Back to Journals » Infection and Drug Resistance » Volume 16
Epidemiology of Nontuberculous Mycobacteria in Nanjing and MAB_0540 Mutations Associated with Clofazimine Resistance in Mycobacterium abscessus
Authors Zhang R, Luo S, Wang N, Zhang H, Wu X
Received 17 February 2023
Accepted for publication 29 April 2023
Published 6 May 2023 Volume 2023:16 Pages 2751—2764
DOI https://doi.org/10.2147/IDR.S408986
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Ruixian Zhang,1 Sha Luo,1 Nan Wang,1 Hongying Zhang,2 Xuping Wu1
1The Second Hospital of Nanjing, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, 210003, People’s Republic of China; 2Nanjing Center for Disease Control and Prevention Affiliated to Nanjing Medical University, Nanjing, Jiangsu, 210008, People’s Republic of China
Correspondence: Xuping Wu, The Second Hospital of Nanjing, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, 210003, People’s Republic of China, Email [email protected] Hongying Zhang, Email [email protected]
Background: Nontuberculous mycobacteria (NTM) are easily misdiagnosed as multidrug-resistant tuberculosis (MDR-TB), and treatment drugs are very limited. The main objective of our study was to evaluate the minimal inhibitory concentration (MIC) in vitro of bedaquiline (BDQ), clofazimine (CFZ), linezolid (LZD), delamanid (DLM), and pretomanid (PA-824) for treatment of M. abscessus and M. intracellulare. Furthermore, we determined whether MAB_1448, MAB_4384, MAB_2299c, MAB_1483, MAB_0540, rplD, rplC, and rrl were related to drug resistance to provide an experimental basis for the use of these five drugs in the treatment of NTM.
Methods: We identified sample characteristics of epidemics in 550 patients with suspected NTM infection in Nanjing from 2019 to 2021 using the PCR-reverse spot hybrid method. Furthermore, we evaluated the MIC of BDQ, CFZ, DLM, LZD, and PA-824 against 155 clinical isolates of NTM using the microbroth dilution method. The resistant isolates were sequenced using Sanger sequencing.
Results: The top three dominant species of NTM distributed in Nanjing were M. intracellulare, M. avium, and M. abscessus. Notably, the proportion of M. abscessus infections increased. The proportion of M. abscessus increased from 12% in 2019 to 18% in 2021. Demographic analysis showed that female infection rates were substantialy greater than male for M. abscessus (P=0.017, < 0.05). Our results demonstrate that NTM are highly sensitive to bedaquiline and clofazimine in vitro. However, delamanid and pretomanid had little effect on M. abscessus and M. intracellulare. In addition, we found 30– 41 nucleotide deletion mutations and some novel point mutations in the MAB_0540 gene of M. abscessus that are resistant to clofazimine.
Conclusion: Bedaquiline, clofazimine, and linezolid were more successful in vitro treatments against M. abscessus and M. intracellulare. The MAB_0540 mutation may be associated with resistance of M. abscessus to clofazimine.
Keywords: nontuberculous mycobacteria, minimal inhibitory concentration, gene mutations
Introduction
Nontuberculous mycobacteria (NTM), a mycobacterium other than the Mycobacterium tuberculosis complex (MTC) and M. leprae, are conditional pathogens widely found in the environment.1 Studies have shown that the probability of NTM transmission is extremely low, and exposure comes primarily from the environment, soil, water, etc.1 In recent years, lung diseases caused by NTM have attracted widespread attention as disease prevalence and mortality have gradually increased. More than 190 species of NTM have been identified, and after 2015, the dominant species of NTM in China gradually changed from M. abscessus to M. intracellulare.1,2 In recent years, NTM have shown growth trends worldwide, especially M. abscessus.3–5 Currently, M. abscessus is the only species with evidence of human-to-human transmission. Furthermore, different species of NTM have different drug susceptibilities, especially M. abscessus, which is naturally resistant to a variety of first-line antituberculosis drugs.3 In clinical practice, NTM are easily misdiagnosed as multidrug-resistant tuberculosis, which is a cause for concern.6 Several studies have shown that immunocompromised elderly people are the primary population of NTM infection.5,7,8 Patients infected with NTM usually have one or more underlying diseases, like tumors, surgical history, previous tuberculosis, and HIV.5,9 Due to displaying similar clinical and radiological characteristics to tuberculosis, NTM lung diseases were misdiagnosed in some cases, causing great inconvenience for clinical treatment and control of the occurrence and increased development of NTM.6 Macrolides are the drugs of choice for the treatment of NTM infection,10 but in recent years, the widespread use of macrolides has also predisposed bacteria to drug resistance, so there is a need to find new therapeutic agents.11
Bedaquiline (BDQ), clofazimine (CFZ), linezolid (LZD), delamanid (DLM), and pretomanid (PA-824) have been recommended by the WHO for the treatment of multidrug-resistant tuberculosis.10 In recent years, some studies have attempted to use bedaquiline, clofazimine, and linezolid for NTM.12–14 However, the in vitro susceptibility of NTM to these five drugs has been poorly reported and limited to local studies. Studies of linezolid resistance in NTM clinical isolates have identified resistance-associated mutations in 23S rRNA (rrl), rplC, and rplD.15 The potential resistance genes of M. abscessus to bedaquiline are MAB_1448, MAB_4384, and MAB_2299c.13,16,17 Whole genome sequencing of M. abscessus against clofazimine revealed that the major genes linked to resistance were MAB 2299c, MAB 1483, and MAB 0540.18 However, there have been no systematic studies on drug resistance-related gene mutations, and the mechanism of drug resistance remains unclear.
In this study, we investigated suspected NTM infections admitted from January 2019 to December 2021 in Nanjing Public Health Medical Center to explore the relationship between different species and age, sex, and previous medical history, further providing a reliable basis for the clinical treatment of nontuberculosis mycobacterial disease. In addition, we conducted in vitro drug susceptibility studies of NTM to bedaquiline, clofazimine, linezolid, delamanid, and pretomanid and sequenced the possible drug resistance-related genes to provide a basis for the development of new drugs for the treatment of NTM.
Materials and Methods
Methods
Species Identification by PCR-Reverse Spot Hybrid Method
M. tuberculosis and NTM were identified by PCR-reverse spot hybridization.19 All reagents were obtained from the Mycobacterium species Identification Kit (Shenzhen Yaneng Bio, China).
DNA Isolation
Fresh colonies were picked for inactivation, and then samples were centrifuged at 13,000 rpm for 5 min. The MTB lotion solution was added to the sediment and centrifuged at 10,000 rpm for 2 minutes. Next, 50 μL of lysate was added and it was placed in a boiling water bath for 10 minutes, then centrifuged at 10,000 r/min for 2 minutes. The supernatant was used for PCR amplification.
PCR Test
PCR was carried out in a 25 μL reaction mixture including 4 μL DNA template. The first step was incubation at 95 °C for 10 min. This was followed by the amplification of the first 30 cycles (95°C for 45s, 68°C for 60s), then perform the amplification of the second 30 cycles (95°C for 30s, 54°C for 30s, 68°C for 60s). Finally, it was extended at 68°C for 10min. The instrument used to perform PCR was the ABI 7500 Quantitative PCR Instrument (Thermo Fisher Scientific, USA).
Hybridization
Next, solution A (2xSSC, 0.1% SDS) is added to the PCR reaction solution and placed in a boiling water bath for 10 min. The solution was removed and put into a hybridization box at 59°C for hybridization for 1.5 hours. The membrane strip was removed and it was continuously washed for 15 min in 40 mL of B solution (0.5 x SSC, 0.1% SDS) pre-warmed to 59°C. Finally, it was washed with supporting reagents and a color developer was added for 10 min to protect it from light. The instrument used in the hybridization process was the Thermostatic Hybridization Instrument (Shenzhen Yaneng Bio, China).
Minimum Inhibitory Concentration (MIC) Testing
NTM isolates (n=155) from 155 patients were cultured and preserved in L-J culture medium (Zhuhai Baso Bio, China) at the Nanjing Public Health Medical Center. Bacterial cultures and related procedures were performed in a negative-pressure laboratory.
NTM in vitro drug susceptibility assays were performed using the CLSI-recommended micro-broth dilution method.20 Bedaquiline (Meilunbio, Dalian, China), clofazimine (Macklin, Shanghai, China), linezolid (Meilunbio, Dalian, China), delamanid (TargetMol, Boston, USA), and pretomanid (TargetMol, Boston, USA) were dissolved in dimethyl sulfoxide (DMSO). The ranges of drug concentration were as follows: bedaquiline, 0.004 to 2.048 μg/mL; clofazimine, 0.016 to 8 μg/mL; linezolid, 0.125 to 64 μg/mL; delamanid, 0.063 to 32μg/mL; and pretomanid, 0.125 to 64 μg/mL.
Middlebrook 7H9 liquid medium was supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC) and catalase solution before clinical use.
Microbroth Dilution Method
A culture plate was prepared on a 96-well plate with negative control set in the first column. Next, 200 μL of medium was added to each well. The second column added 180 μL of 7H9 liquid medium and 20 μL of drug reservoir per well. The third column to the 12th column added 100 μL of medium per well. The positive control is shown in column 12. Starting from column 2, 100 μL of liquid was aspirated and added to the next column, and the drug was diluted multiplicatively.
Bacterial Solution Preparation
Fresh colonies grown for 2–4 weeks were picked with an inoculation loop, and 0.5 McFarland standard concentration was prepared using an ultrasonic dispersion counter.
Inoculation
Except for the negative control wells, the rest of the well plates were incubated by adding 100 μL of bacterial solution, and each bacterium was tested twice in parallel. The rapidly growing Mycobacterium was cultured for 3–5 days, and the slow-growing Mycobacterium was cultured for 7–10 days. In addition, a blank plate was prepared and only 100 μL of culture medium and 100 μL of bacterial solution were added for observation of bacterial growth status.
Observation of Results
Alma Blue solution was prepared with a concentration of 1 mg/mL; 30 μL of Alma Blue solution was added to each well of the 96-well plates, and the results were observed within 24–48h. When the blue color changed to pink or purple, it was judged to be drug resistant, and the drug concentration corresponding to the last blue hole close to the color-changing hole had the lowest inhibitory concentration.
PCR and DNA Sequencing
The primers were designed using NCBI and Primer3plus,21,22 and synthesized by Universalbiol (Anhui, China). PCR amplification of MAB_1448, MAB_4384, MAB_0540, MAB_2299c, and MAB_1483 for rplC, rplD, and rrl for M. abscessus and M. intracellulare, respectively, was conducted under the reaction conditions described in Supplementary Table S1 and Figure S1. The PCR amplification products were purified using 1% agarose gel electrophoresis, the corresponding bands were cut, and the amplification products were sent to the General Biology Company (Anhui, China) for sequencing.
Quality Control
For each MIC experiment, M. abscessus was used to determine Staphylococcus aureus ATCC29213, and M. intracellulare were needed to determine M. intracellulare ATCC13950, to ensure that the MIC concentrations of the quality control strains were within the appropriate range.
Statistical Analysis
A bacterial species distribution study was conducted based on the number of detected bacterial species. Demographic data and clinical risk factor analyses were performed based on the number of patients. Statistical analyses were performed using SPSS22.0 software. All tests were two-sided probability tests, and statistical significance was set at P < 0.05. Sequencing results were analyzed by sequence analysis using SnapGene 6.0.2 software and compared with the sequences of standard strains on NCBI.21 Multiple sequence alignment was performed on the website ESPript 3,23 using M. tuberculosis H37Rv protein as the basis for homologous protein sequence alignment.
Results
Distribution of Species in NTM
Between 2019 and 2021, 550 patients at Nanjing Public Health Medical Center were identified as having a NTM infection. Among them, 37 patients were infected with two NTMs simultaneously, three patients with three NTMs, and one patient with four NTMs. Therefore, 597 cases of NTM were identified.
Ten types of NTM were detected between 2019 and 2021 (Figure 1). The results showed that M. intracellulare (MIN) accounted for up to 50% of cases (95% CI: 46.4%--54.4%), followed by M. avium (MAV) at 17% (95% CI: 14.2%--20.3%), M. abscessus (MAB) at 13% (95% CI: 10.2–15.6%), M. Gordon (MGO) at 7% (95% CI: 5.1–9.3%), and M. kansasii (MKA) at 6% (95% CI: 4.1–7.9%).
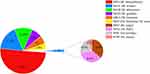 |
Figure 1 Distribution of NTM species. |
In recent three years, M. intracellulare, M. abscessus, and M. avium have been the dominant species of NTM in Nanjing. There has been a decline in NTM infections; however, it should be noted that the prevalence of different strains has also changed (Figure 2). Infections from M. abscessus have shown an increasing trend. The proportion of M. abscessus was 12%, 11%, and 18%, respectively, from 2019 to 2021. Furthermore, the proportion of women infected with M. abscessus has been increasing annually.
Demographic Characteristics of Patients Infected with NTM
Among the 550 patients, 54.2% (298/550) were male and 45.8% (252/550) were female, with a male-to-female ratio of 1.2 to 1 (Table 1). The results showed that the risk factors for NTM infection were related to sex (P=0.003, <0.05), and men were more likely to be infected with NTM. The male age distribution ranged between 30–79 years old, while females were mainly between 50–69 years old. The minimum age of patients infected with NTM was 15 years old, the maximum was 90 years old, the average age was 56.82±16.90, and the median was 60. The population over 50 years of age accounted for 70.7%, and more than 50.5% of the patients were over 60 years old, indicating that NTM infection was related to age. In addition, men aged 30–49 years old were more likely to be infected with NTM than were women of the same age (P < 0.05).
 |
Table 1 Gender and Age of Patients Infected with NTM |
In addition, there was a significant difference between men and women in M. avium, M. kansasii, and M. abscessus infections (P < 0.05) (Table 2). The infection rates of M. avium and M. kansasii were higher in males than in females, whereas those of M. abscessus showed the opposite trend.
 |
Table 2 Gender Distribution Among Different Species Types |
A total of 58.36% (321/550) of the patients were definitively diagnosed with NTM, and 46.55% (256/550) had been diagnosed with or had a history of tuberculosis. Notably, 13.27% of patients had AIDS. Bronchiectasis is a common complication of NTM infections. In addition, 37.27% of the patients had abnormal liver function (Table 1).
MIC Distributions to Bedaquiline and Clofazimine
In this study, the NTM strains saved by Nanjing Public Health Medical Center were collected. The strains cultivated more than once by the same patient were counted as one case, and the redundant, contaminated, and failed strains were deleted, yielding 155 results in total. Among them, there were 30 strains of M. abscessus, 117 strains of M. intracellulare, one strain of M. kansasii, two strains of M. avium, two strains of M. gordonii, and three strains of M. fortuitum / porcine. Finally, 155 NTM isolates showed MIC results.
The MIC distributions of M. abscessus and M. intracellulare to BDQ and CFZ are shown in Figure 3A–D. There were 30 isolates of M. abscessus which showed drug-susceptibility testing for bedaquiline, and most of the MICs were concentrated at 0.256 μg/mL to 1.024 μg/mL. The MIC50 was 0.128μg/mL, and the MIC90 was 2.048μg/mL. There were three strains of M. abscessus having MIC >2.048 μg/mL. No bedaquiline-resistant strains of M. intracellulare were detected. For M. intracellulare, the MIC50 was 0.004μg/mL and the MIC90 was 0.032μg/mL of bedaquiline. The MIC50 and MIC90 of bedaquiline and clofazimine against M. abscessus were higher than those against M. intracellulare. A total of 11 strains of M. abscessus were determined to be clofazimine-resistant. The MIC of clofazimine against M. abscessus was mainly distributed between 0.25μg/mL to 8μg/mL, with a MIC50 at 2μg/mL and MIC90 at 8μg/mL. The MIC of clofazimine against M. intracellulare was mainly distributed from 0.015μg/mL to 0.5μg/mL, with a MIC50 and MIC90 of 0.125μg/mL and 0.5μg/mL, respectively.
MIC Distributions to Linezolid, Delamanid, and Pretomanid
The MIC ranges of linezolid were the same for M. abscessus and M. intracellulare, and 8 strains of M. abscessus and 38 strains of M. intracellulare showed linezolid resistance (Figure 3E and F). The MIC50 of M. abscessus was 8 μg/mL, and MIC90 was 32 μg/mL. Meanwhile, the MIC50 of M. intracellulare was 16 μg/mL, and MIC90 was 32 μg/mL. Linezolid required higher concentrations against M. intracellulare than against M. abscessus.
As shown in Figure 3G and H, the M. intracellulare and M. abscessus included in the study were resistant to delamanid and had MICs ≥ 32 μg/mL. A total of 108 NTM isolates were tested for pretomanid MIC, as shown in Figure 3I. The M. intracellulare and M. abscessus were resistant to pretomanid and had MICs ≥ 64 μg/mL. Notably, two strains of M. gordonii were sensitive to pretomanid.
M. abscessus Resistant Mutations to BDQ, CFZ, LZD
There were 3 strains of M. abscessus strains were resistant to BDQ. MAB_1448, MAB_4384, and MAB_2299c were also sequenced. The results showed that one strain had a base C insertion at position 13 in MAB_1448, which caused a frameshift mutation. In addition, the other two strains failed repeated amplification in MAB_4384. Synonymous mutations were observed only in MAB_4384 and MAB_2299c (Table 3).
 |
Table 3 NTM Mutation M. abscessus of BDQ, CFZ, and LZD |
There were 11 strains of M. abscessus resistant to CFZ. MAB_2299c is associated with cross-resistance between bedaquiline and clofazimine;17 no amino acid mutations have been found in MAB_2299c. We observed no cross-resistance between bedaquiline and clofazimine in the MAB_2299c mutant. In MAB_1483, nucleotide mutations alone did not cause amino acid mutations. It was worth noting that a large amount of nucleotide mutation deletion was found in MAB_0540. Six of the 11 clofazimine-resistant isolates displayed base deletions between positions 30 and 41 in MAB_0540. No mutations were detected in either strain.
Mutations in Genes of M. intracellulare to LZD
There were 8 strains of M. abscessus resistant to LZD. Table 3 presents the results. There were 38 M. intracellulare strains of linezolid with MIC >16 μg/mL classified as phenotypically resistant according to CLSI.20 Five strains that were not amplified were excluded from the analysis. The rplC, rplD, and rrl genes of 33 phenotypically resistant strains were sequenced. The results are presented in Figure 4 and Table 4. The known rplC and rplD protein crystal structures of Mycobacterium tuberculosis H37Rv were used as the basis for multiple sequence alignment of M. abscessus and M. intracellulare, respectively, and the alignment results are shown in Supplementary Figures S2 and S3. The mutation site of MAB_rplD is in a highly variable region, which is poorly correlated with drug resistance.
 |
Table 4 NTM Mutation M. Intracellulare of LZD |
 |
Figure 4 Gene mutations of linezolid in the 23S rRNA (rrl) among M. intracellulare clinical isolates. Abbreviations: n, the number of mutation isolates. -, Base deletion. |
Discussion
Recently, NTM have attracted increasing attention. However, NTM do not meet the mandatory reporting measures for infectious diseases, and there has been no systematic investigation or research internationally or domestically. A previous study showed that the separation rate of NTM in Jiangsu Province in 2008 was 3.37%, slightly lower than the average level in Shanghai.24 However, in the study by Hu et al, the isolation rate of NTM in Nanjing was 15% from 2017 to 2018, indicating that the infection rate of NTM in Nanjing was on the rise.25
In this study, we analyzed the characteristics of NTM infections at Nanjing Public Health Medical Center from 2019 to 2021. M. intracellulare was the main species causing NTM infections in patients in Nanjing from 2019 to 2021, followed by M. avium and M. abscessus. Zhou et al conducted a systematic review study and meta-analysis on the prevalence of NTM in Mainland China from 2000 to 2019, and the study showed that the dominant species in China changed from M. abscessus to M. intracellulare after 2015.2 Hu et al studied M. intracellulare, M. abscessus, and M. avium in patients diagnosed with NTM lung disease in Nanjing from 2017 to 2018, and found that NTM infection was related to age but not gender.25 Our study found that without distinguishing between NTM colonized patients and confirmed patients, there was a significant gender difference in the population with detected NTM, suggesting that the chance of NTM infection was gender-related. Especially in 30–49-year-olds, males are more likely to be infected with NTM than females.
There was a significant difference between in the rate of M. avium and M. kansasii infection between males and females (P=0.000, < 0.05). Males had a higher rate of infection with M. avium and M. kansasii. Among those infected with M. avium and M. kansasii, 46.6% and 22.2% of patients had a history of HIV infection, respectively. Studies have shown that patients with immunodeficiency diseases are more susceptible to M. avium and M. kansasii diseases.7,26 This study found that there was no significant difference in the rate of M. intracellulare infection between males and females, and M. abscessus infection was more likely to occur in female patients. Other studies have shown that NTM is more likely to occur in older female patients.25,27 In this study, M. abscessus infection was more likely to occur in older female patients, which may be related to the NTM species.25,27
In recent years, NTM infection has increased both within China and worldwide, especially M. abscessus infection, which has aroused widespread concern.28–30 Studies have shown that M. abscessus can be transmitted from person to person.3,5 From 2019 to 2021, the number of NTM infected patients in Nanjing decreased, which may be related to the COVID-19 epidemic. Both have aerosol transmission routes, and the risk of NTM infection may be reduced if specific protective measures are taken. However, in this study, it is worth noting that the number of M. abscessus infections has increased, indicating that M. abscessus infection is still not negligible.
In this study, we measured the in vitro activities of bedaquiline, clofazimine, linezolid, delamanid, and pretomanid against M. abscessus and M. intracellulare. We also sequenced correlations between drug resistance-related genes. Bedaquiline showed a great effect on M. intracellulare in vitro, with MICs between 0.004 and 0.512 μg/mL. Bedaquiline is a diarylquinoline compound that mainly acts on the ATP synthase of bacteria, which in turn affects the synthesis of ATP in bacteria.16 In recent years, in vitro studies of bedaquiline against NTM have been reported.31–33 In addition, in these studies, bedaquiline was more active against NTM, with MICs of <1 μg/mL for M. intracellulare and M. avium, and MIC <2 μg/mL for M. abscessus.31–34 Bedaquiline had a two-fold higher MIC against M. abscessus than against M. intracellulare,31 which was also confirmed in our study. Clofazimine, a drug used to treat Mycobacterium leprae, is currently used to treat NTM. In several in vitro drug susceptibility studies,35,36 clofazimine has shown strong activity against NTM. In the present study, we measured the activity of clofazimine against M. abscessus and M. intracellulare. In a study in Korea, the MIC50 and MIC90 for M. abscessus were 4 mg/L and 8 mg/L, respectively.35 In our study, MIC50 at 2μg/mL and MIC90 at 8μg/mL to M. abscessus, whereas clofazimine has a MIC50 and MIC90 of 0.125μg/mL and 0.5μg/mL for M. intracellulare. We found that the MIC50 and MIC90 values of clofazimine for M. abscessus were greater than those for M. intracellulare. Furthermore, in the study in Korea, the MIC of clofazimine is closely related to the clinical prognosis of NTM pulmonary (NTM-PD), and an MIC ≤ 0.25 μg/mL of clofazimine was used for NTM-PD patients.35 In a prospective study on the use of clofazimine against NTM, the addition of clofazimine to the treatment regimen for drug-resistant NTM infection resulted in successful treatment in most patients.33 Linezolid is an oxazolidinone compound used primarily for the treatment of diseases caused by gram-positive bacteria and has been used in recent years for the treatment of multidrug-resistant tuberculosis. In this study, the MIC50 of linezolid against M. abscessus was 8 μg/mL and MIC90 was 32 μg/mL. In some studies, linezolid showed general activity against NTM.37–40 In research by Yu et al and Wen et al,37,38 the susceptibility of slow-and rapidly growing mycobacteria to linezolid was investigated in Beijing, where linezolid had a low MIC value against M. kansasii. An MIC90 value of 32 μg/mL was determined for M. intracellulare as well as Mycobacterium avium complex, along with an MIC90 of 16 μg/mL for M. abscessus. In our study, the results showed an MIC90 of 32 μg/mL for M. abscessus and M. intracellulare for linezolid. Delamanid showed some activity against slow-growing mycobacteria, but little activity against fast-growing mycobacteria, especially M. abscessus. Our study was in agreement with the results of Yu et al.32 Delamanid had strong activity only against M. kansasii.41 Delamanid was active against some slow-growing mycobacteria and almost inactive against fast-growing mycobacteria. A few studies have suggested that delamanid has some activity against the M. avium complex in vitro.42 However, in this study, the MIC of delamanid was >32 μg/mL against M. avium, M. intracellulare, and M. abscessus. In a study by Yu et al, M. gordonii showed sensitivity to delamanid.32 The two strains of M. gordonii used in our study also showed delamanid sensitivity. PA-824 is a drug of the same type as delamanid and is used for the treatment of multidrug-resistant tuberculosis. In a study conducted in Beijing, all NTM included in the study were resistant to PA-824.43 The findings in our study for M. intracellulare and M. abscessus were consistent with this study. Notably, two strains of M. gordonii were found to have low MIC values for PA-824 and delamanid.
The main genes known to be associated with resistance in Mycobacterium tuberculosis to bedaquiline are atpE, Rv0678, Rv1979C, and pepQ.12,44 In M. abscessus, MAB_1448, MAB_4384, and MAB_2299c are the genes reported to be potentially associated with bedaquiline resistance.13,17 For most strains, the minimal bactericidal concentration (MBC) of bedaquiline was higher than the MIC, indicating strong bacterial inhibition.34 In the study by Aguilar-Ayala et al, they sequenced the atpE gene of all strains, and results indicated that mutations in the atpE gene were associated with bedaquiline resistance.34 In a study on the distribution of the MIC of bedaquiline for M. abscessus and determination of the mechanism of reduced drug susceptibility, no atpE mutation was found in M. abscessus.45 MAB_4384 of M. abscessus was homologous to MmpS5/MmpL5 and Rv0678 of Mycobacterium tuberculosis.45 This suggests that the mechanism of resistance to bedaquiline in M. abscessus is different from that of Mycobacterium tuberculosis. Mutations in MAB_4384 resulted in reduced susceptibility to bedaquiline.45 In this study, we found no mutations in MAB_4384. Only one M. abscessus isolate was found to have a code-shifting mutation with a base insertion at site 13 of MAB_1448.
Among the M. abscessus mutations of clofazimine resistance, the main mutated genes were MAB_0540, MAB_1483, and MAB_2299c,18 of which MAB_2299c was confirmed to be associated with cross-resistance to bedaquiline and clofazimine.17 In vitro activity studies of clofazimine against nontuberculous Mycobacterium avium isolated from Beijing revealed unidirectional cross-resistance between bedaquiline- and clofazimine-resistant strains.46 In our study, clofazimine showed strong antibacterial activity against M. abscessus and M. intracellulare. In novel mutation studies of clofazimine resistance in M. abscessus, MAB_2299c, MAB_1483, and MAB_0540 are the major mutated genes.18 In a genome-wide study, a large deletion between position 0 and 45 was found in MAB_0540,18 and a deletion between position 30 and 41 was also found in our sequencing study. In addition, mutations at other sites of MAB_0540 were found in our study, which may have caused amino acid changes. Unfortunately, how this gene affects the mechanism of drug action remains unclear and further studies are needed.
The primary genes associated with linezolid resistance were rplC, rplD, and rrl. Ye et al15 performed a molecular analysis of M. abscessus associated with linezolid resistance and compared it with the standard M. abscessus ATCC19977 strain and found nine mutations in the 23SrRNA gene associated with linezolid resistance. Other mutations in both resistant and sensitive strains were found which were not associated with linezolid resistance.15 In our study, none of the strains of M. abscessus resistant to linezolid were found to have mutations associated with linezolid resistance. In a Korean study, molecular analysis of the linezolid-resistant Mycobacterium avium complex identified two novel mutations associated with the 23SrRNA gene, G2599A and A2137T.47 In our study, neither of these mutations was found in linezolid-resistant strains of M. intracellulare.
Conclusion
In conclusion, we clarified the distribution of NTM in Nanjing from 2019 to 2021 and determined the in vitro drug susceptibilities of NTM to bedaquiline, clofazimine, linezolid, delamanid, and pretomanid. Furthermore, we sequenced the major resistance genes in the drug-resistant strains. From 2019 to 2021, M. intracellulare was the most prevalent strain in Nanjing, followed by M. avium and M. abscessus. M. intracellulare and M. abscessus were more susceptible to bedaquiline, clofazimine, and linezolid in vitro. In addition, we found 30–41 nucleotide deletion mutations and other novel point mutations in the MAB_0540 gene of M. abscessus resistant to clofazimine.
Abbreviations
NTM, nontuberculous mycobacteria; MDR-TB, multi-drug resistant tuberculosis; MIC, minimal inhibitory concentration; BDQ, bedaquiline; CFZ, clofazimine; LZD, linezolid; DLM, delamanid; PA-824, pretomanid; MTC, Mycobacterium tuberculosis complex; CI, confidence interval; HIV, Human immunodeficiency virus; NTM-PD, nontuberculous mycobacteria pulmonary.
Data Sharing Statement
Data relating to this study are contained and presented in this document. Other materials are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study protocol was approved by Nanjing Center for Disease Control and Prevention Ethics Committee (approval number: PJ2020-A001-04; approval date: 17 August, 2020). Patient information collected in the case system did not contain name, address or other personal information, so the patient’s written informed consent was exempt. The study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Medical Science and Technology Development Foundation, Nanjing Department of Health (GrantZKX19048).
Disclosure
The authors declare that they have no competing interests.
References
1. Koh W-J, Schlossberg D. Nontuberculous mycobacteria-overview. Microbiol Spectr. 2017;5(1). doi:10.1128/microbiolspec.TNMI7-0024-2016
2. Zhou L, Xu D, Liu H, Wan K, Wang R, Yang Z. Trends in the prevalence and antibiotic resistance of non-tuberculous mycobacteria in Mainland China, 2000–2019: systematic review and meta-analysis. Front Public Health. 2020;8:295. doi:10.3389/fpubh.2020.00295
3. Johansen MD, Herrmann J-L, Kremer L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol. 2020;18(7):392–407. doi:10.1038/s41579-020-0331-1
4. Dahl VN, Mølhave M, Fløe A, et al. Global trends of pulmonary infections with nontuberculous mycobacteria: a systematic review. Int J Infect Dis. 2022;125:S1201971222005513.
5. Ratnatunga CN, Lutzky VP, Kupz A, et al. The rise of non-tuberculosis mycobacterial lung disease. Front Immunol. 2020;11:303. doi:10.3389/fimmu.2020.00303
6. Shahraki AH, Heidarieh P, Bostanabad SZ, et al. “Multidrug-resistant tuberculosis” may be nontuberculous mycobacteria. Eur J Intern Med. 2015;26(4):279–284. doi:10.1016/j.ejim.2015.03.001
7. McCarthy KD, Cain KP, Winthrop KL, et al. Nontuberculous mycobacterial disease in patients with HIV in Southeast Asia. Am J Respir Crit Care Med. 2012;185(9):981–988. doi:10.1164/rccm.201107-1327OC
8. Henkle E, Winthrop KL. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015;36(1):91–99. doi:10.1016/j.ccm.2014.11.002
9. Ahmed I, Tiberi S, Farooqi J, et al. Non-tuberculous mycobacterial infections-A neglected and emerging problem. Int J Infect Dis. 2020;92S:S46–S50. doi:10.1016/j.ijid.2020.02.022
10. Mirzayev F, Viney K, Linh NN, et al. World Health Organization recommendations on the treatment of drug-resistant tuberculosis, 2020 update. Eur Respir J. 2021;57(6):2003300. doi:10.1183/13993003.03300-2020
11. Wu M-L, Aziz DB, Dartois V, Dick T. NTM drug discovery: status, gaps and the way forward. Drug Discov Today. 2018;23(8):1502–1519. doi:10.1016/j.drudis.2018.04.001
12. Khoshnood S, Goudarzi M, Taki E, et al. Bedaquiline: current status and future perspectives. J Global Antimicrobl Resist. 2021;25:48–59. doi:10.1016/j.jgar.2021.02.017
13. Vesenbeckh S, Schönfeld N, Roth A, et al. Bedaquiline as a potential agent in the treatment of Mycobacterium abscessus infections. Eur Respir J. 2017;49(5):1700083. doi:10.1183/13993003.00083-2017
14. Pfaeffle HOI, Alameer RM, Marshall MH, Houpt ER, Albon DP, Heysell SK. Clofazimine for treatment of multidrug-resistant non-tuberculous mycobacteria. Pulm Pharmacol Ther. 2021;70:102058. doi:10.1016/j.pupt.2021.102058
15. Ye M, Xu L, Zou Y, et al. Molecular analysis of linezolid-resistant clinical isolates of Mycobacterium abscessus. Antimicrob Agents Chemother. 2019;63(2):e01842–01818. doi:10.1128/AAC.01842-18
16. Dupont C, Viljoen A, Thomas S, et al. Bedaquiline inhibits the ATP synthase in Mycobacterium abscessus and is effective in infected zebrafish. Antimicrob Agents Chemother. 2017;61(11):e01225–01217. doi:10.1128/AAC.01225-17
17. Richard M, Gutiérrez AV, Viljoen A, et al. Mutations in the MAB_2299c TetR regulator confer cross-resistance to clofazimine and bedaquiline in Mycobacterium abscessus. Antimicrob Agents Chemother. 2019;63(1):e01316–e01318. doi:10.1128/AAC.01316-18
18. Chen Y, Chen J, Zhang S, et al. Novel mutations associated with clofazimine resistance in Mycobacterium abscessus. Antimicrob Agents Chemother. 2018;62(7):e00544–00518. doi:10.1128/AAC.00544-18
19. Zhang Q, Xiao H, Yan L. PCR-reverse blot hybridization assay in respiratory specimens for rapid detection and differentiation of mycobacteria in HIV-negative population. BMC Infect Dis. 2021;21(1):264. doi:10.1186/s12879-021-05934-x
20. Woods GL, Brown-Elliott BA, Conville PS, et al. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes.
21. Gene, National Library of Medicine (US), National Center for Biotechnology Information. 2004 . Available from: https://www.ncbi.nlm.nih.gov/gene/.
22. Untergasser A, Cutcutache I, Koressaar T, et al. Primer3--new capabilities and interfaces. Nucleic Acids Research. 2012;40(15):e115. doi:10.1093/nar/gks596
23. Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Research. 2014;42(W320–W324). doi:10.1093/nar/gku316
24. Shao Y, Chen C, Song H, et al. The epidemiology and geographic distribution of nontuberculous mycobacteria clinical isolates from sputum samples in the eastern region of China. PLoS Negl Trop Dis. 2015;9(3):e0003623. doi:10.1371/journal.pntd.0003623
25. Hu C, Huang L, Cai M, Wang W, Shi X, Chen W. Characterization of non-tuberculous mycobacterial pulmonary disease in Nanjing district of China. BMC Infect Dis. 2019;19(1):764. doi:10.1186/s12879-019-4412-6
26. Wang D-M, Liao Y, Li Q-F, et al. Drug resistance and pathogenic spectrum of patients coinfected with nontuberculous mycobacteria and human-immunodeficiency virus in Chengdu, China. Chin Med J. 2019;132(11):1293–1297. doi:10.1097/CM9.0000000000000235
27. Lee H, Myung W, Koh W-J, Moon SM, Jhun BW. Epidemiology of nontuberculous mycobacterial infection, South Korea, 2007–2016. Emerg Infect Dis. 2019;25(3):569–572. doi:10.3201/eid2503.181597
28. Floyd K, Glaziou P, Zumla A, Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir Med. 2018;6(4):299–314. doi:10.1016/S2213-2600(18)30057-2
29. Tan Y, Deng Y, Yan X, et al. Nontuberculous mycobacterial pulmonary disease and associated risk factors in China: a prospective surveillance study. J Infect. 2021;83(1):46–53. doi:10.1016/j.jinf.2021.05.019
30. Liu C-F, Song Y-M, He W-C, et al. Nontuberculous mycobacteria in China: incidence and antimicrobial resistance spectrum from a nationwide survey. Infect Dis Poverty. 2021;10(1):59. doi:10.1186/s40249-021-00844-1
31. Pang Y, Zheng H, Tan Y, Song Y, Zhao Y. In vitro activity of bedaquiline against nontuberculous mycobacteria in China. Antimicrob Agents Chemother. 2017;61(5):e02627–02616. doi:10.1128/AAC.02627-16
32. Yu X, Gao X, Li C, et al. In vitro activities of bedaquiline and delamanid against nontuberculous mycobacteria isolated in Beijing, China. Antimicrob Agents Chemother. 2019;63(8):e00031–00019. doi:10.1128/AAC.00031-19
33. Schulthess B, Akdoğan Kittana FN, Hömke R, Sander P. In vitro bedaquiline and clofazimine susceptibility testing in Mycobacterium abscessus. Antimicrob Agents Chemother. 2022;66(5):e02346–02321. doi:10.1128/aac.02346-21
34. Aguilar-Ayala DA, Cnockaert M, André E, et al. In vitro activity of bedaquiline against rapidly growing nontuberculous mycobacteria. J Med Microbiol. 2017;66(8):1140–1143. doi:10.1099/jmm.0.000537
35. Kwak N, Whang J, Yang JS, Kim TS, Kim SA, Yim -J-J. Minimal inhibitory concentration of clofazimine among clinical isolates of nontuberculous mycobacteria and its impact on treatment outcome. Chest. 2021;159(2):517–523. doi:10.1016/j.chest.2020.07.040
36. Luo J, Yu X, Jiang G, et al. In vitro activity of clofazimine against nontuberculous mycobacteria isolated in Beijing, China. Antimicrob Agents Chemother. 2018;62(7):e00072–00018. doi:10.1128/AAC.00072-18
37. Wen S, Gao X, Zhao W, et al. Comparison of the in vitro activity of linezolid, tedizolid, sutezolid, and delpazolid against rapidly growing mycobacteria isolated in Beijing, China. Int J Infect Dis. 2021;109:253–260. doi:10.1016/j.ijid.2021.06.055
38. Yu X, Huo F, Wang F, et al. In vitro antimicrobial activity comparison of linezolid, tedizolid, sutezolid and delpazolid against slowly growing mycobacteria isolated in Beijing, China. Infect Drug Resist. 2021;14:4689–4697. doi:10.2147/IDR.S332835
39. Zhang Z, Lu J, Song Y, Pang Y. In vitro activity between linezolid and other antimicrobial agents against Mycobacterium abscessus complex. Diagn Microbiol Infect Dis. 2018;90(1):31–34. doi:10.1016/j.diagmicrobio.2017.09.013
40. Kim DH, Kim S-Y, Koh W-J, Jhun BW. In vitro activity of oxazolidinone against nontuberculous mycobacteria, including macrolide-resistant clinical isolates. Antimicrob Agents Chemother. 2021;65(7):e02306–e02320. doi:10.1128/AAC.02306-20
41. Kim DH, Jhun BW, Moon SM, et al. In Vitro activity of bedaquiline and delamanid against nontuberculous mycobacteria, including macrolide-resistant clinical isolates. Antimicrob Agents Chemother. 2019;63(8):e00665–00619. doi:10.1128/AAC.00665-19
42. Krieger D, Schönfeld N, Vesenbeckh S, et al. Is delamanid a potential agent in the treatment of diseases caused by Mycobacterium avium-intracellulare? Eur Respir J. 2016;48(6):1803–1804. doi:10.1183/13993003.01420-2016
43. Zheng H, Wang Y, He W, et al. In vitro activity of pretomanid against nontuberculous mycobacteria. Antimicrob Agents Chemother. 2022;66(1):e01810–e01821. doi:10.1128/AAC.01810-21
44. Omar SV, Ismail F, Ndjeka N, Kaniga K, Ismail NA. Bedaquiline-resistant tuberculosis associated with Rv0678 mutations. N Engl J Med. 2022;386(1):93–94. doi:10.1056/NEJMc2103049
45. Li B, Ye M, Guo Q, et al. Determination of MIC distribution and mechanisms of decreased susceptibility to bedaquiline among clinical isolates of Mycobacterium abscessus. Antimicrob Agents Chemother. 2018;62(7):e00175–00118. doi:10.1128/AAC.00175-18
46. Ruth MM, Sangen JJN, Remmers K, et al. A bedaquiline/clofazimine combination regimen might add activity to the treatment of clinically relevant non-tuberculous mycobacteria. J Antimicrob Chemother. 2019;74(4):935–943. doi:10.1093/jac/dky526
47. Kim S-Y, Jhun BW, Moon SM, et al. Genetic mutations in linezolid-resistant Mycobacterium avium complex and Mycobacterium abscessus clinical isolates. Diagn Microbiol Infect Dis. 2019;94(1):38–40. doi:10.1016/j.diagmicrobio.2018.10.022
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


