Back to Journals » OncoTargets and Therapy » Volume 9
Epidermal growth factor receptor and B7-H3 expression in esophageal squamous tissues correlate to patient prognosis
Authors Song J, Shi W, Zhang Y, Sun M, Liang X, Zheng S
Received 29 April 2016
Accepted for publication 7 August 2016
Published 12 October 2016 Volume 2016:9 Pages 6257—6263
DOI https://doi.org/10.2147/OTT.S111691
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Li
Jianxiang Song,1,2,* Woda Shi,1,2,* Yajun Zhang,2 Mingzhong Sun,3 Xiaodong Liang,3,4 Shiying Zheng1
1Department of Cardiothoracic Surgery, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu Province, People’s Republic of China; 2Department of Cardiothoracic Surgery, 3Department of Clinical Laboratory, 4Department of Pathology, The Third People’s Hospital of Yancheng City, Yancheng, Jiangsu Province, People’s Republic of China
*These authors contributed equally to this work
Abstract: Biomarkers that can serve as diagnostic and prognostic indicators of esophageal squamous cell carcinoma (ESCC) are urgently needed to help improve patient outcomes. Here, the expression of epidermal growth factor receptor (EGFR) and costimulatory molecule B7-H3, both of which have been implicated in tumor onset and progression in certain tumors, was investigated in relation to the clinical characteristics and survival outcomes of patients with ESCC. ESCC tissue samples were analyzed for 100 patients. Tumor and patient characteristics were recorded. Tissues were investigated for EGFR and B7-H3 staining by immunohistochemistry. Patients were followed for up to 96 months to determine overall survival (OS) and progression-free survival (PFS). High expression for EGFR (68.0%) and B7-H3 (66.0%) was observed in the majority of cases. High expression of either EGFR or B7-H3 was correlated with tumor invasion depth and clinical stage (P<0.05). Further, high expression of either EGFR or B7-H3 was correlated with worse survival outcomes. The estimated OS (38.1 months) and PFS (13.4 months) of patients with high expression of EGFR were lower than those of patients with low expression (69.3 and 68.1 months, P<0.05). The estimated OS (31.1 months) and PFS (13.1 months) of patients with high expression of B7-H3 were also lower than those of patients with low expression (69.3 and 66.6 months, P<0.05). Indeed, Cox multiple regression showed that OS and PFS were correlated with EGFR (relative risk =1.853, 1.875, respectively) and B7-H3 (relative risk =1.886, 2.061, respectively) (all P<0.05) expression level. Thus, EGFR and B7-H3 are highly expressed in tumor tissues of patients with ESCC. Their expression levels are correlated with tumor severity and survival, and therefore these may be viable biomarkers to aid in prognosis determination.
Keywords: esophageal squamous cell carcinoma, epidermal growth factor receptor, costimulatory molecule B7-H3, prognosis, immunohistochemistry
Introduction
Esophageal cancer is a serious malignancy worldwide that is associated with a morbidity rate ranking fifth among tumors and fourth in mortality rate. Further, the incidence and mortality rate are gradually increasing each year.1 Indeed, in People’s Republic of China, esophageal cancer is the number one cause of cancer mortality.1,2 Interestingly, while the main pathological type of esophageal cancer in Western countries is adenocarcinoma, the main type in Asia is esophageal squamous cell carcinoma (ESCC);1 in People’s Republic of China, ESCC cases account for over 90% of all the esophageal cancer patients.2 Surgery remains the main therapeutic approach to ESCC, but despite treatment ESCC patients at stages IIA to III have a 5-year survival rate of just 20%–30%.3 The application of molecular targeted therapies such as cetuximab, erlotinib, trastuzumab, and bevacizumab is providing more clinical benefit to patients. Although such therapies play an important role in improving the postoperative quality of life and long-term survival, many patients will still experience recurrence or metastasis, and the overall prognosis remains poor.4 Therefore, advancements in diagnosis and treatment are still urgently needed to improve outcomes in affected individuals.
Clinical practice continues to rely on tumor, node, metastasis staging to predict patient prognosis, but the search for molecular indicators and potential therapeutic targets is well underway. For example, worse ESCC outcomes are associated with higher multidrug resistance protein expression, high activity of glutathione S-transferase, and abnormal expression of P53 and RAS.5,6 All of these changes can promote multidrug resistance of the tumor, thereby negatively affecting the efficacy of treatment and worsening the prognosis of ESCC patients.6 Similarly, epidermal growth factor receptor (EGFR) plays a role in cell adhesion and contributes to tumor growth, differentiation, infiltration, and metastases through its various ligands.7 For example, over 90% of squamous cell carcinomas of the head and neck exhibit abnormal EGFR expression; likewise, in ESCC, EGFR expression is upregulated and is correlated with TNM stage.8 Indeed, some of the aforementioned molecular therapies target EGFR. Thus, EGFR may play an important role as a biomarker in ESCC.
Advances in immunooncology indicate that tumors are recognizable by the immune system. Under some circumstances, the immune system can regulate the tumor growth and even clear tumors; therefore, identifying key molecules in immune regulation has become important for advancing cancer treatment. This search has revealed that T-cell activation requires T-cell receptors and costimulatory molecules like B7/CD28.9 Costimulatory molecules are present on the surfaces of T- and B cells, antigen presenting cells, and target cells and participate in immune responses. Negative costimulatory molecules can weaken the early activation of immature T-cells and memory T-cells, lowering the regulatory and clearing activities on tumor cells.10 One of the B7 family of costimulatory molecules, B7-H3, is widely expressed in human tissues and cell strains. Elevated B7-H3 expression can promote tumorigenesis.11 In vitro, inhibition of B7-H3 in ESCC cells suppresses their migration and invasion abilities.12 Thus, B7-H3 may play an important role in ESCC development and/or progression and represents a potential diagnostic/prognostic marker. Here, expression of EGFR and B7-H3 were investigated in ESCC tissues to establish their utility in predicting patient prognosis.
Materials and methods
Clinical materials
Paraffin-embedded esophageal cancer tissue and para-carcinoma tissue specimens were collected from 100 patients between January 2006 and December 2015. Specimens were included only from patients who received surgery as the first-line treatment and thus were free of chemotherapy or radiotherapy. The resected tumor tissues were fixed in formaldehyde, embedded in paraffin, and stored. Patients (68 men and 32 women) were postoperatively given adjuvant therapies such as radiotherapy and chemotherapy according to specific conditions. Their ages ranged from 35 to 75 years, with a median age of 60 years. Clinical characteristics such as age, sex, clinical staging, tumor infiltration depth, differentiation degree, and lymph node metastasis were recorded. The study was approved by the Ethics Committee of The Third People’s Hospital of Yancheng City. All participants provided written informed consent.
Immunohistochemistry
Resected tumor tissue specimens were serially sectioned at 3 μm. The streptavidin-perosidase method was used for immunostaining after sections were dewaxed. Expression of EGFR and B7-H3 was detected using mouse anti-human EGFR monoclonal antibody (Invitrogen, Carlsbad, CA, USA) or sheep anti-human B7-H3 monoclonal antibody (Invitrogen). Diaminobenzidine was used for substrate conversion to visualize staining. Positive staining for either EGFR or B7-H3 was detected as yellow particles in the ESCC cytoplasm or cell membrane. Under a microscope (OLYMPUS BX53, Tokyo, Japan), sections were evaluated for EGFR or B7-H3 staining patterns by the proportion of positively stained tumor cells and the intensity of staining; 100 cells were counted in each field (40×), and five fields were used in evaluation. Two chief physicians in the pathology department were designated to read the images independently, and in case of disagreement, one senior pathologist made the final judgment. Staining intensity was scored as follows: a score of 0 for no color; 1 point for light yellow; 2 points for brown-yellow; and 3 points for brown. The percentage of positive cells was scored as follows: a score of 0 when the positive tumor cell proportion was <10%; 1 point when the positive tumor cell proportion ranged from 10% to 25%; 2 points when the positive tumor cell proportion ranged from 26% to 50%; 3 points when the positive tumor cell proportion ranged from 51% to 75%; and 4 points when the colored tumor cell proportion was >75%. The sum of the two scores served as staining score. The total expression of the two markers was evaluated according to this score with the following criteria: a score of 0 marked negative expression; a score of 1–2 points marked weakly positive; a score of 3–4 points marked positive; and a score of >4 points marked strongly positive. Patients with a negative or weakly positive expression were classified into the low-expression group, and patients with a positive or strongly positive expression were classified into the high-expression group. They were followed through a combination of home visits and telephone calls. Patients’ time of overall survival (OS) and progression-free survival (PFS) were recorded. The deadline for follow-up was October 2015. The follow-up period ranged between 5.2 and 96 months (the median follow-up time was 36 months).
Statistical methods
The Statistical Package for the Social Sciences (SPSS, IBM Corporation, Armonk, NY, USA) 19.0 statistical software was used for statistical analysis. Numerical data are expressed as percentages. A chi-square (χ2) test was used to compare the proportions of EGFR and B7-H3-positive cases with different clinical characteristics. A Kaplan–Meier survival analysis was applied to compare OS and PFS, and a log-rank test was used to compare their differences. Cox multiple regression analysis was used to perform multifactor analysis on the features that influenced OS and PFS. A P<0.05 indicated that the difference was statistically significant.
Results
Expression of EGFR and B7-H3 in ESCC tissues
Using the staining score criteria described earlier, we assessed expression of EGFR and B7-H3 in ESCC tumor sections. In the 100 ESCC tumor samples, 68 patients (68%) presented high expression of EGFR (Table 1), and 66 patients (66%) presented high expression of B7-H3 (Table 2). The normal paracarcinoma tissues had a slightly weak expression (Figure 1).
  | Table 1 EGFR expression levels and clinical characteristics in ESCC tissues (n [%]) |
  | Table 2 B7-H3 expression levels and clinical characteristics in ESCC tissues |
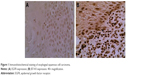  | Figure 1 Immunohistochemical staining of esophageal squamous cell carcinoma. |
As described, patients were classified into high- and low-expression groups based on EGFR and B7-H3 staining scores. We then compared clinical characteristics among high- and low-expression groups (Tables 1 and 2). ESCC patients with different EGFR expression levels had statistically significant differences in tumor infiltration depth and clinical staging (χ2=21.784, 11.505, respectively, both P<0.05). Similarly, ESCC patients with different B7-H3 expression levels had statistically significant differences of tumor infiltration depth and clinical staging (χ2=31.824, 18.522, respectively, both P<0.05) as well as age (χ2=26.471, P<0.05).
Relationship between EGFR and B7-H3 expression and ESCC patient survival time
Finally, we assessed survival outcomes, specifically OS and PFS, among ESCC patients. Within the follow-up period, 66 patients (66%) died. The estimated OS was 42.3 months (95% confidence interval [CI] =38.3–46.3 months), and the estimated PFS was 21.6 months (95% CI =10.9~32.3 months) (Table 3). Kaplan–Meier survival analysis curves for OS and PFS of all the patients are shown in Figure 2.
  | Table 3 Survival outcomes of ESCC patients |
  | Figure 2 Kaplan–Meier survival curve for (A) OS and (B) PFS in patients with ESCC. |
Next, we assessed survival differences between groups classified by their marker expression levels. For patients with high EGFR expression, the estimated OS was 31.8 months (95% CI =26.5~37.1 months), and the estimated PFS was 13.4 months (95% CI =12.3~14.5 months). In comparison, patients with low EGFR expression had OS of 69.3 months (95% CI =59.9~78.7 months) and PFS of 68.1 months (95% CI =56.9~79.4 months). The differences in OS and PFS between the two expression groups were statistically significant (χ2=35.292, 36.271, respectively, both P<0.05; Table 4). Kaplan–Meier survival curves for OS and PFS by EGFR expression level are shown in Figure 3.
For the patients with high B7-H3 expression, the estimated OS was 31.1 months (95% CI =24.9~37.3 months), and the estimated PFS was 13.1 months (95% CI =10.5~15.7 months). For patients with low B7-H3 expression, OS was 69.3 months (95% CI =59.8~78.8 months) and PFS was 66.6 months (95% CI =55.3~77.9 months). The differences in OS and PFS between the two expression groups were statistically significant (χ2=34.481, 36.823, respectively, both P<0.05; Table 5). The Kaplan–Meier survival curves for OS and PFS by B7-H3 expression level are shown in Figure 4.
  | Figure 4 Kaplan–Meier survival curves for (A) OS and (B) PFS of patients classified by B7-H3 expression levels. |
The Cox multiple factor regression analysis indicated that the OS of the ESCC patients correlated with both EGFR expression level (relative risk [RR] =1.853) and B7-H3 expression level (RR =1.886) (P<0.05); similarly, PFS was correlated with both EGFR expression level (RR =1.875) and B7-H3 expression level (RR =2.061) (P<0.05) (Table 6).
Discussion
Molecules involved in growth signal regulation, apoptosis, angiogenesis, and tissue infiltration and metastasis represent important targets for cancer therapy, as well as serving as potential diagnostic and prognostic biomarkers. EGFR, a membrane receptor in the BrbB protein kinase family (also called HER1 or ErbB1), contributes to tumorigenesis and has become an important molecular target in multiple cancer types, including ESCC.7,8,13–15 Consistent with this, our study of ESCC tissues revealed high expression of EGFR in the majority of cases. Further, high expression of EGFR was associated with more advanced tumor infiltration and clinical staging. Survival analyses also indicated that higher EGFR expression was associated with poorer OS and PFS. Thus, EGFR may provide value as a prognostic marker at the time of biopsy.
Our understanding of the role of the immune system in tumorigenesis continues to expand, particularly with recent findings regarding the roles of costimulatory molecules.10 Costimulatory molecules can provide secondary signals for the activation of T-cells in the immune response. The B7 family of molecules, which currently comprises ten members, is expressed in lymph and nonlymph organs and binds with different receptors to mediate positive or negative immune regulation signals. They promote activation and proliferation of T-cells to participate in antitumor immunity; additionally, some negative costimulatory molecules directly participate in the tumor formation through nonimmunoregulatory effects.16 B7-H3 receptors can be expressed inductively on the surface of activated T-cells. In the presence of anti-human CD3 monoclonal antibodies, B7-H3 can increase the generation of CD4+ and CD8+ cells, selectively increase the generation of interferon-γ, and participate in the activity of T- and B cells. In addition, elevated B7-H3 expression can inhibit the regulatory effects of immune system on tumors and promote tumorigenesis.11 Indeed, significant upregulation of serum B7-H3 levels is associated with the onset and progression of bladder cancer and primary liver cancer, as well as invasiveness of glioma and non-small-cell lung cancers.9
Our study supports these previous findings. In ESCC tissues, B7-H3 exhibited high expression in the majority of cases, and this high expression was correlated with patient age as well as invasion depth and clinical stage. Thus, B7-H3 in tumor tissues may inhibit CD8+ T-cells to enable the tumor cells to avoid immunological surveillance. Further, as with EGFR expression, higher B7-H3 expression was associated with poorer survival outcomes. These findings indicate that further investigation into the clinical utility of B7-H3 is warranted for ESCC diagnosis, prognosis, and treatment options.17
Conclusion
In sum, tumor tissues of ESCC patients had high expression of both EGFR and the B7-H3 costimulatory molecule. Expression of these markers was correlated with tumor severity and patient survival outcomes. Thus, assessment of EGFR and/or B7-H3 at the time of biopsy may help guide prognosis.
Disclosure
The authors report no conflicts of interest in this work.
References
Zhang J, Jiang Y, Wu C, et al. Comparison of clinicopathologic features and survival between eastern and western population with esophageal squamous cell carcinoma. J Thorac Dis. 2015;7(10):1780–1786. | ||
Chuang WY, Chang YS, Chao YK, et al. High sex determining region Y-box 2 (SOX2) expression correlates with absence of nodal metastasis in esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2015;8(8):9248–9255. | ||
Zhang W, Liu X, Xiao Z, et al. Efficacy of intensity-modulated radiotherapy for resected thoracic esophageal squamous cell carcinoma. Thorac Cancer. 2015;6(5):597–604. | ||
Kikuchi O, Ohashi S, Nakai Y, et al. Novel 5-fluorouracil-resistant human esophageal squamous cell carcinoma cells with dihydropyrimidine dehydrogenase overexpression. Am J Cancer Res. 2015;5(8):2431–2440. | ||
Ma Q, Liu W, Long H, et al. Right versus left transthoracic approach for lymph node-negative esophageal squamous cell carcinoma. J Cardiothorac Surg. 2015;10(1):123. | ||
Henry MA, Lerco MM, de Oliveira WK, Guerra AR, Rodrigues MA. The Glasgow prognostic score. An useful tool to predict survival in patients with advanced esophageal squamous cell carcinoma. Acta Cir Bras. 2015;30(8):580–585. | ||
Pabla B, Bissonnette M, Konda VJ. Colon cancer and the epidermal growth factor receptor: current treatment paradigms, the importance of diet, and the role of chemoprevention. World J Clin Oncol. 2015;6(5):133–141. | ||
Kuramitsu S, Ohno M, Ohka F, et al. Lenalidomide enhances the function of chimeric antigen receptor T cells against the epidermal growth factor receptor variant III by enhancing immune synapses. Cancer Gene Ther. 2015;22(10):487–495. | ||
Karakatsanis S, Bertsias G, Roussou P, Boumpas D. Programmed death 1 and B and T lymphocyte attenuator immunoreceptors and their association with malignant T-lymphoproliferative disorders: brief review. Hematol Oncol. 2014;32(3):113–119. | ||
Aung LL, Balashov KE. Decreased Dicer expression is linked to increased expression of co-stimulatory molecule CD80 on B cells in multiple sclerosis. Mult Scler. 2015;21(9):1131–1138. | ||
Yan R, Yang S, Gu A, et al. Murine b7-h3 is a co-stimulatory molecule for T cell activation. Monoclon Antib Immunodiagn Immunother. 2013;32(6):395–398. | ||
Zhang S, Chen Z, Yang R, et al. Irinotecan combined with co-stimulatory molecule blockade prolongs survival of cardiac allografts in alloantigen-primed mice. Cell Immunol. 2013;282(2):85–92. | ||
Li N, Li H, Su F, Li J, Ma X, Gong P. Relationship between epidermal growth factor receptor (EGFR) mutation and serum cyclooxygenase-2 Level, and the synergistic effect of celecoxib and gefitinib on EGFR expression in non-small cell lung cancer cells. Int J Clin Exp Pathol. 2015;8(8):9010–9020. | ||
Furugen M, Uechi K, Hirai J, et al. An autopsy case of two distinct, acquired drug resistance mechanisms in epidermal growth factor receptor-mutant lung adenocarcinoma: small cell carcinoma transformation and epidermal growth factor receptor T790M mutation. Intern Med. 2015;54(19):2491–2496. | ||
Liang W, He Q, Chen Y, Zou X, Hamblin L, He J. Discontinuing epidermal growth factor receptor-tyrosine kinase inhibitor during second-line chemotherapy: is the evidence strong enough? Ann Transl Med. 2015;3(14):202. | ||
Han FF, Fan H, Wang ZH, et al. Association between co-stimulatory molecule gene polymorphism and acute rejection of allograft. Transpl Immunol. 2014;31(2):81–86. | ||
Jia J, Wang Z, Li X, Wang Z, Wang X. Morphological characteristics and co-stimulatory molecule (CD80, CD86, CD40) expression in tumor infiltrating dendritic cells in human endometrioid adenocarcinoma. Eur J Obstet Gynecol Reprod Biol. 2012;160(2):223–227. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




