Back to Journals » Infection and Drug Resistance » Volume 17
Epidemiology, Microbiology, and Risk Factors of Bacterial Bloodstream Infections in Patients After Allogeneic Hematopoietic Stem Cell Transplantation
Authors Zhang R, Xiong Y, Zhang L, Liu L
Received 27 November 2023
Accepted for publication 27 March 2024
Published 20 April 2024 Volume 2024:17 Pages 1561—1569
DOI https://doi.org/10.2147/IDR.S451781
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Ruonan Zhang, Yiying Xiong, Linyi Zhang, Lin Liu
Department of Hematology, the First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Correspondence: Lin Liu, Email [email protected]
Purpose: To investigate the clinical characteristics, etiology, and risk factors of bacterial bloodstream infection (BSI) in allogeneic hematopoietic stem cell transplantation (allo-HSCT) recipients. This study also aimed to provide a clinical basis for early identification of high-risk patients and optimization of empirical antimicrobial treatment.
Patients and Methods: This is a retrospective study of clinical data during agranulocytosis from 331 patients with hematological malignancies who underwent allo-HSCT at our institute between January 2016 and December 2022. The incidence, distribution and drug resistance patterns, and the risk factors of BSI were analyzed.
Results: Among the 331 HSCT patients, 250 had febrile neutropenia and 45 cases were found to have BSI. The incidence of BSI in patients with agranulocytosis fever was 18% (45/250). A total of 48 pathogens were isolated during BSI episodes, gram-negative bacteria (GNB) accounted for 70.8% (34/48), gram-positive bacteria (GPB) for 29.2% (14/48). Multivariate analysis revealed that ≥grade 2 acute graft-versus-host disease (aGVHD) and previous BSI within 6 months before HSCT were independently associated with an increased occurrence of BSI. Coagulase-negative staphylococci (CoNS) and Escherichia coli were the most commonly isolated GPB and GNB, respectively. A total of 32 GNB were tested for drug susceptibility, the detection rate of carbapenem-resistant Enterobacteriaceae (CRE) was 12.5% (4/32), and extended-spectrum β-lactamase (ESBL) accounted for 56.3% (18/32).
Conclusion: BSIs are still a common and severe complication after allo-HSCT. In our center, BSIs in allo-HSCT patients are dominated by gram-negative bacteria and the resistance rate to carbapenem drugs is high. Risk factors for BSI during agranulocytosis were previous BSI within 6 months before HSCT and ≥grade 2 aGVHD.
Keywords: allogeneic hematopoietic stem cell transplantation, bloodstream infection, pathogenic bacteria, antibiotic resistance patterns, risk factors
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT), as a mature treatment method, has been widely used in the treatment of various hematological malignancies, bone marrow failure syndrome and severe immune deficiency diseases. Nevertheless, the risk of infection is significantly increased due to immunosuppression, neutrophilic deficiency, long-term central venous catheter retention and mucositis caused by high-dose chemoradiotherapy during the process of transplantation.1
Bacterial bloodstream infection (BSI) refers to systemic inflammatory response syndrome caused by the toxins and metabolites produced by pathogenic microorganisms after they invade the blood circulation system and grow and reproduce, which can lead to sepsis and multiple organ failure in severe cases.2 Specifically, BSI is a common infectious complication in the early stage of allo-HSCT, with a reported incidence of 13.6% to 38.9%.3–5 Despite significant advances in the management of transplant-related infections, BSI remains an important cause of non-relapse death in HSCT patients, and the crude mortality rate can reach up to 50%.6 Additionally, with the widespread utilization of broad-spectrum antibiotics, the emergence and prevalence of multi-drug-resistant bacteria (MDR) has become a major challenge in the field of global public health.
Despite numerous published studies on risk factors for BSI, many controversies exist among them, not all studies are in agreement. Moreover, the resistance patterns differ between transplantation centers, which is of particular importance considering the globally increasing rate of gram-negative BSI in allo-HSCT recipients during the last decades.7 Hence, we conducted a study to evaluate the prevalence, risk factors, distribution and drug resistance of pathogenic bacteria of BSI during neutropenia in allo-HSCT patients.
Materials and Methods
Patients
In this retrospective single-center study, we analyzed all BSI episodes occurring in allo-HSCT patients with neutropenia in the Hematology Department of the First Affiliated Hospital of Chongqing Medical University from January 2016 to December 2022. Patients with a fungal infection, contaminant result, or primary graft failure, excluded from this study. This study also did not evaluate BSI episodes occurring after the day of the second HSCT.
Conditioning Regimens and Graft-versus-Host Disease (GVHD) Prophylaxis
Transplantations were performed according to institutional protocols, and the choice of conditioning regimen was based on the patient’s age, underlying disease and pre-existing comorbidities. The conditioning regimens for most patients with hematological malignancies were based on BUCY (Busulfan + Cyclophosphamide), Modified FC (Fludarabine + Cyclophosphamide) + anti-thymocyte globulin (ATG) were used for severe aplastic anemia. Standard GVHD prophylaxis included either Cyclosporine A (CsA) or Tacrolimus (FK506) in combination with Methotrexate (MTX) and Mycophenolate mofetil (MMF), of which 268 cases were also treated with ATG to prevent GVHD.
Definitions
Febrile neutropenia was defined as a single oral temperature measurement of ≥38.3°C (axillary temperature≥38.0°C) or a temperature of ≥38.0°C (axillary temperature ≥ 37.7°C) for more than one hour and an absolute neutrophil count< 0.5 × 109 /L or expected to fall below <0.5 × 109/L.8 Engraftment was defined as the first of three consecutive days of an absolute neutrophil count of ≥0.5 × 109 /L. Day 0 was defined as the last date of stem cell infusion.
BSI was defined as isolation of a bacterial pathogen from at least 1 blood culture drawn from a neutropenic patient after the start of the conditioning regimen. The pathogens were classified as GPB and GNB. In case of the resident skin flora (such as coagulase-negative staphylococci and Corynebacteria), two or more consecutive blood cultures with the same strain were interpreted as clinically significant. BSI was considered polymicrobial if 2 or more pathogens were isolated in single blood culture or in separate blood cultures detected within 48h of the first BSI. Isolation of the same bacteria within 7 days was considered to be 1 episode.
MDR defined as the strain non-susceptible to at least one agent in ≥3 classes of antibiotics, including carbapenems, combinations of beta-lactams plus beta-lactamase inhibitors, cephalosporins, aminoglycosides, and fluoroquinolones.9 Bacteria reported as intermediate or resistant were considered resistant.
Acute GVHD (aGVHD) was diagnosed based on clinical symptoms and/or biopsies according to standard criteria10 and was graded according to well-established criteria.11
Infection Prophylaxis, Monitoring and Treatment
All patients received fluoroquinolones as antibiotic prophylaxis from the onset of conditioning until neutrophil engraftment. In the case of febrile neutropenia or any clinical manifestations consistent with an infection, blood cultures were drawn, prophylaxis was stopped, and empirical intravenous broad‐spectrum antibiotics treatment were initiated promptly. In our center, we use carbapenems (imipenem and meropenem) as first-line agents for patients with febrile neutropenia, reinforced with vancomycin or tigecycline if necessary GPB coverage. Antibiotic therapy was further modified based on the microbiology, diagnostic test results, and patient’s clinical signs.
Microbiological Investigation
All patients had an indwelling central venous catheter before the initiation of conditioning. When a febrile episode occurred, at least 2 sets of blood cultures (1 aerobic bottle and 1 anaerobic bottle) were drawn from a peripheral vein and from the central catheter. This process was repeated in 3 days when fever persisted. During the study period, the numbers of blood cultures drawn in the event of fever were not fixed, but varied. Blood specimens were processed and incubated using Alert 3D automated blood culture system (Biomerien X, French). Pathogen identification and antibiotic susceptibility analysis was done by VITEK2 COMPACT automatic blood culture instrument (Biomerien X, French). The susceptibility testing was calibrated to clinical breakpoints from the Clinical and Laboratory Standards Institute (CLSI) guidelines.12
Statistical Analysis
In this study, patients were divided into two groups according to whether BSI occurred. To determine the potential factors that may be influencing the occurrence of BSI, we assessed differences in demographic, clinical, and transplantation parameters using Chi-Squared or Continuity Correction test for categorical variables. Univariate models were built to determine the outcomes of interest based on baseline characteristics, including age, gender, diagnosis, history of previous BSI, chemotherapy course, type of transplant, the use of ATG, agranulocytosis time and aGVHD. Variables that were deemed significant (P < 0.1) based on the univariate models were then used to build a multivariate logistic regression model. As a measure of the strength of association, the odds ratio (OR) with 95% confidence intervals (95% CI) were computed. All P-values are two-sided, and P < 0.05 was considered statistically significant. The analysis was performed using SPSS.
Results
Incidence and Clinical Characteristics of BSIs
Finally, 331 patients recruited, the median patient age at transplantation was 33 (9–65) years, and male‐to‐female ratio was 1.1:1. Acute myeloid leukemia (44.7%), acute lymphoblastic leukemia (23.9%), and severe aplastic anemia (10.3%) were the most common underlying diseases, respectively. Among them, there were 222 patients received HLA-haploidentical HSCT, 85 patients underwent fully (donor-recipient) HLA-related matched HSCT and 24 patients underwent HLA-unrelated HSCT.
Among the 331 allo-HSCT patients, 250 patients had febrile neutropenia, and 45 patients were microbiologically diagnosed with BSIs. The incidence of BSI in patients with agranulocytosis fever was 18% (45/250). The median onset of BSI was +5 days after transplantation (range, day −10 to +12). There were 1 patient developed BSI twice and 2 patients had two or more pathogenic bacteria in our cohort.
Causative Pathogens and Antibiotic Resistance
During the study period, Gram-positive bacteria (GNB) predominated over Gram-positive bacteria (GPB) (70.8% vs 29.2%). Escherichia coli was the most common GNB (22/34, 64.7%), followed by Klebsiella pneumoniae (7/34, 20.6%). Coagulase-negative staphylococci (CoNS) was the most frequently isolated pathogen among GPB (9/14, 64.3%). The detailed etiology is shown in Table 1.
 |
Table 1 Resistance Rate of Major Gram-Negative Bacteria to Common Antibacterial Drugs |
We further carried out combined drug susceptibility test for 44 strains of pathogens. The resistance rate of GNB to fluoroquinolones was 78.1%. In GNB, 56.3% (18/32) produced ESBL, 21.9% (7/32) were resistant to carbapenems and Carbapenem-resistant Enterobacteriaceae (CRE) accounted for 12.5% (4/32). In GPB, the nine staphylococci were all reported as methicillin-resistant coagulase-negative Staphylococcus (MRCNS) and the enterococci was sensitive to vancomycin, tigecycline and linezolid.
Risk Factors for BSI
250 patients with agranulocytosis fever were divided into two groups: non-BSI (205) and BSI (45). The following factors were significantly correlated to BSI in univariate analysis using non-BSI as control group: AML (Acute myeloid leukemia), pre-treatment with ATG, ≥grade 2 aGVHD and history of previous BSI before HSCT (Table 2). Further multivariate analysis demonstrated that ≥grade 2 aGVHD and the history of previous BSI in prior 6 months were independent risk factors for the occurrence of BSI (Table 3).
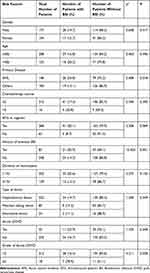 |
Table 2 Univariate Analysis of Risk Factors for BSI |
 |
Table 3 Multivariate Analysis of Risk Factors for BSI |
Other factors including, gender, age, the chemotherapy course, the type of donor, duration of neutropenia and having acute GVHD were not statistically associated with increased risk of BSI.
Outcomes
Among the 45 BSI patients, 4 cases died of complicated septic shock or sepsis, the overall mortality rate of BSIs in our center was 8.9% (4/45). Two of the fatal cases were because of septic shock caused by GNB (stenotrophomonas maltophilia and extended-spectrum beta lactamase producing E. coli), the other two patients had sepsis caused by enterococcus faecium and streptococcus mitis, respectively. A total of 11 patients without BSI died, including 5 patients died of pulmonary infection, 1 patient died of Epstein-Barr virus infection, 1 patient died of central nervous system infection, 1 patient died of intracranial hemorrhage, and 3 patients died of aGVHD. The early (0–100d) mortality of patients without BSI was 5.4%(11/205).
Discussion
BSI is a common complication in patients undergoing allo-HSCT and is remarkably associated with dismal prognosis.6 Due to immunodeficiency, patients with hematological malignancy are predisposed to developing BSI during the granulocytopenia period. Our study documented an incidence of BSI of 18% in febrile neutropenic patients who undergone allo-HSCT, as has been documented the literature.13 Accumulated evidence suggested that the epidemiology of BSI in HSCT patients changed over the past decades, with the reemergence of GNB as the predominant causative pathogens.14,15 Similarly, our study showed that GNB were the main pathogens (70.8%), followed by GPB (29.2%). Moreover, the majority of bacterial BSI episodes in our patients was attributed to gram-negative pathogens such as Escherichia coli and Klebsiella pneumoniae, which is in line with recent studies.16,17
In our cohort, the overall rate of multidrug resistance gram‐negative bacteremia was 78.1% of all GNB isolates, with Escherichia coli being the most commonly isolated pathogen. In the current study, the common Enterobacteriaceae (E. coli and K. pneumoniae) had ESBL detection rates of 61.9% and 42.9%, respectively, and the two isolates had acceptable sensitivity to carbapenems (84.4%).18 Consistent with previous reports, our study reemphasized that carbapenems could still act as the drugs of choice in ESBL associated BSIs in HSCT patients.19 Notably, the majority of GNB were quinolone resistant, raising concerns about the efficacy of the current strategy of fluoroquinolone antibiotic prophylaxis. It is therefore of utmost importance to closely monitor changes in the antibiotic-resistance patterns of BSI pathogens to ensure that breakthrough resistant pathogens do not impede the benefits of fluoroquinolones prophylaxis. Moreover, MDRGN pathogens showed a high resistance rate to available antimicrobial agents, which could be attributed to the increased use of carbapenems and aminoglycosides as front‐line therapy as well as changing global bacterial resistance.20 Accordingly, Clinicians should be alert to the abuse of antibiotics and adjust anti-infection strategies based on the antibacterial spectrum strictly. Although febrile neutropenia should be considered a medical emergency and a prompt administration of empirical antibiotic therapy is mandatory, increased mortality could be seen due to inappropriate empiric antibiotic therapy in setting with high rates of resistant pathogens.21–23 Surprisingly, we found that the sensitivity of Gram-positive isolates to the vancomycin, linezolid, and tigecycline was 100.0% in our setting and the drugs could be considered a good treatment choice as recommended by current guidelines.24
Numerous factors can influence the development of BSI after HSCT, such as the high-risk underlying disease, prolonged and profound neutropenia, severe mucositis, steroid use, and others.6,25–28 Our results unveil some factors potentially affecting the risk of BSIs such as AML, and pre-treatment with ATG. Nevertheless, only ≥grade 2 aGVHD and previous BSI within 6 months before HSCT retained significance in a multivariate analysis. The finding that the presence of a bacterial BSI prior to HSCT contributing to an elevated infectious risk is consistent with reports from other centers.29,30 In addition, some studies have reported that pretransplant infection with resistant GNB was highly predictive of a pre‐engraftment infection by a pathogen with the same susceptibility phenotype.28,31 These reinforcing evidences indicate the difficulty in truly eradicating pathogens and underscore the importance of thorough evaluation of residual infection in patients slated for HSCT. Thus, active and regular surveillance for detecting colonization, strict isolation measures, and prompt tailored therapy might be useful in order to formulate the optimal antimicrobial strategy and avoid horizontal transmission in the high-risk allo-HSCT population where MDR infections are frequent.32–35
GVHD is a series of clinical symptoms caused by the attack of donor T lymphocytes on recipient tissues and organs after allo-HSCT, we generally achieve the goal of reducing GVHD by adding ATG/ALG to remove T lymphocytes in vivo or inhibiting T lymphocyte activation with immunosuppressants. Nevertheless, GVHD and its prophylaxis and control strategies can simultaneously destroy mucosal protective barriers of the skin and gastrointestinal (GI) tract, enabling tremendous highly pathogenic bacteria to enter the systemic circulation and ultimately spread widely.36 It has been recognized through mouse models that acute GVHD targets Paneth cells and leads to a significant loss of intestinal flora diversity, leading to an increased risk of bacterial BSI.37 On top of that, several reports have demonstrated a close relationship between acute GI-GVHD and BSI.5,38,39 Interestingly, several studies identified that only severe acute GVHD is relevant to increased morbidity and mortality of BSI.40–42 In agreement with previous studies,43,44 we demonstrated that bacterial BSI during agranulocytosis was independently associated with a more than three-fold increased risk of subsequent ≥grade 2 aGVHD. Regarding these results, potential explanations include the elaboration of cytokines during BSI favoring the development of aGVHD and/or the immunosuppressive treatment of aGVHD favoring the development of BSI.
The utility of prophylactic antibiotics in patients undergoing HSCT is not completely clear. Girmenia et al28 reported that fluoroquinolone prophylaxis reduced the incidence of GNB bacteremia in auto-HSCT patients by half; however, there was no significant reduction on allo-HSCT patients. In addition, although fluoroquinolone prophylaxis has been associated with lower BSI rates, numerous landmark studies and meta-analyses have failed to demonstrate a reduction in mortality in patients with hematologic malignancies.45 Notably, the majority of GNB (78.1%) were fluoroquinolone‐resistant similar to a study by Mikulska showing a high incidence of fluoroquinolone resistance among GNB (74%).46 Therefore, identification of high-risk patients and regular surveillance of drug-resistance pattern in oncology centers is imperative to balance the potential morbidity and mortality of BSI against emerging drug resistance.
Clearly, the main limitation of our study is that it was a retrospective and single-center study with a geographically defined cohort, so there might be hidden biases in the analysis of the relationship and the results may not be generalizable to other centers or areas. Those limitations notwithstanding, it described the most important characteristics of bacterial BSIs in patients within vulnerable populations, such as those undergoing allo-HSCT.
Conclusion
In summary, this study provides important evidence in relation to the epidemiology, microbiology and risk factors of BSI after allo-HSCT, which is especially crucial since many previous reports were to some extent controversial. Early identification of high-risk recipients is necessary to reduce mortality of BSI and improve the overall efficacy of transplantation. This study demonstrated that ≥grade 2 aGVHD and previous BSI within 6 months before HSCT are risk factors for BSI. Additionally, GNB were the predominant causative pathogens in BSIs, and drug resistance to carbapenem and quinolone antibiotics was relatively high in our center. In this setting, local surveillance of antimicrobial resistance of GNB in HSCT patients are paramount to decide upon empiric antibiotic therapy for patients with neutropenic fever.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Statement
This study, which was in compliance with the Declaration of Helsinki, received ethical approval from the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. As neither individual data were published nor any intervention was performed on patients, patient consent was waived by the Ethics Committee.
Consent for Publication
All authors approved the final manuscript and the submission to this journal.
Funding
This project was supported and funded by the National Natural Science Foundation of China (No. 82070130).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yan CH, Wang Y, Mo XD, et al. Incidence, Risk Factors, Microbiology and Outcomes of Pre-engraftment Bloodstream Infection After Haploidentical Hematopoietic Stem Cell Transplantation and Comparison With HLA-identical Sibling Transplantation. Clin Infect Dis. 2018;67(suppl_2):S162–S173. doi:10.1093/cid/ciy658
2. Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32(3):858–873. doi:10.1097/01.ccm.0000117317.18092.e4
3. Kikuchi M, Akahoshi Y, Nakano H, et al. Risk factors for pre- and post-engraftment bloodstream infections after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2015;17(1):56–65. doi:10.1111/tid.12345
4. Mikulska M, Raiola AM, Galaverna F, et al. Pre-Engraftment Bloodstream Infections after Allogeneic Hematopoietic Cell Transplantation: impact of T Cell-Replete Transplantation from a Haploidentical Donor. Biol Blood Marrow Transplant. 2018;24(1):109–118. doi:10.1016/j.bbmt.2017.08.024
5. Mori Y, Yoshimoto G, Nishida R, et al. Gastrointestinal Graft-versus-Host Disease Is a Risk Factor for Postengraftment Bloodstream Infection in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Biol Blood Marrow Transplant. 2018;24(11):2302–2309. doi:10.1016/j.bbmt.2018.06.002
6. Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR. Blood stream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant. 2007;40(1):63–70. doi:10.1038/sj.bmt.1705690
7. Mikulska M, Viscoli C, Orasch C, et al. Aetiology and resistance in bacteraemias among adult and paediatric haematology and cancer patients. J Infect. 2014;68(4):321–331. doi:10.1016/j.jinf.2013.12.006
8. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):427–431. doi:10.1093/cid/ciq147
9. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol and Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
10. Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100(2):406–414. doi:10.1182/blood.v100.2.406
11. Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828.
12. Woods GL, Brown-Elliott BA, Conville PS, et al. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes.
13. Han TT, Huang XJ, Liu KY, et al. Blood stream infections during agranulocytosis period after hematopoietic stem cell transplantation in one single center. Zhonghua Nei Ke Za Zhi. 2011;50(8):654–658.
14. Weisser M, Theilacker C, Tschudin Sutter S, et al. Secular trends of bloodstream infections during neutropenia in 15 181 haematopoietic stem cell transplants: 13-year results from a European multicentre surveillance study (ONKO-KISS). Clin Microbiol Infect. 2017;23(11):854–859. doi:10.1016/j.cmi.2017.03.020
15. Puerta-Alcalde P, Cardozo C, Marco F, et al. Changing epidemiology of bloodstream infection in a 25-years hematopoietic stem cell transplant program: current challenges and pitfalls on empiric antibiotic treatment impacting outcomes. Bone Marrow Transplant. 2020;55(3):603–612. doi:10.1038/s41409-019-0701-3
16. Cao W, Guan L, Li X, et al. Clinical Analysis of Bloodstream Infections During Agranulocytosis After Allogeneic Hematopoietic Stem Cell Transplantation. Infect Drug Resist. 2021;14:185–192. doi:10.2147/IDR.S280869
17. Gill J, Busca A, Cinatti N, et al. Bacterial Bloodstream Infections after Allogeneic Hematopoietic Stem Cell Transplantation: etiology, Risk Factors and Outcome in a Single-Center Study. Microorganisms. 2023;11(3):742. doi:10.3390/microorganisms11030742
18. Amanati A, Sajedianfard S, Khajeh S, et al. Bloodstream infections in adult patients with malignancy, epidemiology, microbiology, and risk factors associated with mortality and multi-drug resistance. BMC Infect Dis. 2021;21(1):636. doi:10.1186/s12879-021-06243-z
19. Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Machuca I, Pascual A. Treatment of Infections Caused by Extended-Spectrum-Beta-Lactamase-, AmpC-, and Carbapenemase-Producing Enterobacteriaceae. Clin Microbiol Rev. 2018;31(2):e00079–17. doi:10.1128/CMR.00079-17
20. Taur Y, Xavier JB, Lipuma L, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55(7):905–914. doi:10.1093/cid/cis580
21. Retamar P, Portillo MM, López-Prieto MD, et al. Impact of inadequate empirical therapy on the mortality of patients with bloodstream infections: a propensity score-based analysis. Antimicrob Agents Chemother. 2012;56(1):472–478. doi:10.1128/AAC.00462-11
22. Stoma I, Karpov I, Milanovich N, Uss A, Iskrov I. Risk factors for mortality in patients with bloodstream infections during the pre-engraftment period after hematopoietic stem cell transplantation. Blood Res. 2016;51(2):102–106. doi:10.5045/br.2016.51.2.102
23. Martinez-Nadal G, Puerta-Alcalde P, Gudiol C, et al. Inappropriate Empirical Antibiotic Treatment in High-risk Neutropenic Patients With Bacteremia in the Era of Multidrug Resistance. Clin Infect Dis. 2020;70(6):1068–1074. doi:10.1093/cid/ciz319
24. Chinese Society of Hematology, Chinese Medical Association; Chinese Medical Doctor Association, Hematology Branch. Chinese guidelines for the clinical application of antibacterial drugs for agranulocytosis with fever. Zhonghua Xue Ye Xue Za Zhi. 2020;41(12):969–978.
25. Labarca JA, Leber AL, Kern VL, et al. Outbreak of Stenotrophomonas maltophilia bacteremia in allogenic bone marrow transplant patients: role of severe neutropenia and mucositis. Clin Infect Dis. 2000;30(1):195–197. doi:10.1086/313591
26. Herbers AH, de Haan AF, van der Velden WJ, Donnelly JP, Blijlevens NM. Mucositis not neutropenia determines bacteremia among hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2014;16(2):279–285. doi:10.1111/tid.12195
27. Gudiol C, Garcia-Vidal C, Arnan M, et al. Etiology, clinical features and outcomes of pre-engraftment and post-engraftment bloodstream infection in hematopoietic SCT recipients. Bone Marrow Transplant. 2014;49(6):824–830. doi:10.1038/bmt.2014.37
28. Girmenia C, Bertaina A, Piciocchi A, et al. Incidence, Risk Factors and Outcome of Pre-engraftment Gram-Negative Bacteremia After Allogeneic and Autologous Hematopoietic Stem Cell Transplantation: an Italian Prospective Multicenter Survey. Clin Infect Dis. 2017;65(11):1884–1896. doi:10.1093/cid/cix690
29. Ren J, Lin Q, Chen W, et al. G-CSF-primed haplo-identical HSCT with intensive immunosuppressive and myelosuppressive treatments does not increase the risk of pre-engraftment bloodstream infection: a multicenter case–control study. Eur J Clin Microbiol Infect Dis. 2019;38(5):865–876. doi:10.1007/s10096-019-03482-6
30. Youssef A, Hafez H, Madney Y, et al. Incidence, risk factors, and outcome of blood stream infections during the first 100 days post-pediatric allogeneic and autologous hematopoietic stem cell transplantations. Pediatr Transplant. 2020;24(1):e13610. doi:10.1111/petr.13610
31. Girmenia C, Rossolini GM, Piciocchi A, et al. Infections by carbapenem-resistant Klebsiella pneumoniae in SCT recipients: a nationwide retrospective survey from Italy. Bone Marrow Transplant. 2015;50(2):282–288. doi:10.1038/bmt.2014.231
32. Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–1238. doi:10.1016/j.bbmt.2009.06.019
33. Averbuch D, Orasch C, Cordonnier C, et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica. 2013;98(12):1826–1835. doi:10.3324/haematol.2013.091025
34. Girmenia C, Viscoli C, Piciocchi A, et al. Management of carbapenem resistant Klebsiella pneumoniae infections in stem cell transplant recipients: an Italian multidisciplinary consensus statement. Haematologica. 2015;100(9):e373–e376. doi:10.3324/haematol.2015.125484
35. Satlin MJ, Walsh TJ. Multidrug-resistant Enterobacteriaceae, Pseudomonas aeruginosa, and vancomycin-resistant Enterococcus: three major threats to hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2017;19(6):
36. Shono Y, van den Brink MRM. Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat Rev Cancer. 2018;18(5):283–295. doi:10.1038/nrc.2018.10
37. Eriguchi Y, Takashima S, Oka H, et al. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of α-defensins. Blood. 2012;120(1):223–231. doi:10.1182/blood-2011-12-401166
38. Levinson A, Pinkney K, Jin Z, et al. Acute gastrointestinal graft-vs-host disease is associated with increased enteric bacterial bloodstream infection density in pediatric allogeneic hematopoietic cell transplant recipients. Clin Infect Dis. 2015;61(3):350–357. doi:10.1093/cid/civ285
39. Satwani P, Freedman JL, Chaudhury S, et al. A Multicenter Study of Bacterial Blood Stream Infections in Pediatric Allogeneic Hematopoietic Cell Transplantation Recipients: the Role of Acute Gastrointestinal Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2017;23(4):642–647. doi:10.1016/j.bbmt.2017.01.073
40. Petersen J, Lindner C, Hakki M. Incidence and Outcomes of Bacterial Bloodstream Infections during Acute Graft-versus-Host Disease Involving the Gastrointestinal Tract after Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2019;25(8):1648–1653. doi:10.1016/j.bbmt.2019.04.016
41. Modi A, Rybicki L, Majhail NS, Mossad SB. Severity of acute gastrointestinal graft-vs-host disease is associated with incidence of bloodstream infection after adult allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2020;22(1):e13217. doi:10.1111/tid.13217
42. Inoue Y, Okinaka K, Fuji S, et al. Severe acute graft-versus-host disease increases the incidence of blood stream infection and mortality after allogeneic hematopoietic cell transplantation: Japanese transplant registry study. Bone Marrow Transplant. 2021;56(9):2125–2136. doi:10.1038/s41409-021-01291-0
43. Poutsiaka DD, Munson D, Price LL, Chan GW, Snydman DR. Blood stream infection (BSI) and acute GVHD after hematopoietic SCT (HSCT) are associated. Bone Marrow Transplant. 2011;46(2):300–307. doi:10.1038/bmt.2010.112
44. Blennow O, Mattsson J, Remberger M. Pre-engraftment blood stream infection is a risk factor for acute GVHD grades II–IV. Bone Marrow Transplant. 2013;48(12):1583–1584. doi:10.1038/bmt.2013.103
45. Mikulska M, Averbuch D, Tissot F, et al. Fluoroquinolone prophylaxis in haematological cancer patients with neutropenia: ECIL critical appraisal of previous guidelines. J Infect. 2018;76(1):20–37. doi:10.1016/j.jinf.2017.10.009
46. Mikulska M, Del Bono V, Raiola AM, et al. Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: reemergence of Gram-negative rods and increasing antibiotic resistance. Biol Blood Marrow Transplant. 2009;15(1):47–53. doi:10.1016/j.bbmt.2008.10.024
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
