Tau-pSer396 and BACE1 protein expression in the AD rats was elevated relative to the sham-operated rats (p < 0.01), while Tau-pSer396 and BACE1 expression in the AD rats with EGCG intervention was remarkably lower than that in the AD rats in a dose-dependent manner. The results demonstrated that EGCG could improve the learning and memory function of AD rats by means of diminishing Tau phosphorylation as well as the expression of BACE1.
EGCG Reduced BACE1 Activity and Aβ1-42 Expression in the Hippocampus of AD Rats
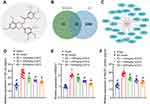
The secondary structure of EGCG was detected in the database PubChem (Figure 4A). A total of 44 targets of EGCG predicted by the SwissTarget database were intersected with 2317 AD-related genes identified by the database CTD (screening Inference Score ≥ 20), revealing 25 intersecting targets (Figure 4B). The drug-target regulatory network was obtained using the Cytoscape 3.5.1 software (Figure 4C). Among them, APP and BACE1 were predicted targets of EGCG.
Previous literature has highlighted the capacity of EGCG to alleviate Aβ-induced neurotoxicity in cultured hippocampal neurons in vitro,26 while the long-term administration of EGCG has been shown to decrease the APP level in mice.27 Compared with the sham-operated rats, Aβ1-42 expression in AD rats was significantly elevated. However, Aβ1-42 expression was significantly lowered in AD rats administered with EGCG than that in the AD rats in a dose-dependent manner (Figure 4D). BACE1 represents a key enzyme in the formation of Aβ in the hippocampus of rats.15 As exhibited in Figure 4E–F, BACE1 mRNA and activity was markedly elevated in the hippocampus of the AD rats when compared with sham-operated rats. In addition, BACE1 mRNA expression and activity in AD rats was notably diminished following treatment with EGCG, especially following treatment with a high dose of EGCG. Taken together, the aforementioned findings suggest that EGCG could improve the learning and memory function of rats by reducing BACE1 activity and Aβ1-42 expression.
EGCG Improved the Antioxidant System and Scavenged Free Radicals in AD Rats
The activities of GPx, and T-SOD and MDA content were subsequently determined. GPx and T-SOD activities were reduced while the MDA content was increased in the AD rats relative to that of the sham-operated rats. Compared with the AD rats, GPx and T-SOD activities were enhanced in the AD rats with EGCG treatment, especially in the AD rats treated with 250 mg/kg EGCG. In contrast, the MDA content was decreased following treatment with EGCG, with the most distinct reduction found in the AD rats treated with 625 mg/kg EGCG (Figure 5A–C). The results suggested that EGCG may scavenge free radicals through antioxidant system to improve the learning and memory function of rats.
EGCG Increased Ach Content Through Reducing AchE Activity
Ach is stored in the presynaptic membrane vesicles after its synthesis in cholinergic neurons. When the nerve impulse reaches the nerve endings, the vesicles and presynaptic membrane fuse to release Ach by means of exocytosis. The released Ach is subsequently hydrolyzed with the balancing effect of AchE. If AchE activity increases, the level of Ach in the synaptic space is reduced, and the transmission of synapses is affected, resulting in learning and memory and cognitive dysfunction.28 Compared with the sham-operated rats, AchE activity in AD rats was increased (Figure 6A), while the Ach content was significantly reduced (Figure 6B). However, when compared with the AD rats, the AchE activity in the AD rats treated with EGCG was markedly decreased (Figure 6A), while the Ach content was notably elevated (Figure 6B), in a dose-dependent fashion. These results indicated that EGCG may improve the learning and memory function of rats by reducing AchE activity and increasing Ach content.
Discussion
EGCG has been well documented to possess the ability to prevent and treat various neurodegenerative diseases by means of pharmacological activities and EGCG treatment could improve dendritic integrity, reduce inflammatory effects and Aβ plaques.29 The alleviating effects of EGCG on coordination and memory abilities have been previously emphasized in AD modeled rats induced by Aβ injection.30 Therefore, inhibition of Aβ deposition and regular neurotransmitter system arrangement represents a promising therapeutic approach for AD.31 However, the relationship and molecular mechanism between EGCG and AD are not well understood. Hence, the current study aimed to elucidate the mechanism by which EGCG influences learning and memory related behaviors of SD rats with AD. The results obtained suggested that EGCG improved the learning and memory function of AD model rats by reducing hyperphosphorylation of Tau protein, downregulating BACE1 activity and expression and Aβ1-42 expression, improving the antioxidant system, inhibiting AchE activity and increasing Ach content.
Our initial observations following intervention with EGCG indicated that the escape latency period was decreased while the time spent at the target quadrant was increased in the control rats when compared to the AD rats, a finding of which was consistent with the findings of Zhang et al.8 In addition, we also observed that EGCG may serve as a scavenger of free radicals through its anti-oxidative properties, resulting in the improvement of learning and memory function of rats. The major components of EGCG give the compound with an antioxidant, anti-inflammatory, anti-cancer, anti-diabetes, and neuroprotective effect.32 Both oral administration and i.p. of EGCG could attenuate cognitive impairment and i.p.-treated animals exhibit a more pronounced benefit in AD mouse models.15 Specifically, dual-drug loaded nanoparticles of EGCG and ascorbic acid have been reported to alleviate neuroinflammation, Aβ plaque burden, as well as enhance spatial learning and memory.33 Additionally, EGCG has been demonstrated to enhance insulin sensitivity and cognitive deficits in mouse model with high-fat diet.34 GTP scavenges oxygen free radicals, augments antioxidant potential, and decreases oxidative DNA damage, thereby repressing cognitive impairment that occurs secondary to chronic cerebral hypoperfusion.35 Similarly, Haque et al asserted that GTP can effectively prevent Aβ-induced cognitive impairment by upregulating antioxidant defense.36 Moreover, Xing et al suggested that EGCG promotes the activities of various antioxidant enzymes including SOD, CAT and GPx in BLM-treated rats, indicating that intervention with EGCG may aid in resisting oxidative stress by means of regulating enzymatic activity.10
On the other hand, our data revealed that EGCG diminished the hyperphosphorylation of Tau protein, BACE1 activity and expression, Aβ1-42 expression, and AchE activity while increasing Ach content in AD rats. A previous study concluded that EGCG and CUR facilitate the clearance of hyperphosphorylated Tau and inhibit Tau β-sheet formation.37 Chesser et al reported that EGCG may promote the clearance of phosphorylated Tau species by specifically upregulating the level of adaptor protein.11 Moreover, EGCG suppresses tau phosphorylation and aggregation in AD.38 GTPs reduced Tau phosphorylation to inhibit the okadaic acid-induced spatial learning and memory impairment in rats,9 which was consistent with our results. Intriguingly, the involvement of increased Tau phosphorylation has been previously determined in AD development.39 More importantly, it was established in previous research that Aβ may induce the phosphorylation of Tau.40 Amyloids have been linked to various neurodegenerative diseases and the aggregation of Aβ into oligomers, fibrils, and plaques represent a key molecular finding strongly implicated in the pathogenesis of AD.41 Aβ is generated by the continuous cleavage of APP by two enzymes, BACE1 and γ-secretase complex.42 Lee et al indicated that EGCG triggered a reduction in Aβ1-42 induced memory dysfunction via modification of secretase activity.16 Evidence has previously demonstrated that EGCG can reduce Aβ-induced neurotoxicity,43 while long-term administration of EGCG has been shown to diminish the level of APP in the hippocampus, as observed in rats.27 Consistent with our observations, a previous study demonstrated that EGCG plays a crucial role in promoting brain health and inhibits AD development by hindering AchE activity.44 Similarly, Ali et al reported that EGCG exerts inhibitory functions on AchE and BchE enzymes, both of which are associated with the treatment of AD.45
Conclusion
Taken together, the key findings of the current study provide evidence elucidating a specific mechanism by which EGCG improves the learning and memory functions of AD rats. In the current study, EGCG treatment was demonstrated to diminish Tau hyperphosphorylation, BACE1 expression, and Aβ1-42 expression to enhance the antioxidant system and reduce AchE activity, which ultimately led to an improvement in the learning and memory function of AD rats (Figure 7). In addition to with further studies, EGCG possesses medicinal properties that could potentially bring about an improvement in learning and memory abilities in AD.
Acknowledgments
We acknowledge and appreciate our colleagues for their valuable efforts made on this work.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Education Department of Jilin Province (No. JJKH20190060KJ).
Disclosure
The authors declare that they have no competing interests.
References
1. DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019;14(1):32. doi:10.1186/s13024-019-0333-5
2. Jin Y, Xu K, Chen Q, et al. Simvastatin inhibits the development of radioresistant esophageal cancer cells by increasing the radiosensitivity and reversing EMT process via the PTEN-PI3K/AKT pathway. Exp Cell Res. 2018;362(2):362–369. doi:10.1016/j.yexcr.2017.11.037
3. Wong MW, Braidy N, Poljak A, Sachdev PS. The application of lipidomics to biomarker research and pathomechanisms in Alzheimer’s disease. Curr Opin Psychiatry. 2017;30(2):136–144. doi:10.1097/YCO.0000000000000303
4. Moosavi F, Hosseini R, Saso L, Firuzi O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des Devel Ther. 2016;10:
5. Obrenovich M, Tabrez S, Siddiqui B, McCloskey B, Perry G. The microbiota-gut-brain axis-heart shunt Part II: prosaic foods and the brain-heart connection in Alzheimer disease. Microorganisms. 2020;8(4). doi:10.3390/microorganisms8040493
6. Jabir NR, Khan FR, Tabrez S. Cholinesterase targeting by polyphenols: a therapeutic approach for the treatment of Alzheimer’s disease. CNS Neurosci Ther. 2018;24(9):753–762. doi:10.1111/cns.12971
7. Islam MT, Tabrez S, Jabir NR, et al. An Insight into the therapeutic potential of major coffee components. Curr Drug Metab. 2018;19(6):544–556. doi:10.2174/1389200219666180302154551
8. Zhang Y, He F, Hua T, Sun Q. Green tea polyphenols ameliorate ethanol-induced spatial learning and memory impairments by enhancing hippocampus NMDAR1 expression and CREB activity in rats. Neuroreport. 2018;29(18):1564–1570. doi:10.1097/WNR.0000000000001152
9. Li H, Wu X, Wu Q, et al. Green tea polyphenols protect against okadaic acid-induced acute learning and memory impairments in rats. Nutrition. 2014;30(3):337–342. doi:10.1016/j.nut.2013.08.021
10. Xing L, Zhang H, Qi R, Tsao R, Mine Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J Agric Food Chem. 2019;67(4):1029–1043. doi:10.1021/acs.jafc.8b06146
11. Chesser AS, Ganeshan V, Yang J, Johnson GV. Epigallocatechin-3-gallate enhances clearance of phosphorylated tau in primary neurons. Nutr Neurosci. 2016;19(1):21–31. doi:10.1179/1476830515Y.0000000038
12. Winer JR, Mander BA, Helfrich RF, et al. Sleep as a potential biomarker of tau and beta-amyloid burden in the human brain. J Neurosci. 2019;39(32):6315–6324. doi:10.1523/JNEUROSCI.0503-19.2019
13. Islam BU, Tabrez S. Management of Alzheimer’s disease-An insight of the enzymatic and other novel potential targets. Int J Biol Macromol. 2017;97:
14. Tackenberg C, Kulic L, Nitsch RM. Familial Alzheimer’s disease mutations at position 22 of the amyloid beta-peptide sequence differentially affect synaptic loss, tau phosphorylation and neuronal cell death in an ex vivo system. PLoS One. 2020;15(9):e0239584. doi:10.1371/journal.pone.0239584
15. Rezai-Zadeh K, Arendash GW, Hou H, et al. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008;1214:
16. Lee JW, Lee YK, Ban JO, et al. Green tea (-)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-kappaB pathways in mice. J Nutr. 2009;139(10):1987–1993. doi:10.3945/jn.109.109785
17. Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25(1):59–70. doi:10.1111/ene.13439
18. Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018;14:
19. Zameer S, Kaundal M, Vohora D, Ali J, Kalam Najmi A, Akhtar M. Ameliorative effect of alendronate against intracerebroventricular streptozotocin induced alteration in neurobehavioral, neuroinflammation and biochemical parameters with emphasis on Abeta and BACE-1. Neurotoxicology. 2019;70:
20. Julien C, Tomberlin C, Roberts CM, et al. In vivo induction of membrane damage by beta-amyloid peptide oligomers. Acta Neuropathol Commun. 2018;6(1):131. doi:10.1186/s40478-018-0634-x
21. Cortes-Gomez MA, Llorens-Alvarez E, Alom J, et al. Tau phosphorylation by glycogen synthase kinase 3beta modulates enzyme acetylcholinesterase expression. J Neurochem. 2020. doi:10.1111/jnc.15189
22. Jabir NR, Shakil S, Tabrez S, Khan MS, Rehman MT, Ahmed BA. In silico screening of glycogen synthase kinase-3beta targeted ligands against acetylcholinesterase and its probable relevance to Alzheimer’s disease. J Biomol Struct Dyn. 2020;1–10. doi:10.1080/07391102.2020.1784796
23. Tabrez S, Damanhouri GA. Computational and kinetic studies of acetylcholine esterase inhibition by phenserine. Curr Pharm Des. 2019;25(18):2108–2112. doi:10.2174/1381612825666190618141015
24. Li X, Wang SW, Li XL, Yu FY, Cong HM. Knockdown of long non-coding RNA TUG1 depresses apoptosis of hippocampal neurons in Alzheimer’s disease by elevating microRNA-15a and repressing ROCK1 expression. Inflamm Res. 2020;69(9):897–910. doi:10.1007/s00011-020-01364-8
25. Morishima-Kawashima M, Hasegawa M, Takio K, et al. Hyperphosphorylation of tau in PHF. Neurobiol Aging. 1995;16(3):365–380. doi:10.1016/0197-4580(95)00027-c
26. Choi YT, Jung CH, Lee SR, et al. The green tea polyphenol (-)-epigallocatechin gallate attenuates beta-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001;70(5):603–614. doi:10.1016/s0024-3205(01)01438-2
27. Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer’s disease. J Neurochem. 2003;87(1):172–181. doi:10.1046/j.1471-4159.2003.01976.x
28. Zhang H, Lai Q, Li Y, Liu Y, Yang M. Learning and memory improvement and neuroprotection of Gardenia jasminoides (Fructus gardenia) extract on ischemic brain injury rats. J Ethnopharmacol. 2017;196:
29. Bao J, Liu W, Zhou HY, et al. Epigallocatechin-3-gallate alleviates cognitive deficits in APP/PS1 mice. Curr Med Sci. 2020;40(1):18–27. doi:10.1007/s11596-020-2142-z
30. Rasoolijazi H, Joghataie MT, Roghani M, Nobakht M. The beneficial effect of (-)-epigallocatechin-3-gallate in an experimental model of Alzheimer’s disease in rat: a behavioral analysis. Iran Biomed J. 2007;11(4):237–243.
31. Ul islam B, Khan MS, Jabir NR, Kamal MA, Tabrez S. Elucidating Treatment of Alzheimer’s disease via different receptors. Curr Top Med Chem. 2017;17(12):1400–1407. doi:10.2174/1568026617666170103163715
32. Pervin M, Unno K, Takagaki A, Isemura M, Nakamura Y. Function of green tea catechins in the brain: epigallocatechin gallate and its metabolites. Int J Mol Sci. 2019;20(15):3630. doi:10.3390/ijms20153630
33. Cano A, Ettcheto M, Chang JH, et al. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J Control Release. 2019;301:
34. Ettcheto M, Cano A, Manzine PR, et al. Epigallocatechin-3-Gallate (EGCG) improves cognitive deficits aggravated by an obesogenic diet through modulation of unfolded protein response in APPswe/PS1dE9 Mice. Mol Neurobiol. 2020;57(4):1814–1827. doi:10.1007/s12035-019-01849-6
35. Xu Y, Zhang JJ, Xiong L, Zhang L, Sun D, Liu H. Green tea polyphenols inhibit cognitive impairment induced by chronic cerebral hypoperfusion via modulating oxidative stress. J Nutr Biochem. 2010;21(8):741–748. doi:10.1016/j.jnutbio.2009.05.002
36. Haque AM, Hashimoto M, Katakura M, Hara Y, Shido O. Green tea catechins prevent cognitive deficits caused by Abeta1-40 in rats. J Nutr Biochem. 2008;19(9):619–626. doi:10.1016/j.jnutbio.2007.08.008
37. Zheng Q, Kebede MT, Kemeh MM, et al. Inhibition of the self-assembly of abeta and of tau by polyphenols: mechanistic studies. Molecules. 2019;24(12):2316. doi:10.3390/molecules24122316
38. Gueroux M, Fleau C, Slozeck M, Laguerre M, Pianet I. Epigallocatechin 3-Gallate as an inhibitor of tau phosphorylation and aggregation: a molecular and structural insight. J Prev Alzheimers Dis. 2017;4(4):218–225. doi:10.14283/jpad.2017.35
39. Li J, Chen W, Yi Y, Tong Q. miR-219-5p inhibits tau phosphorylation by targeting TTBK1 and GSK-3beta in Alzheimer’s disease. J Cell Biochem. 2019;120(6):9936–9946. doi:10.1002/jcb.28276
40. Wu H, Wei S, Huang Y, et al. Abeta monomer induces phosphorylation of Tau at Ser-214 through beta2AR-PKA-JNK signaling pathway. FASEB J. 2020;34(4):5092–5105. doi:10.1096/fj.201902230RR
41. Gremer L, Scholzel D, Schenk C, et al. Fibril structure of amyloid-beta (1-42) by cryo-electron microscopy. Science. 2017;358(6359):116–119. doi:10.1126/science.aao2825
42. Blennow K, Mattsson N, Scholl M, Hansson O, Zetterberg H. Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci. 2015;36(5):297–309. doi:10.1016/j.tips.2015.03.002
43. Prasanth MI, Sivamaruthi BS, Chaiyasut C, Tencomnao TA. Review of the role of green tea (Camellia sinensis) in antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients. 2019;11(2):474. doi:10.3390/nu11020474
44. Jiang Y, Gao H, Turdu G. Traditional Chinese medicinal herbs as potential AChE inhibitors for anti-Alzheimer’s disease: a review. Bioorg Chem. 2017;75:
45. Ali B, Jamal QM, Shams S, et al. In silico analysis of green tea polyphenols as inhibitors of AChE and BChE enzymes in Alzheimer’s disease treatment. CNS Neurol Disord Drug Targets. 2016;15(5):624–628. doi:10.2174/1871527315666160321110607
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.