Back to Journals » Clinical Epidemiology » Volume 11
Epidemiology of heart failure and trends in diagnostic work-up: a retrospective, population-based cohort study in Sweden
Authors Lindmark K , Boman K , Olofsson M , Törnblom M , Levine A , Castelo-Branco A , Schlienger R , Bruce Wirta S, Stålhammar J , Wikström G
Received 12 April 2018
Accepted for publication 24 January 2019
Published 22 March 2019 Volume 2019:11 Pages 231—244
DOI https://doi.org/10.2147/CLEP.S170873
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Vera Ehrenstein
Krister Lindmark,1 Kurt Boman,2 Mona Olofsson,2 Michael Törnblom,3 Aaron Levine,3 Anna Castelo-Branco,3 Raymond Schlienger,4 Sara Bruce Wirta,5 Jan Stålhammar,6 Gerhard Wikström7
1Department of Public Health and Clinical Medicine and Heart Centre, Umeå University Hospital, Umeå, Sweden; 2Research Unit, Medicine-Geriatric, Skellefteå County Hospital, Department of Public Health and Clinical Medicine, Umeå University, Umeå, Sweden; 3Real-World & Analytics Solutions, IQVIA, Solna, Sweden; 4Quantitative Safety & Epidemiology, Novartis Pharma AG, Basel, Switzerland; 5Global RWE Cardio-Metabolics, Novartis Sweden AB, Stockholm, Sweden; 6Department of Public Health and Caring Sciences, Family Medicine and Preventive Medicine, Uppsala University, Uppsala, Sweden; 7Department for Medical Sciences, Uppsala University, Uppsala, Sweden
Purpose: The purpose of this study was to examine the trends in heart failure (HF) epidemiology and diagnostic work-up in Sweden.
Methods: Adults with incident HF (≥2 ICD-10 diagnostic codes) were identified from linked national health registers (cohort 1, 2005–2013) and electronic medical records (cohort 2, 2010–2015; primary/secondary care patients from Uppsala and Västerbotten). Trends in annual HF incidence rate and prevalence, risk of all-cause and cardiovascular disease (CVD)-related 1-year mortality and use of diagnostic tests 6 months before and after first HF diagnosis (cohort 2) were assessed.
Results: Baseline demographic and clinical characteristics were similar for cohort 1 (N=174,537) and 2 (N=8,702), with mean ages of 77.4 and 76.6 years, respectively; almost 30% of patients were aged ≥85 years. From 2010 to 2014, age-adjusted annual incidence rate of HF/1,000 inhabitants decreased (from 3.20 to 2.91, cohort 1; from 4.34 to 3.33, cohort 2), while age-adjusted prevalence increased (from 1.61% to 1.72% and from 2.15% to 2.18%, respectively). Age-adjusted 1-year all-cause and CVD-related mortality was higher in men than in women among patients in cohort 1 (all-cause mortality hazard ratio [HR] men vs women 1.07 [95% CI 1.06–1.09] and CVD-related mortality subdistribution HR for men vs women 1.04 [95% CI 1.02–1.07], respectively). While 83.5% of patients underwent N-terminal pro-B-type natriuretic peptide testing, only 36.4% of patients had an echocardiogram at the time of diagnosis, although this increased overtime. In the national prevalent HF population (patients with a diagnosis in 1997–2004 who survived into the analysis period; N=273,999), death from ischemic heart disease and myocardial infarction declined between 2005 and 2013, while death from HF and atrial fibrillation/flutter increased (P<0.0001 for trends over time).
Conclusion: The annual incidence rate of HF declined over time, while prevalence of HF has increased, suggesting that patients with HF were surviving longer over time. Our study confirms that previously reported epidemiological trends persist and remain to ensure proper diagnostic evaluation and management of patients with HF.
Keywords: diagnosis, heart failure, incidence rate, mortality, prevalence, real-world
Plain language summary
Advances in the management of heart failure (HF), including diagnostic procedures and treatments, together with a growing elderly population, show that it is necessary to assess changing trends in the epidemiology of this debilitating condition. This study assessed trends in the annual incidence rate and prevalence and mortality of HF and examined the diagnostic work-up of patients with HF in Sweden using data derived from national health registers and regional electronic medical records from the counties of Uppsala and Västerbotten. The results show that between 2010 and 2014, the annual incidence rate of HF declined nationally and regionally in Sweden while the prevalence of HF increased, indicating improvements in the management of underlying risk factors and an increasingly aging population living with HF. The overall decreased contribution from ischemic heart disease and myocardial infarction as causes of death further reinforce the improvement in underlying risk factors. Nevertheless, we showed that patients experienced relatively high all-cause and cardiovascular-related mortality. Most patients (almost two thirds) had not received an echocardiogram in the 6 months before and after their HF diagnosis, a trend that was improving over time but still did not exceed half of newly diagnosed patients. These results suggest that there is a growing population of elderly patients with HF in Sweden and that appropriate diagnostic work-up in these patients is not ideal while slowly improving.
Introduction
Heart failure (HF) poses a major clinical and public health challenge globally, affecting ~40 million people worldwide in 2015.1 As one of the leading causes of hospitalizations, morbidity, and mortality, particularly among the elderly, HF is associated with considerable suffering of individual patients and a financial burden for healthcare systems.2
The prevalence of HF depends on the definition used as well as on the age distribution of the population being assessed, but in Western countries it is estimated to affect ~1%–2% of the adult population,3–6 while the incidence rate is generally estimated to be 2–5/1,000 person-years.4,7 A Swedish study conducted in patients hospitalized with HF showed a decrease in hospitalization rates from 1993 to 2000 and a temporal decrease in 1-year mortality.8 In addition, data from an urban population in Sweden between 2006 and 2010 showed no major change in prevalence but a slight decline in annual incidence rate of HF.5 Despite improvements being observed in the survival of patients with HF over recent years, overall prognosis remains poor,2,9 with survival estimates of ~50% at 5 years after initial diagnosis of HF.10–12 Of particular concern is the increasing trend of younger adults (18–54 years) being hospitalized for HF.10,13
Diagnosis of HF is challenging, particularly so in the elderly because many of the characteristic signs and symptoms are non-specific and serve only to raise suspicion of HF rather than to give a definitive diagnosis, thus limiting their diagnostic value.14,15 With evolving changes in patient demographics and overall HF management, including diagnostic procedures and treatment regimens, there is need for an improved understanding of the temporal trends in the epidemiology of HF. In particular, an insight into changes in HF annual incidence rate and prevalence is necessary to inform healthcare stakeholders on the burden of HF to determine its impact on allocation of hospital resources.
In this study, we confirm trends in the incidence rate and prevalence of HF in more recent years, and evaluate the use of recommended diagnostic tests in patients with newly diagnosed HF, and trends in mortality in the HF population. Key questions that this analysis aims to answer include: 1) are the annual incidence rate and prevalence of HF increasing or declining in Sweden, both nationally and regionally? 2) how does the diagnostic work-up of patients in real clinical practice compare with recommendations made in management guidelines and does this change over time? 3) have advances in the treatment of HF translated into patients living longer?
Patients and methods
Study design
This was a retrospective, non-interventional cohort study using national and regional longitudinal, patient-level data for patients with HF in Sweden. Since the care of patients with HF varies, with some patient groups being managed predominantly in a hospital setting and others in primary care, we examined two different cohorts so that we may highlight all aspects of HF management: a national cohort of all patients with HF in secondary care in Sweden (inpatient and outpatient); and a regional cohort consisting of patients from two different counties in Sweden, encompassing electronic medical records (EMR) data from both primary and secondary care. Data were extracted from national health registers, including the National Patient Register (NPR), the National Dispensed Drug Register, and the Cause of Death Register, as well as from EMRs and local echocardiography (echo) registries from the Swedish counties of Uppsala and Västerbotten. Data were linked based on the unique national personal identification numbers issued by the National Board of Health and Welfare. Data were anonymized before the linked database was released to the research group. Ethical approval was obtained from the regional Ethical Review Board in Uppsala, Sweden (2015-045) before data were extracted. No informed consent was required for this retrospective, anonymized study.
Patients
Adult patients who were aged ≥18 years and who had at least two documented HF diagnoses that were treated in primary and secondary care settings were included in the study. The opportunity to analyze both primary and secondary care patients by linking the Swedish NPR data with EMR (PC) data encompasses the challenge of distinct HF diagnosis specificity across the two settings. In order to overcome this challenge, two HF diagnoses were required to reduce the uncertainty surrounding HF diagnosis in primary care and increase overall diagnosis specificity. This criteria has been consistently applied across the two cohorts analyzed. A diagnosis of HF was defined as having International Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) diagnostic codes of I50 (inclusive of all granular codes), I42.0, I42.6, I42.7, I42.9, I11.0, I13.0, or I13.2 as primary or secondary diagnoses. Eligible patients were classified into two cohorts. Cohort 1 (the national cohort) comprised patients with HF diagnoses registered in the NPR from secondary care between January 2005 and December 2013, both hospitalizations and outpatient visits, while cohort 2 (the regional cohort, comprising patients from the counties of Uppsala and Västerbotten) included patients with HF diagnoses registered in EMRs from either primary or secondary care between January 2010 and March 2015. The Pygargus Customized eXtraction Program (CXP 3.0) was used to extract data from EMRs in Uppsala (two hospitals and 46 primary care centers [PCCs]) and Västerbotten (three hospitals and 37 PCCs) that was subsequently linked to data from the national health registers including the NPR. HF phenotype (ie, HF with preserved ejection fraction [HFpEF] and HF with reduced ejection fraction [HFrEF]) was determined based on data from local echo registries for cohort 2 (but not for cohort 1 since local echo data were unavailable in the NPR). HFpEF was defined as a left ventricular ejection fraction (LVEF) of at least 50% and HFrEF as a LVEF of <50%.
Data extraction and study timelines
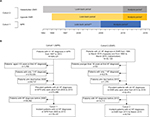
Patient data for cohort 1 were extracted from the NPR based on an observed HF diagnosis between 1997 and 2013 and for cohort 2 from EMRs and NPR on the basis of an observed HF diagnosis in 1994–2015 for Uppsala County and 1992–2016 for Västerbotten County. The study inclusion (analysis) period was January 1, 2005 to December 31, 2013 for cohort 1, which was extended to December 31, 2014 for the analyses of annual incidence rate and prevalence; and January 1, 2010 to March 31, 2015 for cohort 2 (Figure 1A). When defining baseline patient characteristics and evaluating the use of diagnostic procedures, the index date was defined as the date of first HF diagnosis since it was expected that around this time more tests and procedures would be performed. However, for analyzing the incidence rate, prevalence, and mortality, the index date was defined as a patient’s second HF diagnosis to improve accuracy of prevalence and incidence and avoid immortal time bias. Follow-up was defined as the period between the second HF diagnosis and the end of the study, end of EMR collection for those patients who moved to another region, or date of death, whichever came first.
For each cohort, patient records were reviewed during a “look-back” period that preceded the analysis period to exclude prevalent HF cases. For cohort 1, the look-back period in the NPR extended from January 1, 1997 for inpatient care and January 1, 2001 for outpatient care, ending December 31, 2004. For cohort 2, the look-back period in EMR data was from first data available until December 31, 2009, while for NPR data it extended from the same time point as for cohort 1, ending December 31, 2009 (Figure 1). As such, patients in whom HF was diagnosed during the look-back period and who survived into the analysis period were classified as the prevalent HF population, while patients in whom HF was diagnosed during the analysis period were classified as the incident HF population.
Variables analyzed and statistical analyses
All analyses, except for prevalence and trends in mortality for the national prevalent population, were performed on incident patients. Baseline patient demographics and clinical characteristics, as well as baseline laboratory measures, were described using descriptive statistics. Comorbidities, summarized for both cohorts based on a predefined list of ICD-10 codes (Table S1), were collected from both primary (EMR) and secondary care (EMR and NPR) data, using the primary and secondary diagnoses of all healthcare visits that occurred 0–5 years before the index date. The Charlson comorbidity index (CCI) scores at 0–5 years prior to index, ranging from 0 to >10 (where higher scores indicate greater comorbidity), were calculated for each cohort. Crude, age-specific, and age-adjusted annual incidence rate and prevalence of HF were estimated for the years 2010–2014 at a national level (cohort 1; NPR) and regional level for the counties of Uppsala and Västerbotten (cohort 2; NPR and EMR). Data for the population at risk and the distribution of age in the Swedish population were sourced from Statistics Sweden’s population statistics.16 Crude annual incidence rate was estimated as the total number of patients in whom HF was diagnosed in a calendar year divided by the official adult population on 31 December of the year being analyzed. Crude annual prevalence was estimated as the total number of patients with HF who were alive during a calendar year and as a percentage of the total population on 31 December of the year under analysis. Age-adjusted annual incidence rate and prevalence were estimated as the weighted average of age-specific values using population weights based on the Swedish official population from 2015.16 The annual incidence rate is presented as the number of cases per 1,000 inhabitants per year and includes 95% CIs, while prevalence is presented as a percentage of the Swedish adult population and 95% CI.
The diagnostic work-up of patients in cohort 2 was described based on data of performed diagnostic tests, ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) testing and echo. Of note, local echo registries did not include private practices in Uppsala County or the smallest hospital in Västerbotten County. The number of patients in whom each test was performed during the 6 months before and after the first HF diagnosis (index date) are presented by year of diagnosis and stratified by age, sex, and setting of first HF diagnosis.
All-cause mortality, as determined by Kaplan–Meier-estimated death rates, was estimated for both cohorts while cardiovascular disease (CVD)-related mortality was estimated through cumulative incidence function. CVD-related mortality was identified as the underlying main cause of death within the NPR and it is defined as through a list of ICD-10 codes capturing a broad array of cardiovascular-related disease (D50–D53, D55–D64 [anemia], I48 [atrial fibrillation], I60–I69 [cerebrovascular disease], N18 [chronic kidney disease], J40–J44, J47 [COPD], E10–E14 [diabetes], E78 [dyslipidemia], I10–I13, I15 [hypertension], I20–I25 [ischemic heart disease], I73.9 [peripheral artery disease], I61–I64 [stroke]). All-cause mortality was modeled by Cox proportional hazards regression with comparison groups stratified by age group (reference group: 18–54 years), sex (reference group: female), and year of HF diagnosis (reference group: 2005, cohort 1; 2010, cohort 2), as well as setting of HF diagnosis (reference group: primary care), HF phenotype (reference group: HFpEF), and NT-proBNP level (reference group: 0–300 pg/mL) for cohort 2. Similarly, CVD-related mortality was modeled by Fine and Gray model, which accounts for competing risks from other causes of death.17 HRs, adjusted for the variables mentioned, and 95% CIs were estimated for all mortality statistics. Additional analyses of trends in mortality in cohort 1 were conducted, including causes of death in the prevalent HF population, as well as patient characteristics and trends in HF treatment by year of diagnosis in the incident population. Trends in mortality and causes of death, as well as trends in pharmacological management of patients were tested using the Cochran-Armitage test, while trends in patient characteristics were tested using a linear regression method. It should be noted that the trends described for cause of death are based on the number of deaths during a specific year, in relation to the number of patients alive at the beginning of that year.
SAS version 9.3 or higher was used for statistical analysis and data management.
Results
Study population
A total of 584,021 patients with a diagnosis of HF were identified from the Swedish NPR (cohort 1) and 33,120 patients from the Uppsala County and Västerbotten County EMRs (cohort 2; Figure 1B). Of these patients, 273,999 in cohort 1 and 22,812 in cohort 2 had at least two HF diagnoses during the respective analysis periods and were alive at the beginning of the period (Figure 1B). The incident patients of each cohort corresponded to those with no HF diagnoses during the respective look-back periods: 174,537 patients in cohort 1 and 8,702 in cohort 2 (Figure 1B). Among the incident patients in cohort 1, 80% received their first diagnosis in the inpatient setting and 20% in the outpatient specialist setting (Table 1). In comparison, in cohort 2, where also primary care data were available, 53% received their first diagnosis in the inpatient setting, 25% in the outpatient specialist and 17% in the primary care setting (Table 1). Furthermore, in cohort 2, 3,998 patients (46%) had at least one HF diagnosis recorded from primary care, of which 767 patients (9%) had exclusively primary care diagnoses, suggesting that they were never seen by a cardiologist. Altogether, information on HF phenotype was available for 3,167 (36.4%) patients in cohort 2; of these, 2,047 (64.6%) were classified as having HFrEF and 1,120 (35.4%) were classified as having HFpEF (Table 1).
Annual incidence rate and prevalence of HF (2010–2014)
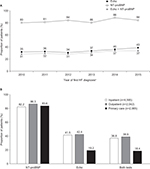
In cohort 1, the age-adjusted annual incidence rate (95% CI) of HF per 1,000 inhabitants in the incident HF population decreased from 3.20 (3.16–3.24) in 2010 to 2.91 (2.87–2.95) in 2014, while the age-adjusted prevalence of HF in the prevalent HF population increased from 1.61% (1.60–1.62) to 1.72% (1.71–1.72) over the same time period (Figure 2A). The age-adjusted annual incidence rate and prevalence of HF were higher in men than in women in every year analyzed (Table S2). In general, when incidence rate and prevalence were analyzed by age group, similar trends in those seen for the overall cohort were observed, and rates were highest for patients aged ≥85 years (Figure 2B).
  | Figure 2 Annual incidence per 1,000 inhabitants and prevalence of HF during 2010–2014 in Sweden (cohort 1) and in the counties of Uppsala and Västerbotten (cohort 2). Notes: (A) Total age-adjusted; and (B) crude incidence and prevalence by age group. Estimates are based on data from the NPR (cohorts 1 and 2) and from primary and secondary care EMRs (cohort 2) for the period 2010–2014. Age-adjusted incidence and prevalence calculated using population weights based on the Swedish population in 2015.16 aSecond HF diagnosis used as the index date. Abbreviations: EMRs, electronic medical records; HF, heart failure; NPr, National Patient Register. |
Taking diagnoses in both primary and secondary care into account, the incidence rate and prevalence of HF were higher in cohort 2 than in cohort 1 (Figure 2A). In 2010, the age-adjusted annual incidence rate of HF was 4.34 (95% CI: 4.15–4.52) in the cohort 2 incident population, which declined to 3.33 (95% CI: 3.16–3.49) in 2014. In the prevalent population, age-adjusted prevalence in 2010 and 2014 were 2.15 (95% CI: 2.11–2.19) and 2.18 (95% CI: 2.14–2.23), respectively (Figure 2A). The overall trends mirrored those seen in cohort 1, and the incidence rate and prevalence were higher in men than in women (Table S1). HF incidence rate and prevalence in cohort 2 were highest in the very elderly patients (aged ≥85 years); however, the prevalence of HF in older patients (aged ≥75 years) in cohort 2 declined slightly in 2014 compared with that in 2010, whereas in patients aged between 18 and 74 years, there was a trend for increasing prevalence over time, as observed for cohort 1 (Figure 2B).
Incident patient baseline demographics and clinical characteristics
In general, patient demographic and clinical characteristics for cohorts 1 and 2 were similar, with a higher proportion of men in each cohort (cohort 1, 53.1%; cohort 2, 54.0%; Table 1). Mean ages in cohorts 1 and 2 were 77.4 and 76.6 years, respectively, and almost 30% of patients in both cohorts were aged ≥85 years. When cohort 2 was stratified by LVEF, patients with HFpEF were found to be older than those with HFrEF (mean, 74.2 and 69.9 years, respectively), although patients with unknown LVEF had the highest mean age (79.5 years; Table 1). Of those with a recorded LVEF, 573 of a total of 1,241 (46.2%) women had HFpEF, compared with 547 of 1,926 (28.4%) men. Notably, the subgroup of patients with HFrEF had higher mean levels of NT-proBNP, ferritin, and hemoglobin, in addition to higher pulse rate and estimated glomerular filtration rate, than patients in the HFpEF and unknown LVEF subgroups (Table 2). Systolic blood pressure was higher in patients with HFpEF and in those with unknown LVEF than in those with HFrEF (Table 2).
The most prevalent comorbidities observed for cohorts 1 and 2 during the 5-year period before the first HF diagnosis were hypertension, atrial fibrillation (AF), and ischemic heart disease (IHD), defined as angina or myocardial infarction (MI) (Table 1). In cohort 2, the prevalence of comorbidities tended to be higher in patients with HFpEF than in those with HFrEF, except for IHD (16.9% vs 22.2% for HFpEF vs HFrEF, respectively), cerebrovascular disease (9.2% vs 9.4%) and peripheral artery disease (2.1% vs 2.6%). Compared with patients in the HFpEF subgroup, those with unknown LVEF had, in general, a higher prevalence of comorbidities, including stroke, hypertension, cerebrovascular disease, IHD, and cognitive disorders (dementia and Alzheimer’s disease).
Analysis of baseline patient characteristics of the incident population by year of HF diagnosis in cohort 1 showed significant trends (P<0.0001) for increasing mean CCI and a decline in the proportion of patients with MI at 0–5 years before diagnosis (Table S3).
Diagnostic work-up in cohort 2
The proportion of patients in cohort 2 receiving NT-proBNP testing, an echo, or both tests generally increased over time between 2010 and 2015, while consistently fewer than half of newly diagnosed patients received an echo across all years (Figure 3A). Overall, during this period, 7,264/8,702 (83.5%) patients underwent NT-proBNP testing in the period starting 6 months before and ending 6 months after the first HF diagnosis. By contrast, an echo was identified for only 3,167 patients (36.4%). Approximately one third of all patients (32.9%) underwent both tests. Patients receiving a diagnosis of HF in primary care were less likely to have an echo than those receiving a diagnosis of HF in either inpatient or outpatient secondary care (Figure 3B); however, echo testing was conducted in fewer than half of the patients with a first diagnosis of HF in secondary care. The use of NT-proBNP testing was similar, regardless of sex (data not shown), although men were more likely than women to have echo testing (n=1,926/4,695 [41.0%] vs n=1,241/4,007 [31.0%], respectively) and to receive both tests (36.6% vs 28.6%).
Trends in all-cause and CVD-related mortality
Cohort 1 (incident patient population)
An increase in 1- and 3-year all-cause mortality and CVD-related mortality (Tables 3 and S4) was observed with increasing age (HR 11.38 [95% CI 10.41–12.44] for all-cause mortality in patients aged 85 and above vs patients aged 18–54 years, and sub-distribution HR (SHR) 14.89 [95% CI 12.97–17.09] for CVD-related mortality comparing the corresponding age groups). Furthermore, both 1-year all-cause and CVD-related adjusted mortality were higher in men compared with women (HR 1.07 [95% CI 1.06–1.09] and SHR 1.04 [95% CI 1.02–1.07], respectively) (Table 3). Finally, 1-year all-cause and CVD-related mortality showed a decreasing trend by year of diagnosis (Table 3). A sensitivity analysis of the mortality trends analyzed in cohort 1 (Tables 3 and S4) has been performed, where the inclusion criteria of having at least two HF diagnoses has been relaxed to only requiring at least one HF diagnosis. The analysis includes a total of 282,893 patients and results are presented in Tables S5 and S6.
Cohort 1 (prevalent patient population)
For the prevalent HF population, there was a gradual but statistically significant decline in all-cause and CVD-related mortality from 2005 (17.7% and 15.0%, respectively) to 2013 (15.5% and 12.9%, respectively; P<0.0001). CVD-related mortality accounted for most all-cause deaths (83.4%–85.0%).
The four most common causes of death in the prevalent HF population in cohort 1 were chronic IHD, MI, HF, and AF/flutter (Figure 4). While death from chronic IHD and MI tended to decline between 2005 and 2013, death resulting from HF and from AF/flutter increased (P<0.0001 for trends over time). Additional common causes of death that changed significantly over time were unspecified diabetes mellitus, which decreased (P=0.0417), and stroke (P=0.0072) and non-rheumatic aortic valve disorder (P=0.0023), both of which increased.
Cohort 2 (incident patient population)
As seen in cohort 1, estimates for all-cause and CVD-related mortality in the incident patient population at 1 and 3 years post-HF diagnosis were higher for women than for men, although no statistically significant difference in the risk (HR) of 1-year mortality was observed between sexes (Tables 4 and S7). Statistically significant associations in 1-year all-cause and CVD-related mortality were found with increasing patient age (aged ≥75 years), diagnosis of HF in secondary care compared to primary care and increased NT-proBNP level around the time of diagnosis (>3,000 pg/mL; Tables 4 and S7). When compared with the HFpEF subgroup, HFrEF was associated with a reduced risk of 1-year all-cause mortality (HR 0.77 [95% CI 0.62–0.96]), with the corresponding HR for HFpEF vs unknown LVEF of 0.85 (95% CI 0.70–1.02). However, the trend was opposite for CVD-related mortality, while the 95% CI spanned 1. (Table 4). Finally, no differences were observed for all-cause or CVD-related 1-year mortality, when comparing year of diagnosis.
Discussion
Our data show that between 2010 and 2014 the age-adjusted annual incidence rate of HF in Sweden was decreasing, while prevalence was increasing both in the national cohort and in the counties of Uppsala and Västerbotten. In addition, the majority of newly diagnosed patients received their first diagnosis in the inpatient setting, while very few were managed exclusively in primary care. There seems to be a discrepancy between recommendations in management guidelines and clinical practice in the diagnostic work-up; NT-proBNP testing was widespread and used for diagnosis in the majority of patients, while echo seemed to be underutilized, especially in patients diagnosed in primary care. Although over time we observed an increasing use of echo to determine diagnosis. Finally evaluation of mortality in both incident and prevalent populations, indicated that patients indeed live longer with their HF, while the mortality rate remains substantial.
The observed overall epidemiological trends align with results from previous reports. In a cross-sectional study using administrative health data from primary and secondary care during 2006–2010, based on data from 2.1 million inhabitants in Stockholm County, Sweden, the prevalence of chronic HF remained largely unchanged, whereas the annual incidence rate declined by 24% in the same period.5 Compared to the study by Zarrinkoub et al,5 the results from this study are newer and reflect the latest trends as well as the whole population instead of a single urban district. Our work also has the benefit of a confirmatory cohort with EMR data to add to the validity. The increase in prevalence that we observe is perhaps the most striking difference. Similarly, a review reporting the results of numerous HF studies showed a decline in the incidence rate of HF since the mid-1990s and an increase in HF prevalence, with additional recent studies confirming these trends.9,18,19 Combined, these findings suggest potential improvements in both the management of IHD, hypertension, and other underlying heart diseases and the treatment of HF following diagnosis, leading to an increasingly aging patient population with HF. Notably, we did not observe an increasing incidence rate among younger adults, as previously described by Barasa et al and Christiansen et al.10,13 In these studies, however, an increasing HF incidence rate was observed in hospitalized patients and therefore may not have reflected a true increased incidence rate in young adults, but a trend for increased inpatient care. Finally, our observation that over half of patients in cohort 2, which covers primary care, received their first diagnosis in the inpatient setting is worrisome, provided the poor prognosis of patients reported by Koudstaal et al20
The increase in HF prevalence over time in the current study complements the small but progressive decline in all-cause mortality observed in the national prevalent HF population between 2005 and 2013. This was also the case for newly diagnosed patients, where relative to 2005, the HR for 1-year all-cause and CVD mortality declined, although still coupled with a high mortality rate. We also examined the most common causes of death between 2005 and 2013 in the national HF population, which were found to be chronic IHD and MI. However, we observed a significant decline in these events over time and an increase in HF as a cause of death. Similar trends have been noted elsewhere, including in the USA, whereby age-adjusted mortality (standardized to the 2,000 US population) attributed to IHD declined nationally between 2000 and 2015, but death attributed to HF saw an upward trend from 2012 to 2015.21 It is plausible that these findings reflect an increased focus and success in managing IHD, MI, and associated risk factors,22 whereas efforts to manage HFpEF and related diseases (eg, AF) have been less successful.23 Analysis of incident HF patient characteristics in cohort 1 according to year of HF diagnosis revealed significant trends in the proportion of patients with prior MI, which decreased from 13.5% in 2006 to 10.8% in 2013 (P<0.0001), suggesting that MI is becoming a less frequent cause of HF. Furthermore, an increase in mean CCI over time in incident patients was observed (from 1.4 in 2006 to 1.7 in 2013; P<0.0001). This increasing comorbidity burden might explain why the 1-year mortality of new onset HF remains high and stresses the need for intense evaluation and care of newly diagnosed patients with HF.
An important finding from this study was the limited use of echo for the diagnostic work-up of HF, particularly in primary care in which fewer than 20% of patients had an echo in the period 6 months before and after first HF diagnosis. A lack of availability of echo testing in the primary care setting may be one potential reason for this finding. There are also other scenarios that could explain this surprisingly low number, including longer than 6 months waiting times for echo validation, or if the patient is suspected of having HF, does not receive a diagnosis at the index visit, but is referred for an echo and is thereafter managed by nurses for more than 6 months, who support up-titration of medicines. By contrast, NT-proBNP testing during diagnostic work-up was used in the majority of patients in cohort 2 (83.5%), signifying a widespread acceptance of this diagnostic measure, which is readily available in primary care. Although the actual proportion of patients receiving an echo close to the time of their diagnosis may be slightly larger due to limitations in data extraction, nevertheless, these findings are notable because recommendations made by the European Society of Cardiology state that an echo is pivotal for the diagnosis of HF, because it provides information such as chamber volumes and ventricular systolic and diastolic function that is pertinent for establishing diagnosis and phenotype, and optimizing the treatment plan for patients.3 A substantial proportion of patients in our study (approximately two thirds) were aged ≥75 years. Elderly patients with HF tend to have a myriad of comorbidities that can interfere with the diagnostic process;3 they are also more likely to be managed by non-cardiologists.24 Indeed we note that patients in cohort 2 that had missing echo data had a considerably higher mean age, compared to both HFrEF and HFpEF.
Finally, from a gender perspective we confirm previously reported epidemiological differences with higher male incidence and prevalence.5 Interestingly, similar to previous studies,5 mortality was elevated among male patients in cohort 1, capturing the secondary care perspective, while this was not the case in the cohort 2, which was ascertained from EMR that also captures patients managed in primary care of Uppsala and Västerbotten counties. Provided this finding, ensuring that also female patients get similar access to echo is important.
A limitation of this study, as with most retrospective studies of this nature, was the challenge of identifying a cohort of patients with a confirmed and validated diagnosis of HF. Although the use of two diagnoses to define HF was expected to increase specificity, it could potentially have led to exclusion of more recent or milder cases of HF, as well as patients with the most severe disease who may have died after a single HF diagnosis. Diagnostic accuracy can also vary by care setting, while only 9% of patients in cohort 2 had diagnoses from primary care only. Another limitation concerned missing data for LVEF in cohort 2, where a large proportion of patients had missing values due to the absence of information on an echo. This may have introduced a level of bias for the diagnostic work-up evaluation, whereby echo use could be underestimated and also for the subgroups based on HF phenotype because it was not known whether missing values for LVEF were equally distributed between phenotypes. This makes it difficult to make broad assumptions on differences in epidemiology between HFpEF and HFrEF. However, patients with unknown LVEF tended to be older and have a high CCI, which indicates that these patients are more similar to patients with HFpEF than HFrEF. Moreover, owing to data constraints, the threshold for defining HFrEF was set as an LVEF of <50%, which is higher than the ESC recommendation of <40%, and therefore would have resulted in a larger number of patients being classified as having HFrEF.
Conclusion
The number of people developing HF annually has declined over time between 2010 and 2014, while those with HF have survived longer with their condition, as reflected by the decreased annual incidence rate and increased annual prevalence of HF. In addition, we witnessed a declining prevalence of prior MI among patients diagnosed in later vs earlier years, while more patients carried a greater comorbidity burden. Diagnosis of HF relied predominantly on NT-proBNP testing and, as such, diagnostic work-up of HF was suboptimal, with the majority of patients not being followed up with guideline-recommended echo investigations. Our data show that many patients receive their first diagnosis in the inpatient setting and that HF is becoming an increasingly common cause of death; this indicates an increasing burden and a need for better coordinated care and management of HF.
Data availability
Major findings from the study will be published in scientific manuscripts only. The data will not be made available in any other format in order to preserve the privacy of the patients in compliance with local laws and regulation.
Acknowledgments
Medical writing support was provided by Sharon Smalley and Carly Sellick of PharmaGenesis London, London, UK, and was funded by Novartis Pharma AG, Basel, Switzerland. The authors would like to thank Dr Gudrun Jonasdottir Bergman for her contributions to data collection and statistical analysis. Uppsala University received research funding from Novartis for conducting this study. IQVIA was commissioned to conduct the study on behalf of Novartis Pharma AG, and has ongoing consulting and research relationships with Novartis Pharma AG. This research was funded by Novartis Pharma AG, Basel, Switzerland.
Disclosure
K Lindmark, K Boman, and M Olofsson received lecture grants and consultant fees from Novartis. J Stålhammar received reimbursement from Novartis via IQVIA for performing the study. R Schlienger is an employee of Novartis Pharma AG, Switzerland, and S Bruce Wirta is an employee of Novartis Sweden AB, Sweden. The authors report no other conflicts of interest in this work.
References
Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of Disease Study 2015. The Lancet. 2016;388(10053):1545–1602. | ||
Roger VL. Epidemiology of heart failure. Circ Res. 2013;113(6):646–659. | ||
Ponikowski P, Voors AA, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;2016(37):2129–2200. | ||
Ohlmeier C, Mikolajczyk R, Frick J, Prütz F, Haverkamp W, Garbe E. Incidence, prevalence and 1-year all-cause mortality of heart failure in Germany: a study based on electronic healthcare data of more than six million persons. Clin Res Cardiol. 2015;104(8):688–696. | ||
Zarrinkoub R, Wettermark B, Wändell P, et al. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail. 2013;15(9):995–1002. | ||
Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93(9):1137–1146. | ||
Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(1):30–41. | ||
Schaufelberger M, Swedberg K, Köster M, Rosén M, Rosengren A. Decreasing one-year mortality and hospitalization rates for heart failure in Sweden; data from the Swedish hospital discharge registry 1988 to 2000. Eur Heart J. 2004;25(4):300–307. | ||
Dunlay SM, Roger VL. Understanding the epidemic of heart failure: past, present, and future. Curr Heart Fail Rep. 2014;11(4):404–415. | ||
Barasa A, Schaufelberger M, Lappas G, Swedberg K, Dellborg M, Rosengren A. Heart failure in young adults: 20-year trends in hospitalization, aetiology, and case fatality in Sweden. Eur Heart J. 2014;35(1):25–32. | ||
Piller LB, Baraniuk S, Simpson LM, et al. Long-term follow-up of participants with heart failure in the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). Circulation. 2011;124(17):1811–1818. | ||
Stewart S, Ekman I, Ekman T, Odén A, Rosengren A. Population impact of heart failure and the most common forms of cancer: a study of 1 162 309 Hospital cases in Sweden (1988 to 2004). Circ Cardiovasc Qual Outcomes. 2010;3(6):573–580. | ||
Christiansen MN, Køber L, Weeke P, et al. Age-specific trends in incidence, mortality, and comorbidities of heart failure in Denmark, 1995 to 2012. Circulation. 2017;135(13):1214–1223. | ||
de Freitas EV, Batlouni M, Gamarsky R. Heart failure in the elderly. J Geriatr Cardiol. 2012;9(2):101–107. | ||
Mant J, Doust J, Roalfe A, et al. Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. Health Technol Assess. 2009;13(32):1–207. | ||
Statistics Sweden. Consumer Price Index [Online]; 2016. Available from: http://www.scb.se/en_/Finding-statistics/Statistics-by-subject-area/Prices-and-Consumption/Consumer-Price-Index/Consumer-Price-Index-CPI. Accessed January 12, 2016. | ||
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. | ||
Conrad N, Judge A, Tran J, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. The Lancet. 2018;391(10120):572–580. | ||
Schmidt M, Ulrichsen SP, Pedersen L, Bøtker HE, Sørensen HT. Thirty-year trends in heart failure hospitalization and mortality rates and the prognostic impact of co-morbidity: a Danish nationwide cohort study. Eur J Heart Fail. 2016;18(5):490–499. | ||
Koudstaal S, Pujades-Rodriguez M, Denaxas S, et al. Prognostic burden of heart failure recorded in primary care, acute hospital admissions, or both: a population-based linked electronic health record cohort study in 2.1 million people. Eur J Heart Fail. 2017;19(9):1119–1127. | ||
Sidney S, Quesenberry CP, Jaffe MG, Sorel M, Go AS, Rana JS. Heterogeneity in national U.S. mortality trends within heart disease subgroups, 2000–2015. BMC Cardiovasc Disord. 2017;17(1):192. | ||
Szummer K, Wallentin L, Lindhagen L, et al. Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: experiences from the SWEDEHEART registry 1995–2014. Eur Heart J. 2017;38(41):3056–3065. | ||
Kotecha D, Lam CS, van Veldhuisen DJ, van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol. 2016;68(20):2217–2228. | ||
Lazzarini V, Mentz RJ, Fiuzat M, Metra M, O’Connor CM. Heart failure in elderly patients: distinctive features and unresolved issues. Eur J Heart Fail. 2013;15(7):717–723. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.